Abstract
Background
Diphencyprone is a universal contact immunotherapy. The mechanism of action is based on an induction of the delayed-type hypersensitivity. Diphencyprone has been used in various forms for treatments of recalcitrant and facial warts, and alopecia areata. However, this treatment modality has not been generally used in immunocompromised patients.
Main observation
The present report demonstrated the efficacy of diphencyprone immunotherapy on the treatment of generalized molluscum contagiosum in a human immunodeficiency virus (HIV)-infected patient. Minimal and transient side effects including pruritus, postinflammatory hyperpigmentation and irritation were noted.
Conclusion
Diphencyprone contact immunotherapy appears to be a possible alternative treatment of widespread molluscum contagiosum in immunocompromised patients.
Keywords: AIDS, diphencyprone, DPC, HIV, molluscum contagiosum, poxvirus, treatment, virus
Introduction
Cutaneous molluscum contagiosum is a common viral skin infection which is caused by a DNA virus from the poxviruses (Poxviridae) family. It is most frequently found in children. However, it is also found in adolescents by sexually transmission, which usually appears on the genital area. Extragenital molluscum contagiosum occurs almost exclusively in HIV-infected patients or immunocompromised patients. Basically, molluscum contagiosum in healthy people is a self limiting disease which can be left to heal naturally. Therapy is mandatory for accelerating the healing process and concerning about spreading to other areas of the body and to other people. Contradictorily, it behaves differently in HIV infected patients. The more immunodeficiency is progressed, the more common and resistance to therapy is increased.[1] Many treatment options such as physical destruction of the lesions (i.e. cryotherapy, extraction and curettage), topical agents (i.e. those applied directly to the lesions) and systemic treatment (i.e. those affecting the whole body) have been used for molluscum contagiosum.[1] However, there is no standard treatment for molluscum contagiosum. The choice of treatment depends on the extension and location of the lesions and cooperation of the patients. Our patient presented with generalized molluscum contagiosum and HIV infection. Judging from our review, diphencyprone (DPC) which is a contact immunotherapy was decided upon for use in our patient. The mechanism of action is based on an induction of the delayed-type hypersensitivity. DPC has been used in various forms for treatments of recalcitrant and facial warts, and alopecia areata.[2] However, this treatment modality has not been generally used in immunocompromised patients. The present report demonstrated the efficacy of DPC on the treatment of generalized molluscum contagiosum in an HIV patient.
Case Report
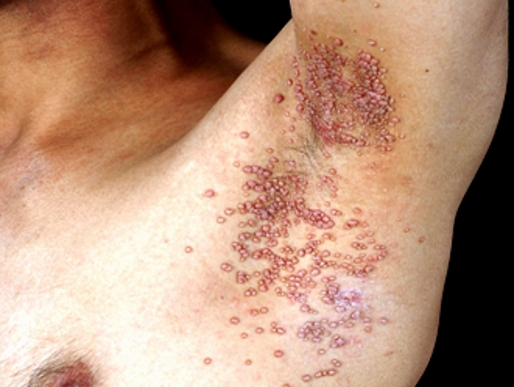
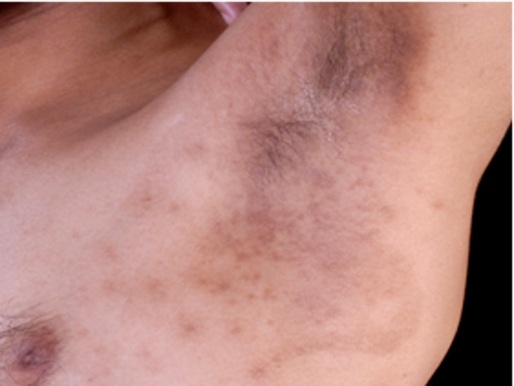
A 32-year-old patient presented with widespread umbilicated papules on genital and extragenital area for 7 months. At that time, he did not have other cutaneous disorders of HIV infection such as oral hairy leukoplakia, oral thrush, papular pruritic eruption, Kaposi sarcoma. Skin biopsy was performed on the lesion of the trunk and demonstrated molluscum bodies. A through history revealed that patient was HIV positive. He had a CD4+ count of 47 cell/mm3 but an HIV RNA level was not investigated at a previous hospital. He has also received the antiretroviral therapy (lamivudine, tenofovir, efavirenz) and multidrug resistant therapy for tuberculosis (ethambutol, isoniazid, rifampicin, pyrazinamide, clarithromycin) for 7 months. His lesions did not improve when the patient was on the antiretroviral medications. The molluscum lesions were widespread on the eyelids, trunk and genital areas [Fig. 1]. The total number of lesions was more than 100. Thus, the curettage, extraction or cryotherapy are cumbersome treatments especially on the eyelid area. Due to the widespread locations of the lesions, we decided to treat this patient with DPC. The instruction about treatment was conveyed to the patient including that he would be sensitized with 2% DPC on the left inner upper arm. He was instructed to keep this area dry for 6 hours. The desirable side-effects such as pruritus, erythema or allergic contact dermatitis, and undesirable side-effects, such as vesicle, dyspigmentation were explained to the patient. The left inner upper arm was chosen because of cosmetic concerns. The duration of treatment was 1-2 week intervals. After understanding the protocol of treatment, he decided to choose this method to treat his molluscum lesions. The patient was sensitized with 2% DPC on his left inner upper arm. After 2 weeks, a sensitization had occurred by showing the eczematous lesion on sensitized area. Then, his lesions were later treated with a concentration of 0.001%. Ten percent of the lesions were applied with DPC on each followup visit. The concentrations of DPC were gradually increased from 0.001% to 2.0%, depending on the patient's tolerability. The frequency of application was 1-2 week intervals. All of the lesions on the eyelids cleared within 2 months of treatment. At that time, He had a CD4+ count of 76 cell/mm3. The other lesions on the trunk and the genitalia gradually decreased in size and resolved with consecutive applications of DPC [Fig. 2]. Minimal and transient side effects, including pruritus, postinflammatory hyperpigmentation and irritation were noted.
Figure 1.
Molluscum contagiosum lesions in the axilla.
Figure 2.
Axilla after therapy with diphencyprone.
Discussion
The tri-ethyleneiminobenzoquinone (TEIB or trenimon) was the first topical contact sensitizer that was used to treat multiple superficial basal and squamous cell carcinomas in 1965.[2] Unfortunately, it is carcinogenic, as just as the nitrogen mustard which had been used to treat cutaneous T-cell lymphoma. The other allergens such as poison ivy, nickel and formalin are unsuitable for topical immunotherapy because they can cross-react with other substances and common in environment.[2] Therefore, the good contact sensitizer should be safe, lack of potential to induce cross-sensitization to other substances and not be a carcinogen. Nowadays, dinitrochlorobenzene (DNCB), squaric acid dibutyl ester (SADBE) and DPC are most common contact sensitizers. (SADBE is considerably more expensive than other agents (2).) There are many reports of their efficacy to treat benign and malignant dermatoses such as alopecia areata, viral warts, basal and squamous cell carcinoma and cutaneous T-cell lymphoma.
DPC was first synthesized in 1959. It is prepared by bromination of dibenzylketone and then cyclized with base to yield DPC.[2] The standard solvent of DPC is acetone which is a strong Ultraviolet (UV) light absorber. UV radiation and heat cause degradation to DPC. Thus, the dilutions of DPC are usually collected in brown UV-opaque bottles and stored at room temperature. Basically, its shelf life is probably no longer than 6 months. It does not appear to cross-react with other chemicals and is not mutagenic in Ames assay. DPC is a universal contact sensitizer in which the response to treatment can occur in remote areas other than those of topical application. It has been widely used in alopecia areata and viral warts. Some studies reported successful treatment in molluscum contagiosum, vitiligo, primary and secondary malignant melanoma.[2–7] The mechanisms of action include alterations in cytokine levels, nonspecific inflammation and reversal of CD4:CD8 ratio.[8] The common side effects are generalized eczema, urticaria, local blistering and swelling and regional lymphadenopathy. The uncommon side effects are erythema multiforme, fever, palpitations, flulike symptoms, headache, vitiligo, and dyschromia in confetti.[2,8–13] In HIV-seropositive patients, DNCB can be used as an alternative treatment because it appears to have a beneficial clinical and immunologic outcome. A pilot study of DNCB and acquired immune deficiency syndrome (AIDS) was done in 1990.[14,15] The immunologic profile of DNCB sensitized patients showed increasing of cytotoxic CD8 Tcells, natural killer cells and decreasing of HIV RNA levels.[15] More important, the death rate in DNCB compliant group was lower than the non-compliant group.[14] Traub and Margulis collected data on HIV-infected patients with topical DNCB in Brazil. After 21 months of treatment, 19 patients had significantly increased CD4 and CD8 T-cell counts and decreased parasitic infections.[16] Our patient has an HIV infection and generalized molluscum contagiosum. Kang HS et al reported complete clearance of multiple molluscum contagiosum with DPC in 14 of 22 children (63.6%).[3] Regard to DPC and HIV-infected patients, DPC sensitization also demonstrates diagnostic utility as a functional measure of immune competence but offers limited data about clinical efficacy.[17] Following from these rationales, DPC has been tried in our patient, although there has not been any previous report from PubMed database for the DPC usage in treatment of molluscum contagiosum in an HIV patient. Good efficacy and minimal side effects were noted in our patient.
Conclusion
Topical DPC is easy and inexpensive method that can produce complete or partial regression in generalized molluscum contagiosum. It should be considered as another option in immunocompetent and immunocompromised patients, when other therapies are contraindicated.
References
- van der Wouden JC, van der Sande R, van Suijlekom-Smit LW, Berger M, Butler C, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2009;7:CD004767. doi: 10.1002/14651858.CD004767.pub3. [DOI] [PubMed] [Google Scholar]
- Buckley DA, Du Vivier AW. The therapeutic use of topical contact sensitizers in benign dermatoses. Br J Dermatol. 2001;145:385–405. doi: 10.1046/j.1365-2133.2001.04399.x. [DOI] [PubMed] [Google Scholar]
- Kang SH, Lee D, Hoon Park J, Cho SH, Lee SS, Park SW. Treatment of molluscum contagiosum with topical diphencyprone therapy. Acta Derm Venereol. 2005;85:529–530. doi: 10.1080/00015550510034948. [DOI] [PubMed] [Google Scholar]
- Aghaei S, Ardekani GS. Topical immunotherapy with diphenylcyclopropenone in vitiligo: a preliminary experience. Indian J Dermatol Venereol Leprol. 2008;74:628–631. doi: 10.4103/0378-6323.45108. [DOI] [PubMed] [Google Scholar]
- Damian DL, Thompson JF. Treatment of extensive cutaneous metastatic melanoma with topical diphencyprone. J Am Acad Dermatol. 2007;56:869–871. doi: 10.1016/j.jaad.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Yoshino T, Asada H, Ando Y, Fujii H, Yamaguchi Y, Yoshikawa K, Itami S. Impaired responses of peripheral blood mononuclear cells to T-cell stimulants in alopecia areata patients with a poor response to topical immunotherapy. Br J Dermatol. 2001;145:415–421. doi: 10.1046/j.1365-2133.2001.04398.x. [DOI] [PubMed] [Google Scholar]
- Orecchia G, Douville H, Santagostino L, Rabbiosi G. Treatment of multiple relapsing warts with diphenciprone. Dermatologica. 1988;177:225–231. doi: 10.1159/000248568. [DOI] [PubMed] [Google Scholar]
- van der Steen P, van de Kerkhof P, der Kinderen D, van Vlijmen I, Happle R. Clinical and immunohistochemical responses of plantar warts to topical immunotherapy with diphenylcyclopropenone. J Dermatol. 1991;18:330–333. doi: 10.1111/j.1346-8138.1991.tb03093.x. [DOI] [PubMed] [Google Scholar]
- Gordon PM, Aldrige RD, McVittie E, Hunter JA. Topical diphencyprone for alopecia areata: evaluation of 48 cases after 30 months' follow-up. Br J Dermatol. 1996;134:869–871. [PubMed] [Google Scholar]
- Hatzis J, Georgiotouo K, Kostakis P, Anastasiadis G, Tosca A, Varelzidis A, Straigos J. Treatment of alopecia areata with diphencyprone. Australas J Dermatol. 1988;29:33–36. doi: 10.1111/j.1440-0960.1988.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Lane PR, Hogan DJ. Diphencyprone. J Am Acad Dermatol. 1988;19:364–365. doi: 10.1016/s0190-9622(88)80258-5. [DOI] [PubMed] [Google Scholar]
- Monk B. Induction of hair growth in alopecia totalis with diphencyprone sensitization. Clin Exp Dermatol. 1989;14:154–157. doi: 10.1111/j.1365-2230.1989.tb00915.x. [DOI] [PubMed] [Google Scholar]
- van der Steen P, Happle R. 'Dyschromia in confetti' as a side effect of topical immunotherapy with diphenylcyclopropenone. Arch Dermatol. 1992;128:518–520. doi: 10.1001/archderm.128.4.518. [DOI] [PubMed] [Google Scholar]
- Stricker RB, Goldberg B, Epstein WL. Topical immune modulation (TIM): a novel approach to the immunotherapy of systemic disease. Immunol Lett. 1997;59:145–150. doi: 10.1016/s0165-2478(97)00114-4. [DOI] [PubMed] [Google Scholar]
- Stricker RB, Zhu YS, Elswood BF, Dumlao C, Van Elk J, Berger TG, Tappero J, Epstein WL, Kiprov DD. Pilot study of topical dinitrochlorobenzene (DNCB) in human immunodeficiency virus infection. Immunol Lett. 1993;36:1–6. doi: 10.1016/0165-2478(93)90060-f. [DOI] [PubMed] [Google Scholar]
- Traub A, Margulis SB, Stricker RB. Use of dinitrochlorobenzene (DNCB) as an immune modulator in HIV-positive patients: A pilot study from Brazil [Abstract PB0304]. International Conference on AIDS; 1994 Aug 7-12; Irmandade da Santa Casa de Misericordia de Porto Alegre, Brazil. [Google Scholar]
- Levis WR. Evaluation of delayed-type hypersensitivity in patients with suspected immunodeficiency. Am J Med. 2008;12:e13. doi: 10.1016/j.amjmed.2007.10.029. [DOI] [PubMed] [Google Scholar]