Abstract
Background
Reflectance confocal laser scanning microscopy (R-CSLM) is a new diagnostic technique which allows visualization of "optical intersections" within the epidermis and superficial layers of the dermis. Outlines of cells and their architecture are imaged and may be analyzed both horizontally and vertically to the skin surface. The method proved useful in early melanoma detection. We evaluated the potential usefulness of this method in a short series of patients with hair diseases.
Main observations
Two healthy persons and 6 patients with hair diseases (1 with alopecia areata, 1 with androgenic alopecia and 4 with genetic hair shaft abnormalities) were examined with the use of Vivascope 1500. In all patients one scalp location and one location in the mid forearm were evaluated. R-CSLM examination gave in all cases high quality images of the hair shaft intersections, at 1µm intervals, which allowed detailed analysis of the hair structure. Hair follicles could be partly visualized at a depth of up to 200µm, which allowed analysis of only superficial parts of the hair follicles. An additional hurdle was bright reflection within the follicular ostia, which decreased the perception of details in these images.
Hair could be best visualized, when analyzed on flat surfaces. Receiving good quality images from convex surfaces on the scalp required additional effort from the patient (to not move) and from the physician (to obtain best possible fit of the "optic window" to the scalp).
Conclusions
These preliminary data show that R-CSLM may develop into a valuable tool in evaluation of hair shaft diseases. Further development is needed to apply this technique in abnormalities of the hair follicle and the perifollicular area.
Keywords: alopecia areata, androgenic alopecia, confocal microscopy, dermoscopy, hair, pili torti, trichoscopy, trichothiodystrophy
Introduction
Reflectance confocal laser scanning microscopy is an optical technique that allows non-invasive imaging of the upper portion of the skin at a resolution that permits visualization of cellular details with near histological resolution in real time. With a penetration depth of about 200µm, the technique allows non-invasive imaging of the epidermis and upper dermis.[1,2,3,4]
R-CSLM has been used for the assessment of benign and malignant pigmented lesions, in particular for early diagnosis of melanoma. Recent studies showed that the method has great potential in other skin tumors, such as basal cell carcinoma,[5,6,7] sebaceous gland hyperplasia,[8] eccrine poroma,[9] trichoepithelioma,[10] cherry angioma,[11] mycosis fungoides,[12] and in inflammatory skin diseases, such as psoriasis,[13] irritant and allergic contact dermatitis,[14,15] pemphigus foliaceus,[16] tinea,[17] scabies,[18,19] herpes virus infection,[20] and folliculitis.[21] Autoimmune connective tissue diseases, including systemic sclerosis[22] and discoid lupus erythematosus have been evaluated with confocal microscopy[23], and onychomycosis[24] was confirmed by this technique. The characteristics of R-CSLM images of precancerous lesions, such as actinic keratosis were described.[25,26]
Despite wide use of imaging techniques in diagnosing hair diseases, R-CSLM is currently not being used in trichology. By some authors, presence of hairs in an RCSLM image is even considered an artifact.[27]
We assessed the potential usefulness the method of R-CSLM for evaluation of hairs and hair follicles in patients with selected hair diseases.
Case Reports
Two healthy persons and 6 patients with hair diseases (1 with alopecia areata, 1 with androgenic alopecia, 1 with uncombable hair syndrome, 1 with pili torti, 2 with trichothiodystropy) with diagnoses confirmed based on clinical evaluation, trichoscopy and laboratory results were examined with the use of Vivascope 1500 (Lucid Inc, Rochester, New York). One scalp location and one location in the mid forearm were evaluated. In a proportion of patients hair was evaluated ex-vivo, after placing it on a sheet of paper.
R-CSLM examination gave in all cases high quality images of the hair shaft intersections, at 1µm intervals, which allowed detailed analysis of the hair structure [Fig. 1]. Hair shaft thickness, as well as the thickness of the medulla, cortex and cuticle could be evaluated in a majority of terminal hair. Hair shaft structure abnormalities could be visualized, as in trichoscopy, however with greater detail. In the patient with trichothiodystrophy hair R-CLSM showed a non-homogenous structure imitating grains of sand throughout the hair shaft [Fig. 2], which appeared to form a tiger tail-like arrangement in some images. In some images, the hairs had a slightly wavy contour.
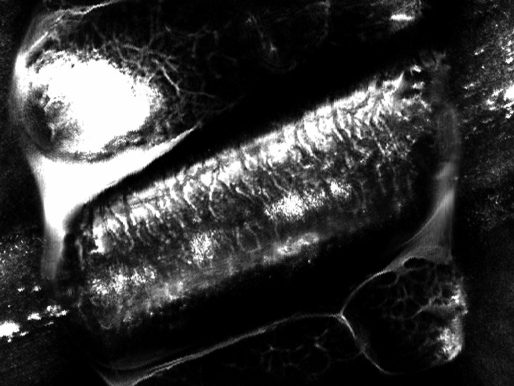
Figure 1.
A R-CSLM image of a healthy hair with intact cuticle layer.
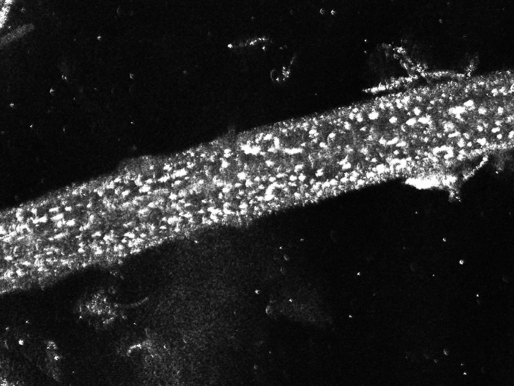
Figure 2.
A R-CSLM image of a hair from a patient with trichothiodystrophy shows characteristic grains throughout the hair shaft. This pattern is only rarely seen in patients with other hair diseases and in healthy persons.
In pili torti twists were seen at irregular intervals along the long axis of the shaft in some images. Upon R-CSLM hair shaft structure in healthy controls, patient in alopecia areata, and patient with androgenic alopecia had the same appearance, however in the patient with androgenic alopecia hair shaft thickness heterogeneity could be visualized.
In one of the patients additionally structures resembling head lice nits were observed, despite lack of clinical or trichoscopic symptoms indicating hair lice.
Hair could be best visualized, when analyzed on flat surfaces, either in vivo (forearm) or ex vivo (on a white sheet of paper). Receiving good quality images from the scalp required additional effort to obtain best possible fit of the "optic window" to the scalp. This was best achievable, when scalp and hair were evaluated at the vertex, while the patient was laying on the back.
Hair follicles could be only partly visualized and analyzed. The method gives best quality images at a depth of only 200µm, which allows visualization of superficial parts of the hair follicle [Fig. 3]. An additional hurdle is the bright reflection of keratin, which decreases the perception of hair follicle details in the image. Superficial hair follicle abnormalities, such as follicular ostia with cadaverized hair in alopecia areata could be analyzed in detail [Fig. 4].
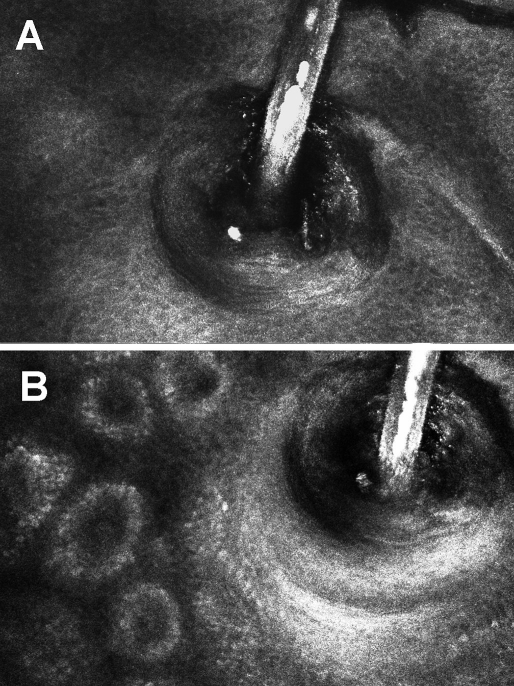
Figure 3.
A R-CSLM image of a hair follicle pictured at the level of the granular cell layer of the epidermis (A) and at the level of basal cell layer with visible intersections of dermal papillae (B). Full thickness of the hair shaft is visible with bright reflection of the medulla.
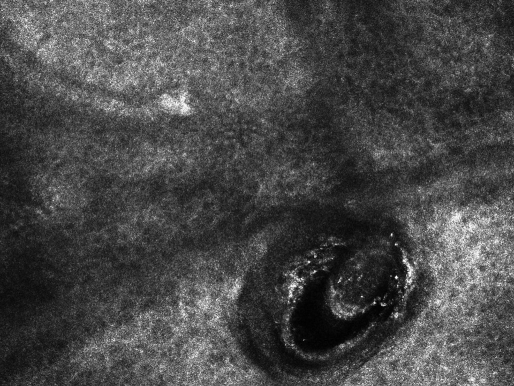
Figure 4.
A R-CSLM image of a cadaverized hair in hair follicle, as observed in the patient with alopecia areata.
Discussion
Diagnosis and therapy of hair and scalp diseases were in the recent years subject to significant progress. One of the major developments was employing imaging techniques, especially hair and scalp dermoscopy (trichoscopy) in dermatological practice.[28,29,30]
Trichoscopy allows in vivo visualization of hair and scalp structures at optical magnifications ranging up to 70-fold and more. Characteristic trichoscopy features of various hair and scalp diseases have been described. These include alopecia areata,[31] female androgenic alopecia,[32] trichotillomania,[28] tinea capitis,[33] monilethrix,[34,35] Netherton syndrome,[36] pili torti,[36] pili anulati,[36] trichthiodystrophy,[36] cicatricial alopecia,[37] and the "Tosti variant" of alopecia areata incognita.[38] The method is non-invasive and technically easy to perform in clinical practice.
An additional advantage of trichoscopy performed with the use of a digital videodermoscope is the possibility to perform measurement of visualized structures. A disadvantage of this method is the fact that in some cases structures of different histopathologic nature may give an undistinguishable trichoscopy appearance. Recently, it has been shown that "yellow dots", which were believed to represent hyperkeratotic plugs in hair follicles,[28,30,31] are frequently present in patients with female androgenic alopecia and may represent sebaceous glands or rather epidermal "sebum lakes".[32] Also the nature of "white dots", which are generally believed to represent perifollicular fibrosis has been questioned at a recent hair dermoscopy session ("La Dermatoscopia Del Cuoio Capelluto", Bologna, Italy, 2008).
These uncertainties generate a need for a new non-invasive technique, which would clarify the nature of structures, which can not be clearly identified by trichoscopy.
We performed a preliminary study in a small series of patients and healthy persons to evaluate, whether R-CLSM may fulfill this gap.
R-CLSM is an optical technique that can image thin slices within living intact human tissue, in a noninvasive manner, with near-histological resolution and high contrast. It has a penetration depth of about 200µm and allows visualization of the epidermis, papillary and superficial reticular dermis with near histological resolution.[1,2,3,4] The system generates 500x500µm fields of vision from a total 8x8mm area, which may be analyzed in a single session. These optical intersections may be evaluated in horizontal blocks, vertical stacks or "cubes", giving a pseudo-3D visualization of analyzed structures. The image contrast is mainly caused by variations in singly back-scattered light resulting from variations in the refractive index of tissue microstructures. Tissue structures with high refractive index act as an "endogenous stain" and appear white on confocal microscopy. Tissues with a low refractive index appear dark.[39]
R-CLSM, performed with the newly developed Vivascope 1500 equipment, allows direct comparison of trichoscopy (dermoscopy) images with an in vivo reflectance confocal microscopy life image performed in exactly the same location.
According to a recently published consensus terminology glossary[27] hair shafts appear in R-CLSM as a cylindrical or tubular long structure that has uniform high refractivity with no cellular pattern centrally, although some cellular pattern may be seen at its periphery. Usually it is seen emanating from a circular nonrefractive area that appears multidimensional, consistent with a hair follicle ostium (lumen). Authors of this paper consider hair as artifacts.[27]
In this preliminary study we evaluated R-CLSM hair images of healthy persons and patients with various hair diseases. Our major observation is that R-CLSM allows receiving high quality images of the hair shaft intersections, at 1µm intervals, which allows detailed analysis of the hair structure. Measurements of hair shaft structures may be performed. In particular hair shaft thickness, as well as the thickness of the medulla, cortex and cuticle may be measured in a majority of terminal hairs. Hair shaft structure abnormalities can be visualized, as in trichoscopy, however with greater detail.
An example may be trichothiodystrophy. R-CLSM showed in trichothiodystrophy a non-homogenous structure resembling grains of sand throughout the hair shaft, which may be present, but is less obvious in trichoscopy.[36] In some images these grains seemed to form a tiger tail-like arrangement, however this finding was less evident.
These grains may reflect severe cuticular and secondary cortical degeneration along almost the entire length of the hair shaft in sulfur-deficient patients with trichthidystrophy, shown previously by Venning et al[40] by means of scanning electron microscopy. According to these authors the cuticle protects hair from environmental damage and thus, cuticle damage may lead to the characteristic hair phenotype of trichothiodystrophy.
It has been later demonstrated that the outside surface of the hair shaft in trichothiodystrophy is markedly irregular and disorganized giving an appearance of hills, valleys, and gaps.[41] The proportion of hair shafts displaying structural abnormalities was related to sulfur content.[41]
Upon R-CSLM hair shaft structure in healthy controls, patient in alopecia areata, and patient with androgenic alopecia had the same appearance, however in the patient with androgenic alopecia hair shaft thickness heterogeneity could be visualized. This is consistent with data, which show that there is no hair shaft structure abnormality in alopecia areata and androgenic alopecia. Microexclamation mark hairs, which may be visualized in trichoscopy of active alopecia areata were not observed in our patient. This, however was a patient with a stable disease in the phase of hair re-growth.
It has to be emphasized that this equipment is generated to evaluate flat skin surfaces. Thus, hair could be best visualized, when analyzed on flat surfaces. Body hair and hair follicle ostia on flat skin surfaces, like the forearm, could be easily analyzed. Receiving good quality images from the scalp was significanly more complex and required additional time to obtain best possible fit of the "optic window" to the scalp. In some patients this was very difficult to achieve. Not in all cases were we able to receive good quality images of the scalp. A technique should be developed to help reduce patient's unintentional movements. It seems unlikely that R-CSLM, which requires a stable position for several minutes, could be easily used in small children. In this regard, trichoscopy has the advantage of shorter examination time.
To more precisely re-evaluate scalp hair we than placed a few hairs on a flat surface. It may be postulated that in patients suspected of hair shaft abnormalities single hairs may be chosen for R-CSLM by prior evaluation by trichoscopy.
Problems with scalp evaluation cause also difficulties in detailed evaluation of scalp hair follicles and scalp skin.
Hair follicles appear in R-CLSM as structures characterized by cells of different sizes in an ordered pattern consistent with cellular differentiation. The cells vary from small (ovoid or polygonal) basal differentiating cells to central larger (flatter) cells. Follicles are commonly associated with a hair shaft and a central lumen.[27]
Hair follicles on flat body surfaces, such as the forearms were examined effortless. Hair follicles may be examined up to a total deepness of 200µm, which is the upper part of the hair follicle.
According to literature data in vivo confocal microscopy allows visualization of infiltrating neutrophils of a subcorneal pustule in superficial folliculitis.[21] Our observations show that perifollicular hyperkeratosis may also be visualized by R-CLSM. In cases of intense perifollicular hyperkeratosis bright reflection slightly decreased the visibility of detailed perifollicular structures.
In conclusion, our preliminary data show that in vivo reflectance confocal scanning laser microscopy may develop into a valuable tool in evaluation of hair shaft diseases. Further development is needed to apply this technique in abnormalities of the hair follicle and the perifollicular area.
References
- González S, Gilaberte-Calzada Y. In vivo reflectance-mode confocal microscopy in clinical dermatology and cosmetology. Int J Cosmet Sci. 2008;30:1–17. doi: 10.1111/j.1468-2494.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- Branzan AL, Landthaler M, Szeimies RM. In vivo confocal scanning laser microscopy in dermatology. Lasers Med Sci. 2007;22:73–82. doi: 10.1007/s10103-006-0416-8. [DOI] [PubMed] [Google Scholar]
- González S, Gilaberte-Calzada Y, González-Rodríguez A, Torres A, Mihm MC Jr. In vivo reflectance-mode confocal scanning laser microscopy in dermatology. Adv Dermatol. 2004;20:371–387. [PubMed] [Google Scholar]
- Selkin B, Rajadhyaksha M, Gonzalez S, Langley RG. In vivo confocal microscopy in dermatology. Dermatol Clin. 2001;19:369–377. doi: 10.1016/s0733-8635(05)70274-6. [DOI] [PubMed] [Google Scholar]
- González S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J Am Acad Dermatol. 2002;47:869–874. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- Ahlgrimm-Siess V, Horn M, Koller S, Ludwig R, Gerger A, Hofmann-Wellenhof R. Monitoring efficacy of cryotherapy for superficial basal cell carcinomas with in vivo reflectance confocal microscopy: a preliminary study. J Dermatol Sci. 2009;53:60–64. doi: 10.1016/j.jdermsci.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Agero AL, Busam KJ, Benvenuto-Andrade C, Scope A, Gill M, Marghoob AA, González S, Halpern AC. Reflectance confocal microscopy of pigmented basal cell carcinoma. J Am Acad Dermatol. 2006;54:638–643. doi: 10.1016/j.jaad.2005.11.1096. [DOI] [PubMed] [Google Scholar]
- Propperova I, Langley RG. Reflectance-mode confocal microscopy for the diagnosis of sebaceous hyperplasia in vivo. Arch Dermatol. 2007;143:134. doi: 10.1001/archderm.143.1.134. [DOI] [PubMed] [Google Scholar]
- Tachihara R, Choi C, Langley RG, Anderson RR, González S. In vivo confocal imaging of pigmented eccrine poroma. Dermatology. 2002;204:185–189. doi: 10.1159/000057879. [DOI] [PubMed] [Google Scholar]
- Ardigo M, Zieff J, Scope A, Gill M, Spencer P, Deng L, Marghoob AA. Dermoscopic and reflectance confocal microscope findings of trichoepithelioma. Dermatology. 2007;215:354–358. doi: 10.1159/000107631. [DOI] [PubMed] [Google Scholar]
- Aghassi D, Anderson RR, González S. Time-sequence histologic imaging of laser-treated cherry angiomas with in vivo confocal microscopy. J Am Acad Dermatol. 2000;43:37–41. doi: 10.1067/mjd.2000.105560. [DOI] [PubMed] [Google Scholar]
- Agero AL, Gill M, Ardigo M, Myskowski P, Halpern AC, González S. In vivo reflectance confocal microscopy of mycosis fungoides: A preliminary study. J Am Acad Dermatol. 2007;57:435–441. doi: 10.1016/j.jaad.2007.02.026. [DOI] [PubMed] [Google Scholar]
- González S, Rajadhyaksha M, Rubinstein G, Anderson RR. Characterization of psoriasis in vivo by reflectance confocal microscopy. J Med. 1999;30:337–356. [PubMed] [Google Scholar]
- Astner S, Gonzalez E, Cheung A, Rius-Diaz F, González S. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986–992. doi: 10.1016/j.jaad.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Astner S, González E, Cheung AC, Rius-Diaz F, Doukas AG, William F, González S. Non-invasive evaluation of the kinetics of allergic and irritant contact dermatitis. J Invest Dermatol. 2005;124:351–359. doi: 10.1111/j.0022-202X.2004.23605.x. [DOI] [PubMed] [Google Scholar]
- Angelova-Fischer I, Pfeuti T, Zillkens D, Rose C. In vivo confocal laser scanning microscopy for non-invasive diagnosis of pemphigus foliaceus. Skin Res Technol. 2009;15:40–44. doi: 10.1111/j.1600-0846.2008.00332.x. [DOI] [PubMed] [Google Scholar]
- Markus R, Huzaira M, Anderson RR, González S. A better potassium hydroxide preparation? In vivo diagnosis of tinea with confocal microscopy. Arch Dermatol. 2001;137:1076–1078. [PubMed] [Google Scholar]
- Ahlgrimm-Siess V, Koller S, El Shabrawi-Caelen L, Hofmann-Wellenhof R, Kerl H. New diagnostic methods in dermatopathology: in vivo reflectance confocal microscopy. J Dtsch Dermatol Ges. 2008;6:591–592. doi: 10.1111/j.1610-0387.2008.06762.x. [DOI] [PubMed] [Google Scholar]
- Longo C, Bassoli S, Monari P, Seidenari S, Pellacani G. Reflectance-mode confocal microscopy for the in vivo detection of Sarcoptes scabiei. Arch Dermatol. 2005;141:1336. doi: 10.1001/archderm.141.10.1336. [DOI] [PubMed] [Google Scholar]
- Goldgeier M, Alessi C, Muhlbauer JE. Immediate noninvasive diagnosis of herpesvirus by confocal scanning laser microscopy. J Am Acad Dermatol. 2002;46:783–785. doi: 10.1067/mjd.2002.120616. [DOI] [PubMed] [Google Scholar]
- González S, Rajadhyaksha M, González-Serva A, White WM, Anderson RR. Confocal reflectance imaging of folliculitis in vivo: correlation with routine histology. J Cutan Pathol. 1999;26:201–205. doi: 10.1111/j.1600-0560.1999.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Sauermann K, Gambichler T, Jaspers S, Radenhausen M, Rapp S, Reich S, Altmeyer P, Clemann S, Teichmann S, Ennen J, Hoffmann K. Histometric data obtained by in vivo confocal laser scanning microscopy in patients with systemic sclerosis. BMC Dermatol. 2002;2:8. doi: 10.1186/1471-5945-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardigò M, Maliszewski I, Cota C, Scope A, Sacerdoti G, Gonzalez S, Berardesca E. Preliminary evaluation of in vivo reflectance confocal microscopy features of Discoid lupus erythematosus. Br J Dermatol. 2007;156:1196–2103. doi: 10.1111/j.1365-2133.2007.07808.x. [DOI] [PubMed] [Google Scholar]
- Hongcharu W, Dwyer P, Gonzalez S, Anderson RR. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42:214–216. doi: 10.1016/S0190-9622(00)90128-2. [DOI] [PubMed] [Google Scholar]
- Horn M, Gerger A, Ahlgrimm-Siess V, Weger W, Koller S, Kerl H, Samonigg H, Smolle J, Hofmann-Wellenhof R. Discrimination of actinic keratoses from normal skin with reflectance mode confocal microscopy. Dermatol Surg. 2008;34:620–625. doi: 10.1111/j.1524-4725.2008.34195.x. [DOI] [PubMed] [Google Scholar]
- Ulrich M, Maltusch A, Rius-Diaz F, Röwert-Huber J, González S, Sterry W, Stockfleth E, Astner S. Clinical applicability of in vivo reflectance confocal microscopy for the diagnosis of actinic keratoses. Dermatol Surg. 2008;34:610–619. doi: 10.1111/j.1524-4725.2007.34117.x. [DOI] [PubMed] [Google Scholar]
- Scope A, Benvenuto-Andrade C, Agero AL, Malvehy J, Puig S, Rajadhyaksha M, Busam KJ, Marra DE, Torres A, Propperova I, Langley RG, Marghoob AA, Pellacani G, Seidenari S, Halpern AC, Gonzalez S. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644–658. doi: 10.1016/j.jaad.2007.05.044. [DOI] [PubMed] [Google Scholar]
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- Inui S, Nakajima T, Nakagawa K, Itami S. Clinical significance of dermoscopy in alopecia areata: analysis of 300 cases. Int J Dermatol. 2008;47:688–693. doi: 10.1111/j.1365-4632.2008.03692.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Olszewska M, Rudnicka L. Trichoscopy criteria for diagnosing female androgenic alopecia. Nature Precedings. 2008 [hdl:10101/npre.2008.1913.1] [Google Scholar]
- Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, Lukomska M, Olszewska M, Szymanska E. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008;59:S77–S79. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222–224. [PubMed] [Google Scholar]
- Liu CI, Hsu CH. Rapid diagnosis of monilethrix using dermoscopy. Br J Dermatol. 2008;159:741–743. doi: 10.1111/j.1365-2133.2008.08705.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J Dermatol Case Rep. 2008;2:14–20. doi: 10.3315/jdcr.2008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui S, Nakajima T, Shono F, Itami S. Dermoscopic findings in frontal fibrosing alopecia: report of four cases. Int J Dermatol. 2008;47:796–799. doi: 10.1111/j.1365-4632.2008.03681.x. [DOI] [PubMed] [Google Scholar]
- Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, Micali G. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64–67. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha M, González S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- Venning VA, Dawber RP, Ferguson DJ, Kanan MW. Weathering of hair in trichothiodystrophy. Br J Dermatol. 1986;114:591–595. doi: 10.1111/j.1365-2133.1986.tb04066.x. [DOI] [PubMed] [Google Scholar]
- Liang C, Morris A, Schlücker S, Imoto K, Price VH, Menefee E, Wincovitch SM, Levin IW, Tamura D, Strehle KR, Kraemer KH, DiGiovanna JJ. Structural and molecular hair abnormalities in trichothiodystrophy. J Invest Dermatol. 2006;126:2210–2216. doi: 10.1038/sj.jid.5700384. [DOI] [PubMed] [Google Scholar]