Abstract
Background
Trichoscopy is a newly developed method of hair image analysis, based on videodermoscopy of hair and scalp.
Objective
The aim of the study was to establish normal values and set the standard for trichoscopy in female population.
Patients and methods
A total of 60 healthy females with no symptoms of hair or scalp diseases in anamnesis, upon clinical examination and in classic hair diagnostic techniques were included into the study. Mean age of these females was 36.5 (19-64) years. Trichoscopy was performed with the use of Fotofinder II. In all patients trichoscopy was performed in four locations (frontal area, occipital area, left and right temporal area). Hair and perifollicular area were evaluated. Measurements were performed with the application of the MoleAnalyzer software.
Results
Mean hair thickness was 0.061mm±0.008mm in frontal area vs. 0.057mm±0.007mm in occiput (p<0,001) and vs. 0.058mm±0.008mm in left temporal area and 0.059mm±0.008mm in right temporal area (p>0.005). The percentage of thin hairs (below 0.03mm) was 5%±4.3 in frontal area vs. 5.5%±4.8 in occiput vs. 6.4%±5.7 in right temporal area. The highest proportion of single-hair pilosebaceous units was observed in the temporal areas (29.1±16.2 vs. 23.2±13.5 in frontal and 18.4±12.1 in occipital areas; p<0.005). Based on study results, the norms for parameters measured in trichoscopy were assessed: mean hair thickness bigger than 0,053mm in frontal area and bigger than 0,050mm in others; percentage of thin hairs should be less than 10% in frontal and occipital area and less than 13% in temporal areas. The percentage of pilosebaceous units with single hair should be less than 35% in frontal area, 30% in occiput and 40% in temporal areas. Yellow dots were seen sporadically and they shouldn't be in a higher number than 3 in 4 fields of vision with 70-fold magnification in frontal area and only 1 in others. Perifollicular discoloration should be lower than 25% for frontal area, lower than 15% in occiput and 20% for temporal areas.
Conclusion
A standard procedure to perform trichoscopy (hair and scalp videodermoscopy) for diagnostic purposes was developed. Norms of measurable parameters were established for the population of adult white females.
Keywords: alopecia, dermoscopy, hair, trichoscopy, women, videodermoscopy
Introduction
Trichoscopy[1,2] is a newly developed method of hair image analysis, based on videodermoscopy of hair and scalp. The method allows visualization of hair shafts at high magnification and performing measurements, such as hair shaft thickness, without the need of removing hair for diagnostic purposes. It also allows in vivo visualization of the epidermal portion of hair follicles and perifollicular epidermis.[3,4]
The usual working magnifications are 20-fold to 70- fold. While the handheld dermoscope with 10-fold magnification may give easy and quick evaluation of hair, it does not precisely measure or document.
Several reports raise the issue of potential usefulness of this technique in diagnosing hair and scalp disorders, such as microsporiasis,[5] androgenic alopecia,[6] alopecia areata,[7,8] lipedematous alopecia,[9] pediculosis[10] or inherited hair shaft abnormalities,[11,12] but the method has not been standardized yet and there is little about what is normal value.
The aim of the study was to standardize the trichoscopy method and to establish the norms for measurable parameters in healthy females.
Patients and methods
A total of 60 female patients were included into the study. The mean age was 36.5 (range 19-64) years.
Trichoscopy was performed with the Fotofinder II videodermoscope, which permits scalp visualization at 20-160-fold magnification. The device is equipped with software which allows carrying out measurements of structures visualized in magnified images and provides results in real scale. Images of the scalp were taken at a 20- fold magnification which allows high quality enlargement of 1cm2 of scalp area to the size of a computer screen and at 70-fold magnification which magnifies in a similar manner an area of 9mm2.
In each patient one image at 20-fold magnification and 4 images at 70-fold magnification were taken each of following four areas: frontal, occipital, right temporal and left temporal.
Hair thickness was measured at 70-fold magnification, in direct proximity to follicular orifices. Hairs have been identified as "thin hairs" (below 0.03mm), "medium-sized hairs" (0.03-0.05mm) and "thick hairs" (above 0.05mm).
The images have been evaluated in accordance with the scheme presented in Table I.
Table I. Scheme of the trichoscopic images evaluation in presented study.
| Parameter | Method of the evaluation |
|---|---|
| Vellus hairs | number of vellus hairs calculated in one field of vision (FOV) at 20-fold magnification total number of vellus hairs in four FOVs at 70-fold magnification highest result in one FOV from the above (at 70-fold magnification) |
| Distribution of hair thickness | percentage of thin hairs (less than 0.03mm) percentage of medium-sized hairs (0.03mm to 0.05mm) percentage of thick hairs (above 0.05mm) mean hair thickness (mm) |
| Pilosebaceous units | percentage of single-hair units (at 20-fold magnification) percentage of double-hairs units percentage of triple-hairs units |
| Yellow dots | number of yellow dots per FOV calculated at 20-fold magnification number of yellow dots per FOV calculated in four fields of vision at 70-fold magnification the highest result from the above (at 70-fold magnification) |
| Perifollicular yellow discoloration (hyperpigmentation) | percentage of follicular ostia with perifollicular yellow discoloration calculated 20-fold magnification number of follicular ostia with perifollicular yellow discoloration in four FOVs at 70-fold magnification the highest result from the above (at 70-fold magnification) |
| Other | white dots (scarified follicular ostia), cadaverized hairs, broken hairs desquamation (rated 0-4) presence of abnormal blood vessels presence of exclamation mark hairs |
The norm's interval was set as the range of one standard deviation from mean value, two-sided.
Statistical analysis was performed with the use of Q Friedman test and W Wilcoxon analysis.
The study design was approved by the local ethics committee.
Results
Hair thickness
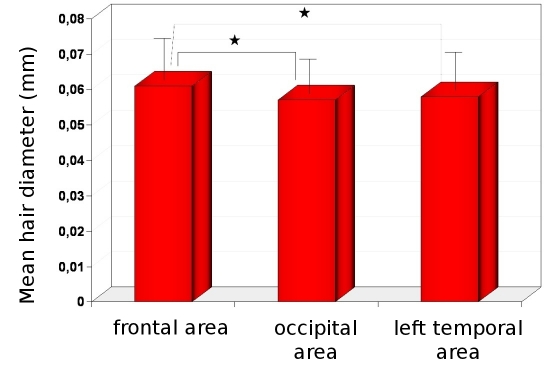
The thickest hairs have been observed in the frontal area, whereas the thinnest in the occipital area. Mean hair thickness was 0.061mm±0.008mm vs. 0.057mm ±0.007mm; respectively (p<0.001). The mean hair thickness in both temporal areas was similar to the thickness in occiput and the differences were not statistically significant (0.058mm±0.008mm in left temporal area and 0.059±0.008mm in right temporal area; p>0.005, Fig. 1).
Figure 1.
Mean hair diameter in frontal, occipital and temporal areas. Asterix indicates the most important, statistically significant differences between (p<0.001).
The norms for this parameter are 0.053-0.070mm for frontal area, 0.050-0.064mm for occipital area and 0.050- 0.067mm for both temporal areas.
Only the lower value is important from the clinical point of view, thus the norm for this parameter should be: mean hair thickness bigger than 0.053mm in frontal area and more than 0.050 mm in others.
Differences between left and right temporal area were statistically not significant in any of the investigated parameters. Thus, results for only one (left) temporal area are presented in figures.
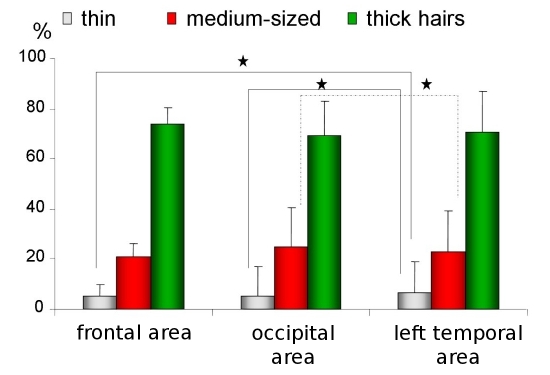
The thin hairs (below 0.03mm) were observed in similar percentage in all areas (5%±4.3 in frontal area vs. 5.5%±4.8 in occiput vs. 6.4%±5.7 in left temporal area, Fig. 2). The evaluated norms for this parameter are 0%- 10% for frontal and occipital area and 0%-13% for both temporal areas.
Figure 2.
Percentage of thin, medium-sized and thick hairs in frontal, occipital and temporal areas. Asterix marks statistically significant differences (p<0.005).
Only the higher value is important from the clinical point of view, thus the norm for this parameter should be: the percentage of thin hairs less than 10% in frontal and occipital area and less than 13% in temporal areas.
The thick hairs in all areas were in the highest percentage (74%±12.8 in frontal, 69%±16,6 in occipital, 70.9%±16,6 for left temporal areas, Fig. 2) and the norms for this parameter were set as 61%- 86% in frontal area, 52%-86% in occiput and 54%-87% for temporal areas.
Only the lower value is important from the clinical point of view, thus the norm for this parameter should be: the percentage of thick hairs bigger than 60% in frontal area and bigger than 50% in occipital and temporal areas.
All calculated normal values are listed in Table II.
Table II. Standarized scheme of hair and scalp evaluation in trichoscopy and norms for evaluated parameters.
| Frontal area | Occipital area | Temporal area | |
|---|---|---|---|
| number of vellus hairs calculated in one field of vision (FOV) at 20-fold magnification | 2 or less | 2 or less | 3 or less |
| total number of vellus hairs in four FOVs at 70-fold magnification | 0-7 | 0-6 | 0-9 |
| percentage of thin hairs (less than 0.03mm) | 0-10% | 0-10% | 0-13% |
| percentage of medium-sized hairs (0.03mm to 0.05mm) | 10-30% | 12-42% | 4-40% |
| percentage of thick hairs (above 0.05mm) | >60% | >50% | >50% |
| mean hair thickness (mm) | >0,053 | >0,050 | >0,050 |
| hair units (%) | Single hair units <35% Double hair units 46-70 Triple hair units >10% |
Single hair <30% Double hair units 45-73% Triple hair units >10% |
Single hair <40% Double hair units 50-75% Triple hair units >10% |
| Yellow dots (number in 4 fields of vision in 70-fold magnification | 0-3 | 0-1 | 0-1 |
| Percentage of follicular ostia with perifollicular yellow discoloration calculated in 20-fold magnification | <25% | <15% | <20% |
| Other parameters | absent | absent | absent |
Short hairs and vellus hairs
The analysis concerning only the hair root thickness does not permit to fully reflect the active processes observed during alopecia. The number of short and thin hairs can be counted on dermoscopic image in 20-fold magnification and results from our patients are: 4.1±2.7 in frontal area, 3.9±2.41 in occipital area and 4.7±4.13 in temporal area. The norms for this parameter are: less or equal 7 in frontal area, less or equal 6 in occipital area and 9 in temporal area.
The calculation in 4 field of vision at 70-fold magnification gives the results: 0.95±1.4 (norm: 0-2.35) in frontal area, 0.98±1.25 (norm: 0-2.23) in occipital area and 1.6±1.7 (norm: 0-2.85) in temporal area.
Based on these results it was established, that normal value for this parameter (calculated on dermoscopic image in 20-fold magnification) are: 2 short hairs in frontal and occipital area and 3 in temporal area.
Pilosebaceous units
Hairs usually are present in groups of few hair roots growing from one follicular orifice. The number of hairs in pilosebaceous units has been evaluated by trichoscopy at the 20-fold magnification. The percentage of singlehair, double-hair and triple-hair units was evaluated.
From the clinical point of view the most important is percentage of pilosebaceous units with single hair.
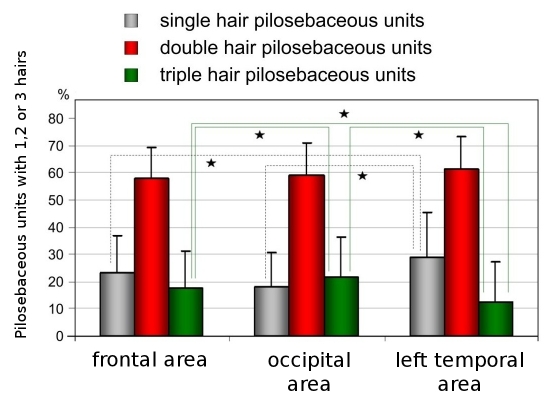
The highest proportion of single-hair pilosebaceous units was observed in the temporal areas (29.1%±16.2 vs. 23.2%±13.5 in frontal and 18.4%±12.1 in occipital areas; p<0.005, Fig. 3).
Figure 3.
Percentage distribution of pilosebaceous units with one, two and three hairs in frontal, occipital and temporal areas. Asterix marks statistically significant differences (p<0.005).
The biggest percentage in all areas is for pilosebaceous units with double hairs, pilosebaceous units with triple hairs are seen less common but gives information about hair condition (mean percentage 17.6±13.48 in frontal area, 21.4±14.9 in occiput and 12.3±14.9 in temporal areas, Fig. 3).
Mean percentage of pilosebaceous units with triple hairs in all areas of our patients was over 10.
The norms were established as listed in Table II.
"Yellow dots"
"Yellow dots", the parameter important in diagnosis of FAGA and alopecia areata, were evaluated at 20-fold magnification and at 70-fold magnification. Results are given as total number of yellow dots in one field of vision (FOV) at 20-fold magnification and as total number seen in four FOVs at 70-fold magnification. However, in general, the number of yellow dots per 1mm2 was on average 20% higher when evaluated at 70-fold magnification, as compared to 20-fold magnification. This difference resulted from better visualization of small trichoscopy structures at higher magnifications.
In both methods the yellow dots were seen occasionally. In method based on counting yellow dots in 4 images from 70-fold magnification the mean results were lower than 1 with median 0, but in frontal area standard deviation was 2.0. The norms for this parameter are only 1 for occipital and temporal areas and 3 for frontal area.
Perifollicular discoloration
The percentage of hair-containing units with perifollicular discoloration was evaluated at 20-fold magnification and in 4 FOVs at 70-fold magnification. Both methods yielded highly comparable results. Thus, results are presented for the 20-fold magnification only.
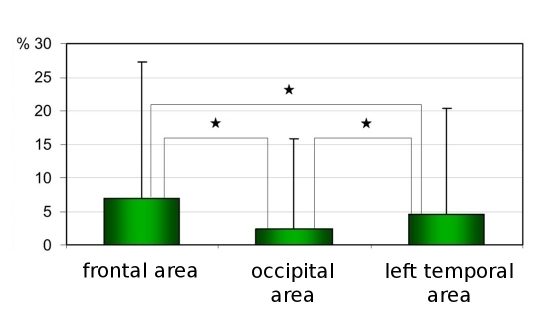
Perifollicular discoloration was found in a mean percentage of 7%±20.2 for frontal area, 2.4%±13.4 for occiput and 4.6±15.7% for left temporal area [Fig. 4]. The norms were assessed as less than 25% for frontal area, less than 15% for occiput and 20% for temporal areas.
Figure 4.
Percentage of pilosebaceous units with perifollicular discoloration evaluated on dermoscopic picture with 20-fold magnification. Asterix marks statistically significant differences (p<0.005).
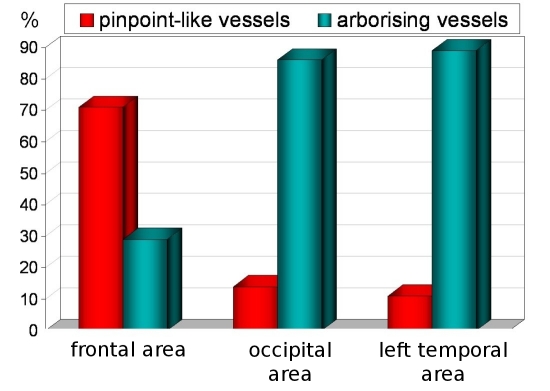
Types of blood vessels
Two types of blood vessels were seen - pinpoint-like vessels, which most commonly were seen in frontal scalp, and arborizing vessels, which dominates in occipital and temporal areas [Fig. 5].
Figure 5.
Distribution of arborising vessels and pinpoint-like vessels in frontal, occipital and temporal areas.
Other parameters
Other parameters (white dots, cadaverized hairs, broken hairs, degree of desquamation, presence exclamation mark hairs) were evaluated in accordance with the scheme presented in Table I and gave results which were nonsignificant. Only one exclamation mark hair, one cadaverized hair and one follicular defragmentation were seen in all dermoscopy images from our patients.
Discussion
Chronic hair loss frequently affects female patients, but there is little or no objective technology available to aid the dermatologist in setting a proper diagnosis and in monitoring treatment efficacy.[13] In particular, it may be difficult to differentiate between female androgenic alopecia (FAGA), a subtype of female pattern hair loss, and chronic telogen effluvium (CTE).[14] A scalp biopsy examination is performed usually to assessed the diagnosis in clinically doubtful cases.
The semi-invasive technique of hair root analysis (trichogram) has a decreasing number of advocates among dermatologists.[15]
Typically, punch biopsies are taken for vertical and horizontal embedding. These are a few millimeters in size and contain only a sample number of hair follicles. Thus, some authors suggest performing multiple biopsies for representative sampling, what increases the invasiveness of this diagnostic technique and makes it even less useful for monitoring of treatment efficacy.[15]
The need for non-invasive, but precise tool for effluvium diagnosis led us implement trichoscopy for diagnostic purposes in hair loss. Videodermoscopy of hair and scalp during last years became more popular, but there is no standardized method for this examination established.
We propose the examination scheme based on one dermoscopy picture in 20-fold magnification and four in 70- fold magnification. We also propose the quantification of all important parameters according to the Table I. Normal values for measurable parameters were established in this study based on a representative group of healthy adult white females and are presented in Table II.
A novel approach to quantify hair density in practical dermatology is evaluation of the number of hairs in one pilosebaceous unit. This was previously not possible with classical diagnostic method or the recently developed phototrichogram.[16] The number of hairs in one pilosebaceous unit varies from 1-3 in healthy persons. Occasionally four-hair units may be found. Interestingly, it has been observed in healthy controls that the temporal areas have the highest number vellus hairs and single-hair pilosebaceous units, indicating that physiologically these areas have lowest hair density, but in our observation (unpublished data) the most significant difference is a bigger distance between pilosebaceous units in this area.
Another important trichoscopy finding is perifollicular (discoloration) of the skin. This feature, called by some authors "hyperpigmentation" or "peripilar sign" is reflecting the presence of perifollicular lymphocytic infiltrates in early androgenic alopecia.[17] Our results confirm the presence of perifollicular discoloration also in healthy persons.
In conclusion, in this study standardized method for performing trichoscopy was established and normal values for white females were determined, which allows better understanding in clinical practice what is abnormal in hair and scalp videodermoscopy.
Acknowledgments
This study was performed as part of my PhD thesis and a fraction of a general project about trichoscopy, coordinated by professor Lidia Rudnicka and conducted at CSK MSWiA and Private Office "Dermatology Specialists" in Warsaw, Poland. I would like to thank all colleagues from both institutions for sharing with me their experience and supporting my work.
References
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Majsterek M, Czuwara J, Slowinska M. Presence and future of dermoscopy. Expert Rev Dermatol. 2006;1:769–772. [Google Scholar]
- Lacarrubba F, Dall'Oglio F, Rita Nasca M, Micali G. Videodermatoscopy enhances diagnostic capability in some forms of hair loss. Am J Clin Dermatol. 2004;5:205–208. doi: 10.2165/00128071-200405030-00009. [DOI] [PubMed] [Google Scholar]
- Ross EK, Vincenzi C, Tosti A. Videodermoscopy in the evaluation of hair and scalp disorders. J Am Acad Dermatol. 2006;55:799–806. doi: 10.1016/j.jaad.2006.04.058. [DOI] [PubMed] [Google Scholar]
- Slowinska M, Rudnicka L, Schwartz RA, Kowalska-Oledzka E, Rakowska A, Sicinska J, Lukomska M, Olszewska M, Szymanska E. Comma hairs: a dermatoscopic marker for tinea capitis: a rapid diagnostic method. J Am Acad Dermatol. 2008;59 (5 suppl):S77–79. doi: 10.1016/j.jaad.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005;4:637–640. [PubMed] [Google Scholar]
- Inui S, Nakajima T, Itami S. Dry dermoscopy in clinical treatment of alopecia areata. J Dermatol. 2007;34:635–639. doi: 10.1111/j.1346-8138.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- Tosti A, Whiting D, Iorizzo M, Pazzaglia M, Misciali C, Vincenzi C, Micali G. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59:64–67. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- Piraccini BM, Voudouris S, Pazzaglia M, Rech G, Vicenzi C, Tosti A. Lipedematous alopecia of the scalp. Dermatol Online J. 2006;12:6. [PubMed] [Google Scholar]
- Di Stefani A, Hofmann-Wellenhof R, Zalaudek I. Dermoscopy for diagnosis and treatment monitoring of pediculosis capitis. J Am Acad Dermatol. 2006;54:909–911. doi: 10.1016/j.jaad.2005.11.1083. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J Dermatol Case Rep. 2008;2:14–20. doi: 10.3315/jdcr.2008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222–224. [PubMed] [Google Scholar]
- Van Neste D. Female patients complaining about hair loss: documentation of defective scalp hair dynamics with contrast- enhanced phototrichogram. Skin Res Technol. 2006;12:83–88. doi: 10.1111/j.0909-752X.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Sinclair R, Jolley D, Mallari R, Magee J. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189–199. doi: 10.1016/s0190-9622(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Van Neste MD. Assessment of hair loss: clinical relevance of hair growth evaluation methods. Clin Exp Dermatol. 2002;27:358–365. doi: 10.1046/j.1365-2230.2002.01003.x. [DOI] [PubMed] [Google Scholar]
- Leroy T, Van Neste D. Contrast enhanced phototrichogram pinpoints scalp hair changes in androgen sensitive areas of male androgenetic alopecia. Skin Res Technol. 2002;8:106–111. doi: 10.1034/j.1600-0846.2002.00329.x. [DOI] [PubMed] [Google Scholar]
- Deloche C, de Lacharrière O, Misciali C, Piraccini BM, Vincenzi C, Bastien P, Tardy I, Bernard BA, Tosti A. Histological features of peripilar signs associated with androgenetic alopecia. Arch Dermatol Res. 2004;295:422–428. doi: 10.1007/s00403-003-0447-y. [DOI] [PubMed] [Google Scholar]