Abstract
Background
Lichen sclerosus is a chronic muco-cutaneous inflammatory disorder of uncertain etiology. The prevalence of lichen sclerosus affecting only the oral mucosa is extremely rare and only 26 cases with histologically verified oral lichen sclerosus have been reported.
Main observations
A 60-year-old man was referred to our hospital for complaints of white lesions in the oral cavity, which was otherwise asymptomatic and did not have any cutaneous or anogenital lesions. Clinical examination revealed white patches with reddish areas on the buccal mucosa bilaterally and on the maxillary posterior gingiva. Microscopic analysis of the lesion showed atrophied epithelium with basal cell degeneration, hyalinized connective tissue stroma with minimal chronic inflammatory cell infiltrate. Verhoeff's staining revealed scantiness of elastic fibers in the connective tissue stroma. On the basis of these histological findings, the final diagnosis was given as Lichen Sclerosus. LS is rare in the oral cavity, particularly in the absence of simultaneous cutaneous and anogenital lesions.
Conclusions
Only 7 cases of oral LS have been reported involving the gingiva. To our knowledge, this is the eigth case to be reported with gingival involvement and the first case to be reported with bilateral involvement of buccal mucosa and gingiva.
Keywords: gingiva, lichen sclerosus, mucous membrane
Introduction
Lichen sclerosus (LS) is a chronic muco-cutaneous inflammatory disorder, preferentially affecting the skin and the genitals.[1] LS may occur in adults of all races and is five to ten times more common in females than in males.[2] The underlying etiology remains obscure, although various infections, trauma, genetic factors, hormonal and auto-immune related disorders have been theorized.[2]
Skin lesions of LS are clinically characterized by flat, stiff, shiny, porcelain white, polygonal papules that coalesce to form plaques. Most of the skin lesions are generally confined to the neck and trunk and are usually asymptomatic, although pruritus may occur.[3]
LS tends to affect the skin of the anogenital region and are characterized by areas of pallor. In contrast to LS of the skin, which rarely itches, there is often severe pruritus in the lesions involving vulvar region, which may lead to erosions and purpura. In men, the foreskin and glans penis are usually affected and often result in phimosis.[3]
Oral lesions of LS are rare and occur with or without lesions of the skin or vulva. Oral mucosal lesions appear as well demarcated, ivory or whitish, flat lesions similar to those seen in vulva but striae, erosions and ulcers clinically indistinguishable from lichen planus are also seen. The buccal, labial and palatal mucosa are the most common intraoral sites affected and the sex distribution is almost equal with no racial preferences.[4–10]
Histopathology of oral LS is characterized by gradual atrophic epithelium, subepithelial homogenization and hyalinization of the collagen fibers with an underlying band like lymphocytic inflammatory infiltrate. Hyperkeratosis may be absent in oral lesions but is always present in skin lesions.[3]
Treatment of oral LS consists of topical corticosteroids or excision. Treatment is unnecessary, if the patient is asymptomatic.[3]
The present report describes a case of LS manifesting only as lesions of oral mucosa, without any simultaneous involvement of cutaneous and anogenital region.
Case Report
A 60-year-old man presented with asymptomatic, white, non-scrapable patches and reddish areas in the right and left buccal mucosa and gingiva of upper and lower molar region. The duration was 6 months. The patient was otherwise well and not under any medication. No history of alcoholism, smoking and familial occurrence of similar disease were reported.
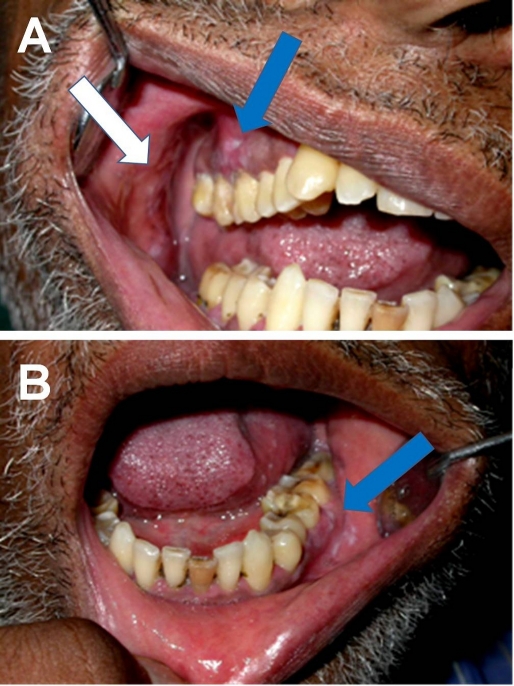
Clinical examination revealed white patches with reddish areas on the buccal mucosa bilaterally. The lesions were well delineated and approximately 2x2 cm in size. The gingiva in relation to 14, 15 and 16 [Fig. 1A] revealed similar lesions, approximately 2x3 cm in size. The gingiva and vestibular mucosa in relation to 35, 36 and 37 [Fig. 1B] also showed white patches with reddish areas. The teeth present in the involved region did not show any tooth mobility and the overall oral hygiene was poor. The clinical diagnosis was given as lichen planus. The patient was referred to a dermatologist and ruled out cutaneous and ano-genital manifestations.
Figure 1.
Clinical picture showing whitish patches with reddish areas involving the gingiva extending from right maxillary first premolar to maxillary molar (A). Whitish patches with reddish areas involving the gingiva and vestibular mucosa extending from left mandibular second premolar to second molar (B).
The incisional biopsy of the lesions from the left gingiva and buccal mucosa were submitted for histopathological evaluation. Microscopic examination of hematoxylin and eosin stained sections showed hyperplastic stratified squamous epithelium with areas of progressive atrophy, along with evidence of hydropic degeneration of basal cells in few areas. The subjacent connective tissue showed dense and evenly hyalinized band of fibrosis along with patchy areas of inflammatory cell infiltrate. Scantiness of elastic fibers in the connective tissue stroma was revealed by Verhoeff elastic stain. Based on the clinical and histopathological features, a diagnosis was given as LS. Subsequently, incisional biopsies from other affected intra-oral regions were also consistent with LS.
The patient underwent complete oral prophylaxis and was given instructions for oral hygiene maintenance. As the lesions were asymptomatic, no treatment instituted and the patient was under follow-up. Cutaneous and anogenital lesions did not develop during the follow-up period of 2 years.
Discussion
According to the reports in literature, LS affecting only the oral mucosa is extremely rare. Only 26 cases have been reported with verified histopathology [Table 1]. No cutaneous or genital lesions developed after the follow-up period in any of the reported cases or in our case. This suggests that oral LS may appear without accompanying skin or genital lesions.[10]
Table 1. Reported cases of isolated oral lichen sclerosus.
| S. No | Author(s) | Year of publication | Patient's age & sex |
Oral site(s) | Extra oral lesions |
|---|---|---|---|---|---|
| 1 | Miller et al.4 | 1957 | 48 F | Buccal mucosa | Genital, perineal, trunk, neck and extremities |
| 2 | Ravits et al.5 | 1957 | 24 M 42 M |
Buccal mucosa, Gingiva Buccal mucosa, palate, anterior tonsillar pillar |
None Genital |
| 3 | Kaminsky et al.29 | 1974 | 26 F | Upper lip w/extension intraorally onto labial mucosa and gingiva | Skin of lower lid and lateral nasal area |
| 4 | de Araujo et al.6 | 1985 | 26 F | Maxillary gingiva midline extending into vestibular mucosa | None for 1 year follow-up period |
| 5 | Siar et al.11 | 1985 | 25 M | Buccal mucosa | No comment |
| 6 | Macleod et al.7 | 1991 | 57 F | Tongue and palate | None |
| 7 | Schulten et al.8 | 1993 | 59 F 12 M |
Labial mucosa, commissure and tongue Labial mucosa |
None None |
| 8 | Brown et al.9 | 1997 | 44 M 18 M |
Soft palate midline Lower lip mucosa and vermilion |
None None for 10 month follow-up period |
| 9 | Buajeeb et al.10 | 1999 | 22 F | Gingiva, oral mucosa | None |
| 10 | Kaur et al.33 | 2002 | 16 M | Upper lip and Gingiva | Present |
| 11 | Jensen et al.3 | 2002 | 10 F | Buccal mucosa | None |
| 12 | Jimenez et al.13 | 2002 | 19 F | Gingiva, buccal sulcus and lip | None |
| 13 | Mendonca et al.28 | 2004 | 20 F | Lower lip, labial mucosa | None |
| 14 | Rajlawat et al.34 | 2004 | 14 F | Lower lip | None |
| 15 | Chaudhry et al.31 | 2006 | 60 F | Dorsum of the tongue | Present |
| 16 | Kelly et al.30 | 2006 | 10 F | Lower lip, labial mucosa | None |
| 17 | Walsh et al.35 | 2008 | 16 M | Vermillion border of lip and labial mucosa | Present |
| 18 | Jimenez et al.32 | 2008 | 31 F | Gingiva, buccal mucosa and labial mucosa | Present |
| 19 | Azevedo et al.36 | 2009 | 19 M 34 F 11 F 38 F 31 F 28 F |
Buccal and labial mucosa Upper lip and labial mucosa Vermillion upper lip and labial mucosa Buccal mucosa and labial mucosa Lower labial mucosa extending to lateral border of tongue Dorsum of tongue, buccal and labial mucosa |
None None None Present Present None |
| 20 | Present case | 2009 | 60 M | Gingiva and buccal mucosa | None |
Oral LS has been described on labial and buccal mucosa, lip, gingiva, palate, tongue and anterior tonsillar pillar. These lesions manifest as white macules or plaques[3,9] with reticular striations[2,11] and superficial ulceration.[7,12] LS is usually asymptomatic,[10] as in our case. Only seven cases of oral LS have been reported involving the gingiva [Table 1]. To our knowledge, this is the eighth case to be reported with gingival involvement and the first case to be reported with bilateral involvement of buccal mucosa and gingiva.
Clinically, it may be difficult to distinguish oral LS from other oral white lesions, especially lichen planus, leukoplakia and localized scleroderma.[13] Oral lichen planus usually presents as white reticular striations and only a minority of patients present with dense white homogenous plaques. Oral leukoplakias shows a variety of appearances, ranging from fine white flecks to dense thick plaques and are commonly seen in smokers and users of smokeless tobacco. Distinguishing oral LS from localized scleroderma clinically is difficult and histopathology may be helpful in establishing the correct diagnosis.[10]
The histologic features of cutaneous LS are characteristic, including follicular plugging, atrophy of epidermis with vacuolar degeneration of the basal layer, edema, homogenization of the collagen and scantiness or loss of elastic fibers in the upper dermis, and an inflammatory infiltrate in the mid derms.[14] The histologic features of oral LS are sufficiently similar to those of LS in the skin.[10]
De Araujo et al. have concluded that the characteristic features of oral LS are pronounced edema and homogenization of the collagen fibers within the lamina propria, together with hydropic degeneration of the basal cells6 which was also evident in our case. Direct immunofluorescent studies may reveal immunoglobulin G, C3 and fibrinogen at the basement membrane level.[15,16]
The cause of LS is unknown. Over the years, a number of etiologies have been proposed. Several studies have linked borrelial or other infections with LS, yet other studies have disputed this and the current reports along this line are few. Genetic and autoimmune factors have been explored without identification of consistent, reproducible patterns. Local irritation seems to play a role in some cases. But the sequence of events that leads to the altered fibroblast function, microvascular changes, and hyaluronic acid accumulation in the upper dermis continues to be researched. Inflammation and altered fibroblast function in the papillary dermis leads to fibrosis of the upper dermis. Several studies have recently identified the presence of autoantibodies to the glycoprotein extracellular matrix protein 1 (ECM1).[17]
Moreover, other recent studies have identified circulating autoantibodies against the ECM1 protein in most patients with lichen sclerosus. ECM1 thus serves as a target antigen such disorders. Within the epidermis, ECM1 has a role in the control of keratinocyte differentiation. Within the dermis, ECM1 binds to the major heparan sulphate proteoglycan, perlecan. In this way, ECM1 may act as "biological glue" in the dermis, helping to regulate basement membrane and interstitial collagen fibril macro-assembly and growth factor bindin.[18]
The onset and persistence of cutaneous lichen sclerosus (LS) are linked to the presence of an inflammatory infiltrate of CD3+ T cells that includes CD4+ and CD8+ cells. Furthermore, a notable number of Granzyme B+ activated Cytotoxic lymphocytes associated with hydropic degeneration of the basal cell layer were found within the dermal infiltrate and at the dermoepidermal interface. A cell-mediated immune response may play an important role in the pathogenesis of the disease.[19]
Interleukin-6 (IL-6) is a multifunctional cytokine that participates in the inflammatory and immune responses. In human skin, keratinocytes produces IL-6 and the presence of IL-6 was demonstrated in normal epidermis and atrophic skin diseases like LSA. In normal skin, there was moderate intercellular and intracellular reactivity detected using a high antibody concentration. In specimens with epidermal atrophy, intense cytoplasmic and intercellular immunostaining was detected using a lower antibody concentration. Increased IL-6 in the epidermis suggests that IL-6 may be related to the pathophysiology of cutaneous LS characterized by epidermal atrophy.[20]
The use of a potent topical corticosteroid in the treatment of genital LS is now commonly recognized as effective, with minimal adverse effects.[21] Topical corticosteroids, especially in the super-potent class, have been found to be useful in genital lichen sclerosus in both sexes and in all age groups.[22] A variety of destructive procedures have been reported to be of benefit, although follow-up studies often do not show the same efficacy as original pilot reports. Cryotherapy of affected genital lesions is also reported to reduce the area involved after one or a series of treatments.[23] Narrow-band UVB, psoralen plus UVA (PUVA), and photodynamic therapy using a photosensitizer with laser light activation are also reported to be anecdotally beneficial by various authors.[24,25]
Treatment of Oral Lichen sclerosus is usually unnecessary because of its asymptomatic nature, benign behavior, few cosmetic concerns and no evidence of recurrence.[3] Topical application of corticosteroids has been reported, the outcome being variable.[10] Literature shows successful treatment of refractory generalized extragenital lichen sclerosus with pulsed high-dose corticosteroids combined with methotrexate.[26] Histopathological confirmation of oral LS is necessary to rule out other whitish lesions such as lichen planus and localized scleroderma which require different management.
The association between genital SCC and genital LS is well known. On the vulva, this risk has been evaluated at 14.8% with a relative risk of 246.6.[27] Although no cases of malignant change associated with oral LS have been reported, regular long term follow-up is indicated.[28]
Conclusion
LS is rare in the oral cavity, particularly in the absence of simultaneous cutaneous and genital lesions. Only 7 cases of oral LS have been reported exclusively involving the gingiva. To our knowledge, this is the eighth case to be reported with gingival involvement and the first case to be reported with bilateral involvement of buccal mucosa and gingiva.
References
- Powell JJ, Wojnarowska F. Lichen sclerosus. Lancet. 1999;353:1777–1783. doi: 10.1016/s0140-6736(98)08228-2. [DOI] [PubMed] [Google Scholar]
- Meffert JJ, Davis BM, Grimwood RE. Lichen sclerosus. Journal of Am Acad Dermatol. 1995;32:393–416. doi: 10.1016/0190-9622(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Jensen T, Worsaae N, Melgaard B. Oral Lichen sclerosus et atrophicus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:702–706. doi: 10.1067/moe.2002.129185. [DOI] [PubMed] [Google Scholar]
- Miller RF. Lichen sclerosus et atrophicus with oral involvement; histopathologic study and dermabrasive treatment. AMA Arch Derm. 1957;76:43–55. doi: 10.1001/archderm.1957.01550190047010. [DOI] [PubMed] [Google Scholar]
- Ravits HG, Welsh AS. Lichen sclerosus et atrophicus of the mouth. AMA Arch Derm. 1957;76:56–58. doi: 10.1001/archderm.1957.01550190060011. [DOI] [PubMed] [Google Scholar]
- de Araújo VC, Orsini SC, Marucci G, de Araújo NS. Lichen sclerosus et atrophicus. Oral Surg Oral Med Oral Pathol. 1985;60:655–657. doi: 10.1016/0030-4220(85)90370-6. [DOI] [PubMed] [Google Scholar]
- Macleod RI, Soames JV. Lichen sclerosus et atrophicus of oral mucosa. Br J Oral Maxillofac Surg. 1991;29:64–65. doi: 10.1016/0266-4356(91)90180-d. [DOI] [PubMed] [Google Scholar]
- Schulten EA, Starink TM, van der Waal I. Lichen sclerosus et atrophicus involving the oral mucosa: report of two cases. J Oral Pathol Med. 1993;22:374–377. doi: 10.1111/j.1600-0714.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Brown AR, Dunlap CL, Bussard DA, Lask JT. Lichen sclerosus et atrophicus of the oral cavity: report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:165–170. doi: 10.1016/s1079-2104(97)90064-0. [DOI] [PubMed] [Google Scholar]
- Buajeeb W, Kraivaphan P, Punyasingh J, Laohapand P. Oral lichen sclerosus et atrophicus: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:702–706. doi: 10.1016/s1079-2104(99)70013-2. [DOI] [PubMed] [Google Scholar]
- Siar CH, Ng KH. Oral lichen sclerosus et atrophicus: report of case. J Oral Med. 1985;40:148–150. [PubMed] [Google Scholar]
- Dalziel K, Reynolds AJ, Holt PJ. Lichen sclerosus et atrophicus with ocular and maxillary complications. Br J Dermatol. 1987;116:735–737. doi: 10.1111/j.1365-2133.1987.tb05909.x. [DOI] [PubMed] [Google Scholar]
- Jiménez Y, Bagán JV, Milián MA, Gavaldá C, Scully C. Lichen sclerosus et atrophicus manifesting with localized loss of periodontal attachment. Oral Dis. 2002;8:310–313. doi: 10.1034/j.1601-0825.2002.02858.x. [DOI] [PubMed] [Google Scholar]
- Jaworsky C. Connective tissue diseases. In: Lever's Histopathology of Skin (Elder DE ed) Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 317–319. [Google Scholar]
- Bushkell LL, Friedrich EG Jr, Jordon RE. An appraisal of routine direct immunofluorescence in vulvar disorders. Acta Derm Venereol. 1981;61:157–161. [PubMed] [Google Scholar]
- Dickie RJ, Horne CH, Sutherland HW, Bewsher PD, Stankler L. Direct evidence of localized immunological damage in vulvar lichen sclerosus at atrophicus. J Clin Pathol. 1982;35:1395–1397. doi: 10.1136/jcp.35.12.1395-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N, Chan I, Neill SM, Hamada T, South AP, Wessagowit V, Wojnarowska F, D'Cruz D, Hughes GJ, Black MM, McGrath JA. Autoantibodies to extracellular matrix protein 1 in lichen sclerosus. Lancet. 2003;362:118–123. doi: 10.1016/S0140-6736(03)13863-9. [DOI] [PubMed] [Google Scholar]
- Chan I. The role of extracellular matrix protein 1 in human skin. Clin Exp Dermatol. 2004;29:52–56. doi: 10.1111/j.1365-2230.2004.01440.x. [DOI] [PubMed] [Google Scholar]
- Gross T, Wagner A, Ugurel S, Tilgen W, Reinhold U. Identification of TIA-1+ and granzyme B+ cytotoxic T cells in lichen sclerosus et atrophicus. Dermatology. 2001;202:198–202. doi: 10.1159/000051636. [DOI] [PubMed] [Google Scholar]
- Romero LI, Pincus SH. In situ localization of interleukin-6 in normal skin and atrophic cutaneous disease. Int Arch Allergy Immunol. 1992;99:44–49. doi: 10.1159/000236334. [DOI] [PubMed] [Google Scholar]
- Renaud-Vilmer C, Cavelier-Balloy B, Porcher R, Dubertret L. Vulvar lichen sclerosus: effect of long-term topical application of a potent steroid on the course of the disease. Arch Dermatol. 2004;140:709–712. doi: 10.1001/archderm.140.6.709. [DOI] [PubMed] [Google Scholar]
- Fischer G, Rogers M. Treatment of childhood vulvar lichen sclerosus with potent topical corticosteroid. Pediatr Dermatol. 1997;14:235–258. doi: 10.1111/j.1525-1470.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Stucker M, Grape J, Bechara FG, Hoffmann K, Altmeyer P. The outcome after cryosurgery and intralesional steroid injection in vulvar lichen sclerosus corresponds to postoperative histopathological findings. Dermatology. 2005;210:218–222. doi: 10.1159/000083513. [DOI] [PubMed] [Google Scholar]
- Kreuter A, Gambichler T. Narrowband UV-B phototherapy for extragenital lichen sclerosus. Arch Dermatol. 2007;143:1213. doi: 10.1001/archderm.143.9.1213-a. [DOI] [PubMed] [Google Scholar]
- Romero A, Hernández-Núñez A, Córdoba-Guijarro S, Arias-Palomo D, Borbujo-Martínez J. Treatment of recalcitrant erosive vulvar lichen sclerosus with photodynamic therapy. J Am Acad Dermatol. 2007;57(2 Suppl):S46–47. doi: 10.1016/j.jaad.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Kreuter A, Tigges C, Gaifullina R, Kirschke J, Altmeyer P, Gambichler T. Pulsed high-dose corticosteroids combined with low-dose methotrexate treatment in patients with refractory generalized extragenital lichen sclerosus. Arch Dermatol. 2009;145:1303–1308. doi: 10.1001/archdermatol.2009.235. [DOI] [PubMed] [Google Scholar]
- Carli P, Cataneo A, De Magnis A, Biggeri A, Taddei G, Giannotti B. Squamous cell carcinoma arising in vulval lichen sclerosus: a longitudinal cohort study. Eur J Cancer Prev. 1995;4:491–495. doi: 10.1097/00008469-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Mendonça EF, Ribeiro-Rotta RF, Silva MA, Batista AC. Lichen sclerosus et atrophicus of the oral mucosa. J Oral Pathol Med. 2004;33:637–640. doi: 10.1111/j.1600-0714.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Kaminsky CA, De Kaminsky AR, Abulafia J. Liquen escleroso y atrofico de piel y mucosa buccal. Med Cutan Ibero Lat Am. 1974;2:87–92. [PubMed] [Google Scholar]
- Kelly SC, Helm KF, Zaenglein AL. Lichen sclerosus of the lip. Pediatr Dermatol. 2006;23:500–502. doi: 10.1111/j.1525-1470.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry SI, Morgan PR, Neill SM. An unusual tongue. Clin Exp Dermatol. 2006;31:831–832. doi: 10.1111/j.1365-2230.2006.02219.x. [DOI] [PubMed] [Google Scholar]
- Jiménez Y, Gavaldá C, Carbonell E, Margaix M, Sarrión G. Lichen sclerosus of the oral mucosa: a case report. Med Oral Patol Oral Cir Bucal. 2008;13:403–406. [PubMed] [Google Scholar]
- Kaur S, Thami GP, Kanwar AJ, Mohan H. Linear oro-facial lichen sclerosus. Clin Exp Dermatol. 2002;27:467–470. doi: 10.1046/j.1365-2230.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- Rajlawat BP, Triantafyllou A, Field EA, Parslew R. Lichen sclerosus of the lip and buccal mucosa. Clin Exp Dermatol. 2004;29:684–685. doi: 10.1111/j.1365-2230.2004.01653.x. [DOI] [PubMed] [Google Scholar]
- Walsh SN, Jorizzo JL, Haverstock C, Sangüeza OP. A linear orofacial macule. Am J Dermatopathol. 2008;30:194–195. doi: 10.1097/DAD.0b013e318162eaca. [DOI] [PubMed] [Google Scholar]
- Azevedo RS, Romañach MJ, de Almeida OP, Mosqueda-Taylor A, Vega-Memije ME, Carlos-Bregni R, Contreras-Vidaurre E, López-Jornet P, Saura-Inglés A, Jorge J. Lichen sclerosus of the oral mucosa: clinicopathological features of six cases. Int J Oral Maxillofac Surg. 2009;38:855–860. doi: 10.1016/j.ijom.2009.03.710. [DOI] [PubMed] [Google Scholar]