Abstract
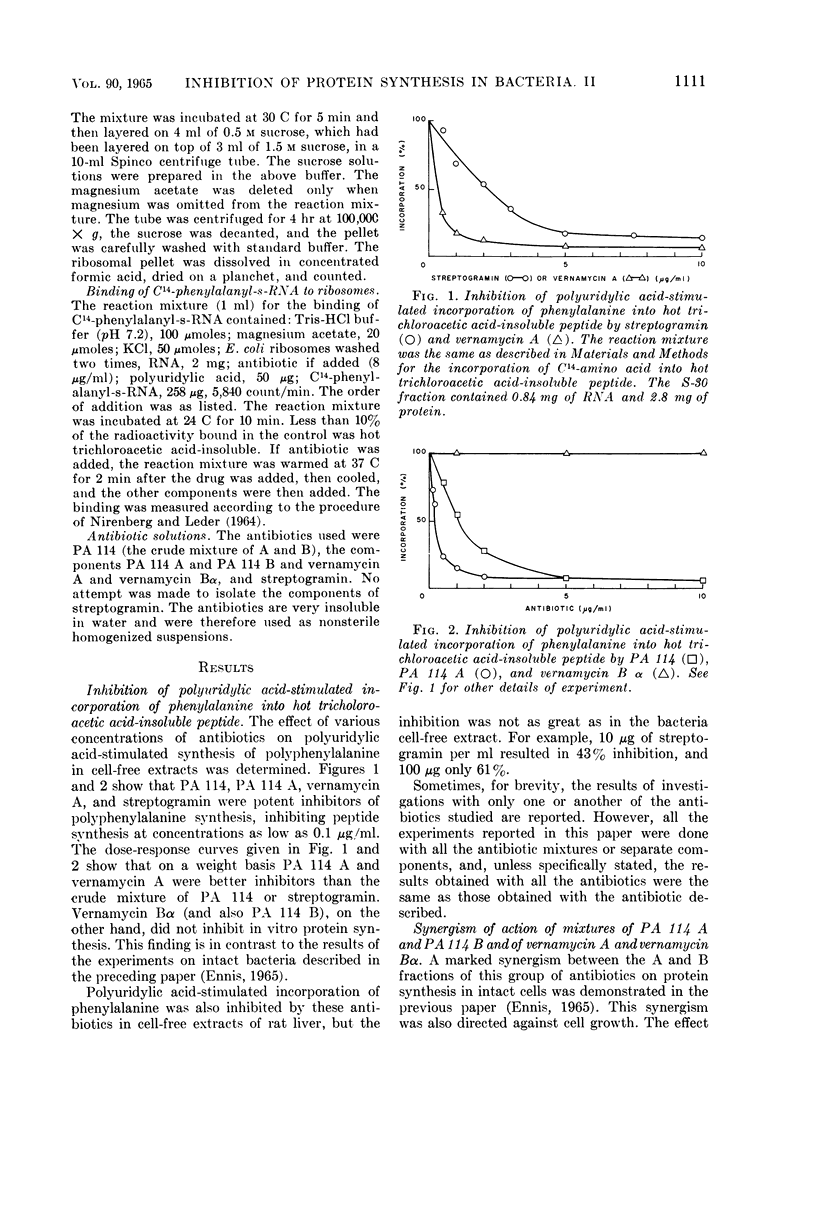
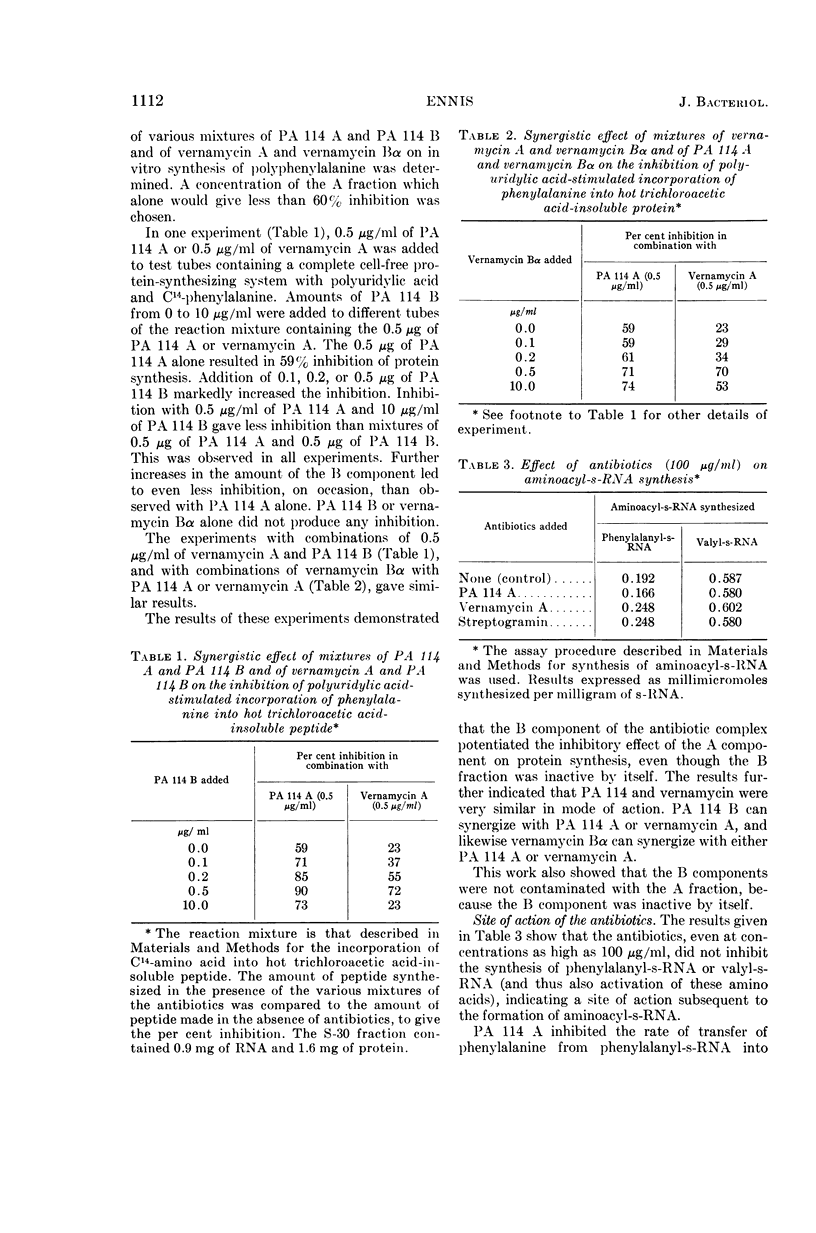
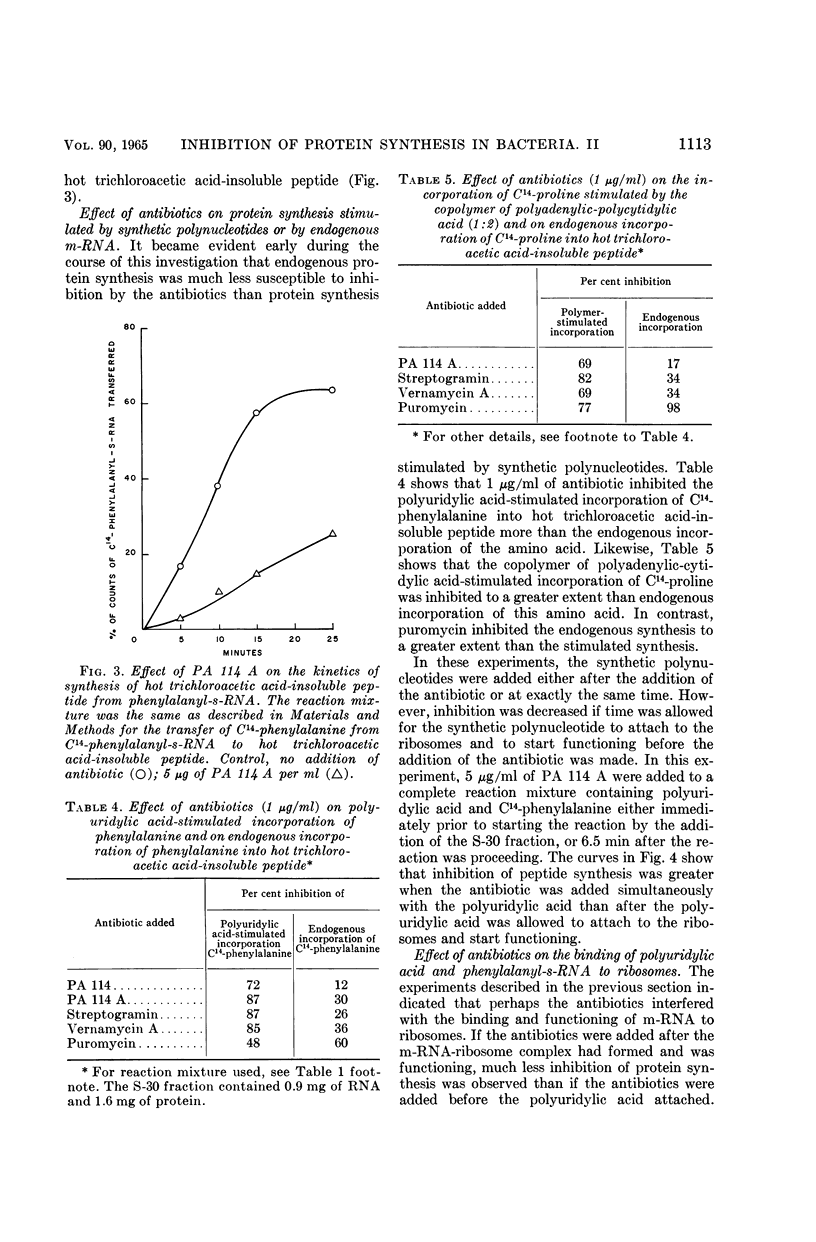
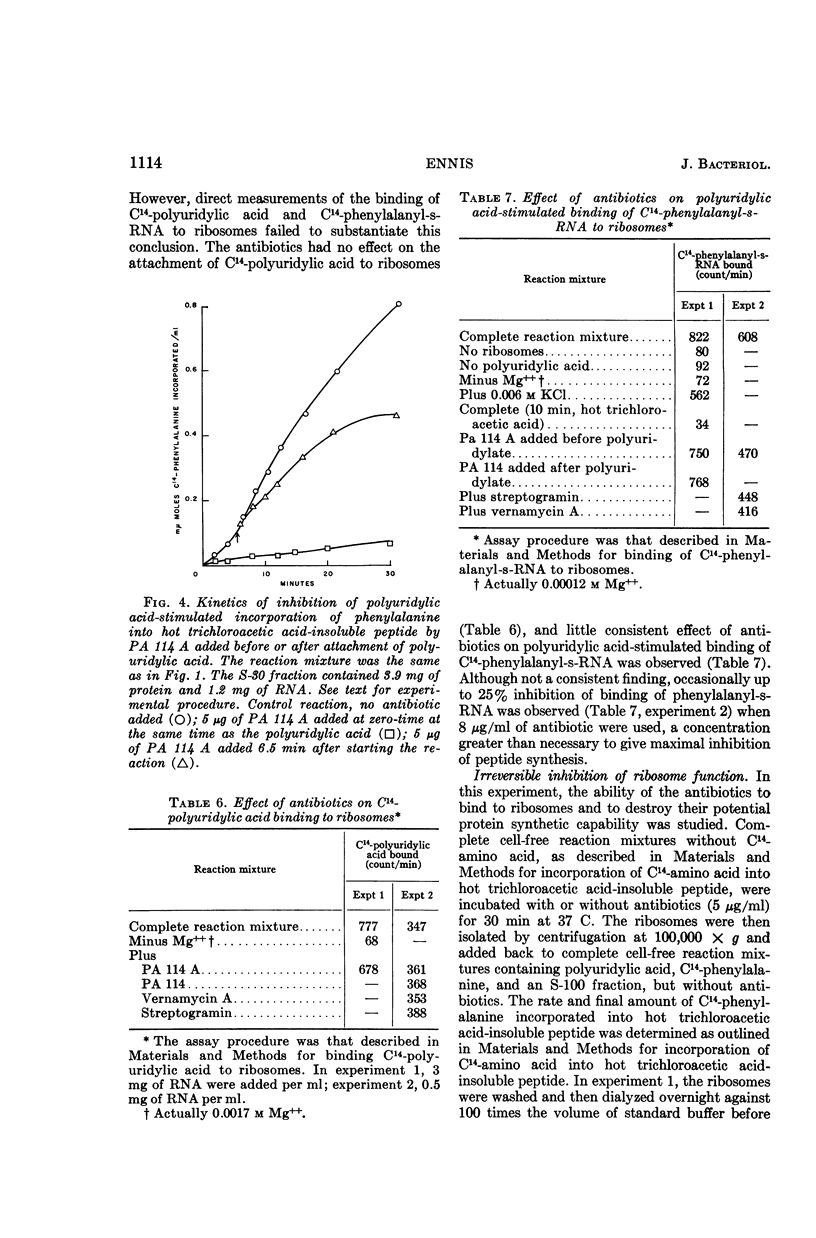
Ennis, Herbert L. (St. Jude Children's Research Hospital, Memphis, Tenn.). Inhibition of protein synthesis by polypeptide antibiotics. II. In vitro protein synthesis. J. Bacteriol. 90:1109–1119. 1965.—This investigation has shown that the polypeptide antibiotics of the PA 114, vernamycin, and streptogramin complexes are potent inhibitors of the synthetic polynucleotide-stimulated incorporation of amino acids into hot trichloroacetic acid-insoluble peptide. The antibiotics inhibited the transfer of amino acid from aminoacyl-soluble ribonucleic acid (s-RNA) to peptide. The A component of the antibiotic complex was active alone in inhibiting in vitro protein synthesis, whereas the B fraction was totally inactive. However, the A component, when in combination with the B component, gave a greater degree of inhibition than that observed with the A fraction alone. On the other hand, the endogenous incorporation of amino acid was much less susceptible to inhibition than the incorporation of the corresponding amino acid in a system stimulated by synthetic polynucleotide. In addition, synthesis of polyphenylalanine stimulated by polyuridylic acid was inhibited to a greater extent when the antibiotics were added before the addition of polyuridylic acid to the reaction mixture than when the antibiotics were added after the polynucleotide had a chance to attach to the ribosomes. However, the antibiotics apparently did not inhibit the binding of C14-polyuridylic acid or C14-phenylalanyl-s-RNA to ribosomes. The antibiotics did not affect the normal release of nascent protein from ribosomes and did not disturb protein synthesis by causing misreading of the genetic code. The antibiotics bind irreversibly to the ribosome, or destroy the functional identity of the ribosome. The antibiotic action is apparently a result of the competition between antibiotic and messenger RNA for a functional site(s) on the ribosome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BODANSZKY M., ONDETTI M. A. STRUCTURES OF THE VERNAMYCIN B GROUP OF ANTIBIOTICS. Antimicrob Agents Chemother (Bethesda) 1963;161:360–365. [PubMed] [Google Scholar]
- DAVIES J. E. STUDIES ON THE RIBOSOMES OF STREPTOMYCIN-SENSITIVE AND RESISTANT STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Apr;51:659–664. doi: 10.1073/pnas.51.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES J., GILBERT W., GORINI L. STREPTOMYCIN, SUPPRESSION, AND THE CODE. Proc Natl Acad Sci U S A. 1964 May;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis H. L. Inhibition of protein synthesis by polypeptide antibiotics. I. Inhibition in intact bacteria. J Bacteriol. 1965 Oct;90(4):1102–1108. doi: 10.1128/jb.90.4.1102-1108.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIEROWSKI M. INHIBITION OF PROTEIN SYNTHESIS BY CHLORTETRACYCLINE IN THE E. COLI IN VITRO SYSTEM. Proc Natl Acad Sci U S A. 1965 Mar;53:594–599. doi: 10.1073/pnas.53.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHAEI J. H., NIRENBERG M. W. Characteristics and stabilization of DNAase-sensitive protein synthesis in E. coli extracts. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1580–1588. doi: 10.1073/pnas.47.10.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS A. J., SCHWEET R. S. Release of soluble protein from reticulocyte ribosomes. Biochim Biophys Acta. 1961 Feb 18;47:415–416. doi: 10.1016/0006-3002(61)90310-9. [DOI] [PubMed] [Google Scholar]
- MORRIS A., ARLIGHAUS R., FAVELUKES S., SCHWEET R. INHIBITION OF HEMOGLOBIN SYNTHESIS BY PUROMYCIN. Biochemistry. 1963 Sep-Oct;2:1084–1090. doi: 10.1021/bi00905a030. [DOI] [PubMed] [Google Scholar]
- NATHANS D., von EHRENSTEIN, MONRO R., LIPMANN F. Protein synthesis from aminoacyl-soluble ribonucleic acid. Fed Proc. 1962 Jan-Feb;21:127–133. [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- TANAKA N., MIYAIRI N., WATANABE K., SHINJO N., NISHIMURA T., UMEZAWA H. Biological studies on mikamycin. II. Laboratory investigations of mikamycin A and mikamycin B. J Antibiot (Tokyo) 1959 Nov;12:290–297. [PubMed] [Google Scholar]
- VAZQUEZ D. Studies on the mode of action of streptogramin. Biochim Biophys Acta. 1962 Nov 26;61:849–851. doi: 10.1016/0926-6550(62)90075-0. [DOI] [PubMed] [Google Scholar]
- VON EHRENSTEIN G., LIPMANN F. Experiments on hemoglobin biosynthesis. Proc Natl Acad Sci U S A. 1961 Jul 15;47:941–950. doi: 10.1073/pnas.47.7.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI H. Action mechanism of mikamycins. I. Effects of mikamycins on protein and nucleic acid metabolisms. J Antibiot (Tokyo) 1961 Nov;14:313–323. [PubMed] [Google Scholar]
- YAMAGUCHI H., TANAKA N. SELECTIVE TOXICITY OF MIKAMYCINS, INHIBITORS OF PROTEIN SYNTHESIS. Nature. 1964 Feb 1;201:499–501. doi: 10.1038/201499a0. [DOI] [PubMed] [Google Scholar]


