Abstract
Background
Linear IgA bullous disease (LABD) is a rare mucocutaneous autoimmune subepidermal blistering disease that can affect children mostly of pre-school age. As many as two-thirds of LABD are related to drug ingestion, particularly certain antibiotics, non-steroidal anti-inflammatory drugs and diuretics.
Main observation
We describe a 3-year-old boy who presented a CMV infection followed by LABD induced by trimtheporim-sulfametoxazole. To our knowledge, this is the first reported case of trimethoprim-sulfamethoxazole that was confirmed by a rechallenge.
Conclusions
Most cases of drug-induced LABD are patients being treated with multiple systemic drugs that could induce the LABD. In the lack of suitable alternative treatment, the identification of the causative drug can be achieved by a rechallenge under close medical surveillance.
Keywords: autoimmune bullous disease, childhood, drug-induced, LABD, linear IgA bullous disease, trimethoprim-sulfamethoxazole
Introduction
Linear immunoglobulin A (IgA) bullous disease (LABD) is a rare mucocutaneous autoimmune subepidermal blistering disease that can affect children mostly of pre-school age.[1,2] LABD and chronic bullous disease of childhood are now considered to be a single entity.[3] The diagnosis of LABD is confirmed by detecting a linear band of IgA deposit at the basement membrane.[2] As many as two-thirds of LABD are related to drug ingestion, particularly certain antibiotics, non-steroidal anti-inflammatory drugs (NSAID) and diuretics.[1] In this case report, we describe a drug-induced LABD secondary to trimethoprim-sulfamethoxazole (TMP-SMX) which was possibly triggered by a CMV infection.
Case Report
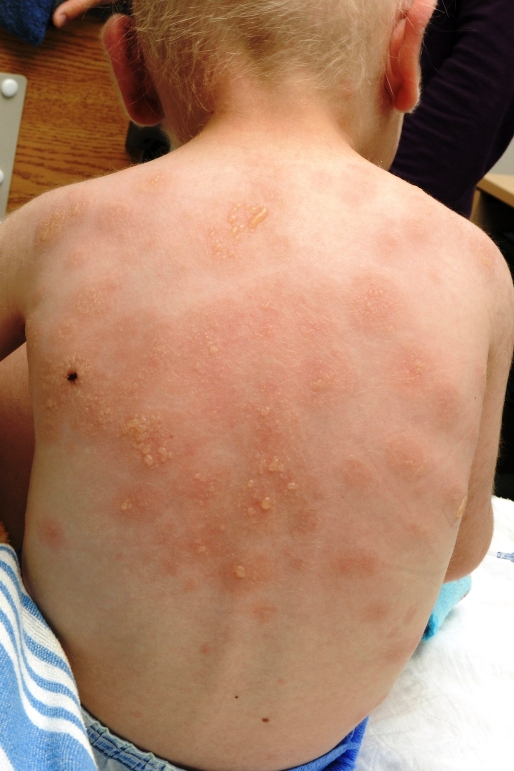
A 3-year-old Caucasian boy with idiopathic congenital thromobocytopenia presented a cutaneous blistering eruption. He had a history of two bonemarrow transplants and splenectomy. He was hospitalized for an infection with cytomegalovirus (CMV) that was treated with gancyclovir for 3 days prior to the onset of the skin eruption. The patient was also on prophylactic TMP-SMX (3 times a week) and penicillin V since 3 months for prevention of infections. On initial examination, the child presented a thoracic erythema-multiformelike targetoid maculopapular eruption. Crops of tense blisters in a characteristic "crown of jewels" pattern appeared progressively over the next 48 hours on the trunk with additional involvement of his ears, face and genital area. The blisters, filled with clear fluid, measured from 0.3 to 2.0 cm in diameter [Fig. 1]. There was no mucosal involvement and the patient maintained a good general status.
Figure 1.
Clusters of blisters arranged in a "crown of jewels" fashion over the posterior thorax.
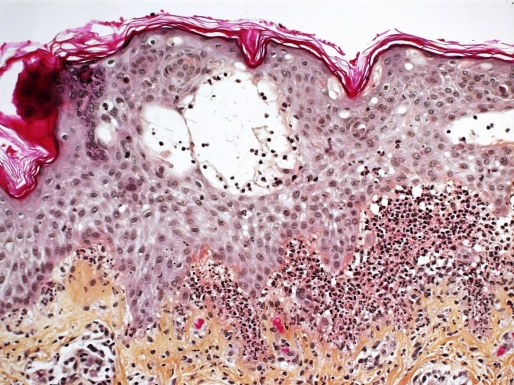
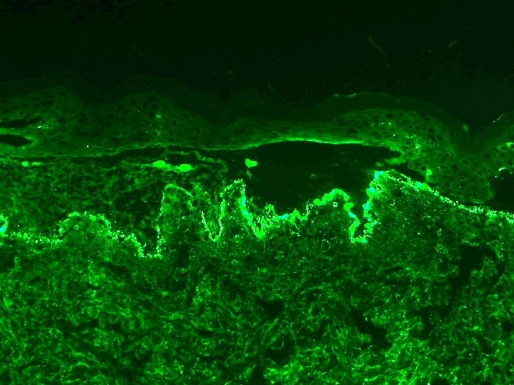
A skin biopsy of a dorsal lesion showed sub-epidermal bullae with neutrophilic abscesses within the dermal papillae [Fig. 2]. Direct immunofluorescence revealed a granular and linear deposition of IgA at the basement membrane zone [Fig. 3]. There was no evidence of CMV on histology or immunohistochemistry. Blood tests, including complete blood count, hepatic and renal functions were normal. A druginduced IgA bullous disease was suspected. Thus, gancyclovir, TMP-SMX and penicillin V were immediately stopped. The eruption kept progressing for 3 days then became stable after adding topical corticosteroids. Gancyclovir was reintroduced at day 5 of the eruption because the viral charge of the CMV was increasing. Subsequently, there was no exacerbation of the LABD. TMP-SMX was reintroduced 10 days after the onset of the eruption, which by then had almost completely resolved. Two days later, new erythematous plaques with blisters appeared over the patient's thighs. The recurrence of the lesions after the reintroduction of TMPSMX and the resolution after stopping this antibiotic while gancyclovir was continued, confirmed the diagnosis of LABD induced by TMP-SMX.
Figure 2.
Sub-epidermal blisters with neutrophilic abscesses, x200 hematoxylin phloxine saffron.
Figure 3.
Granular and linear deposits of IgA at the basement membrane zone within the dermal papillae, x200 direct immunofluorescence.
Discussion
The prevalence of LABD in childhood is unknown. The age of onset in children ranges from 1 to 10 years, with 64% of childhood cases presenting by the age of 6 to 8 years.[1,3] Clinically, drug-induced LABD may mimic bullous pemphigoid, erythema multiforme, dermatitis herpetiformis and toxic epidermal necrolysis.[1,4,5] In our patient, the initial eruption was erythema multiforme-like. The lack of oral and conjuctival involvement, as in our case, is suggestive of druginduced LABD.[3,5,6] However, mucosal lesions can be noted in up to 40% of drug-induced cases, which is still lower than the 80% involvement observed in the classical LABD.[7,11]
Childhood LABD has been mostly associated with infections or drug ingestion and rarely with other conditions such as ulcerative colitis and autoimmune lymphoproliferative disease.[2,9] The most frequently reported drugs that can induce LABD in adults are antibiotics such as vancomycin, penicillin G, ampicillin, cefamandole, TMP-SMX, rifampin and sulfisoxazole, NSAID such as piroxicam, naproxen and diclofenac, interferon-gamma, interleukin-2, phenytoin, amiodarone, captopril, iodine contrast agent, somatostatin, lithium, furosemide and cyclosporine.[1,4,5] Drug-induced LABD is not as well documented in childhood as it is in adults. The suspected causative drugs in the reported pediatric cases are NSAID and antibiotics such as amoxicillin-clavulanic acid and TMP-SMX.[1,3] To our knowledge, there have been 3 reports of LABD suspected to be induced by TMP-SMX in the medical literature.[3,10,11] The only pediatric possible TMPSMX- induced LABD was a 5-year-old child presenting acute lymphoblastic leukemia (ALL) in remission who received prophylactic treatment with TMP-SMX.[3] Our patient is the first case of LABD induced by TMP-SMX that was confirmed by a rechallenge. Adverse cutaneous drug reactions related to TMP-SMX are almost exclusively due to the SMX component and are more frequent in children receiving full dose therapy rather than prophylactic dose.[12]
The exact mechanism by which drugs can stimulate the immune system of a susceptible individual to produce IgA antibodies against the basement membrane in LABD is still unknown.[1,4] Some drugs may act as haptens, complexing with dermal/epidermal proteins, eliciting the autoimmune response.[1]
Idiopathic and drug-induced LABD may be triggered by a precipitating environmental cofactor such as physical trauma, burns, or infections.[3] The CMV infection of our patient might have predisposed him to develop the TMP-SMX-induced LABD. The gradual resolution of the blisters after stopping TMP-SMX and their recurrence after the rechallenge with TMP-SMX, confirm that TMP-SMX rather than the CMV infection caused the LABD.
The time interval between the introduction of the offending drug and the onset of LABD eruption ranges typically from 2 to 28 days.[1] The eruption appeared 3 months after starting prophylactic TMP-SMX in our patient and after 26 months of prophylactic TMP-SMX in the pediatric patient with ALL.[3] This might be due to the fact that the drug was given 3 times a week and not on a daily basis in both cases. Moreover, the CMV infection noted a few days prior to the drug-induced autoimmune reaction might have acted as a promoting cofactor in our case.
Resolution of the lesions of drug-induced LABD occurs spontaneously within 2 to 7 weeks following withdrawal of the offending medication without requiring LABD's specific treatment such as dapsone.[4,7,8] However, the lesions may continue evolving for several weeks due to persistent autoimmunity that might take months to subside.[1,10] Our patient healed progressively within 2 weeks after stopping TMP-SMX.
Conclusion
We describe a rare case of childhood LABD induced by prophylactic TMP-SMX which was confirmed by a positive rechallenge. Drug-induced LABD should be ruled out as an etiologic cause of LABD in children treated with systemic medications. Most cases of drug-induced LABD have been reported in patients treated with multiple systemic drugs and the determination of the causative drug is sometimes problematic.[8] In the lack of suitable alternative treatment in such patients, the identification of the causative agent can be achieved by a rechallenge under close medical surveillance. Life-threatening cutaneous drug eruptions such as anaphylaxis, Stevens-Johnson syndrome, toxic epidermal necrolysis and severe bullous diseases are contraindications for rechallenge.
References
- Ho JC, Ng PL, Tan SH, Giam YC. Childhood linear IgA bullous disease triggered by amoxicillin-clavulanic acid. Pediatr Dermatol. 2007;24:E40–43. doi: 10.1111/j.1525-1470.2007.00438.x. [DOI] [PubMed] [Google Scholar]
- Wong CS, Arkwright PD, Rieux-Laucat F, Cant AJ, Stevens RF, Judge MR. Childhood linear IgA disease in association with autoimmune lymphoproliferative syndrome. Br J Dermatol. 2004;150:578–589. doi: 10.1111/j.1365-2133.2004.05850.x. [DOI] [PubMed] [Google Scholar]
- Polat M, Lenk N, Kürekçi E, Oztaş P, Artüz F, Alli N. Chronic bullous disease of childhood in a patient with acute lymphoblastic leukemia: possible induction by a drug. Am J Clin Dermatol. 2007;8:389–391. doi: 10.2165/00128071-200708060-00010. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Pace JL. Drug-induced linear immunoglobulin-A bullous dermatosis. Clin Dermatol. 1998;16:389–391. doi: 10.1016/s0738-081x(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Acostamadiedo JM, Perniciaro C, Rogers RS. Phenytoin-induced linear IgA bullous disease. J Am Acad Dermatol. 1998;38:352–356. doi: 10.1016/s0190-9622(98)70582-1. [DOI] [PubMed] [Google Scholar]
- Panasiti V, Rossi M, Devirgiliis V, Curzio M, Bottoni U, Calvieri S. Amoxicillin-clavulanic acid-induced linear immunoglobulin A bullous dermatosis: case report and review of the literature. Int J Dermatol. 2009;48:1006–1010. doi: 10.1111/j.1365-4632.2009.04104.x. [DOI] [PubMed] [Google Scholar]
- Egan CA, Zone JJ. Linear IgA bullous dermatosis. Int J Dermatol. 1999;38:818–827. doi: 10.1046/j.1365-4362.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- Wiadrowski TP, Reid CM. Drug-induced linear IgA bullous disease following antibiotics. Australas J Dermatol. 2001;42:196–199. doi: 10.1046/j.1440-0960.2001.00515.x. [DOI] [PubMed] [Google Scholar]
- Handley J, Shields M, Walsh M, Bingham A. Chronic bullous disease of childhood and ulcerative colitis. Br J Dermatol. 1992;127:67–68. doi: 10.1111/j.1525-1470.1993.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Kuechle MK, Stegemeir E, Maynard B, Gibson LE, Leiferman KM, Peters MS. Drug-induced linear IgA bullous dermatosis: report of six cases and review of the literature. J Am Acad Dermatol. 1994;30:187–192. doi: 10.1016/s0190-9622(94)70015-x. [DOI] [PubMed] [Google Scholar]
- Paul C, Wolkenstein P, Prost C, Caux F, Rostoker G, Heller M, Wechsler J, Revuz J, Roujeau JC. Drug-induced linear IgA disease: target antigens are heterogeneous. Br J Dermatol. 1997;136:406–411. [PubMed] [Google Scholar]
- Karpman E, Kurzrock EA. Adverse reaction of nitrofurantoin, trimethoprim and sulfamethoxazole in children. J Urol. 2004;172:448–458. doi: 10.1097/01.ju.0000130653.74548.d6. [DOI] [PubMed] [Google Scholar]