Abstract
Access to quality cancer care is often unavailable not only in low- and middle-income countries but also in rural or remote areas of high-income countries. Teleoncology (oncology applications of medical telecommunications, including pathology, radiology, and other related disciplines) has the potential to enhance both access to and the quality of clinical cancer care as well as education and training. Its implementation in the developing world requires an approach tailored to priorities, resources, and needs. We believe that teleoncology can best achieve its proposed goals through programmatic, consistent, and long-term application. Here we review teleoncology initiatives that have the potential to decrease cancer care inequality between resource-poor and resource-rich institutions and offer guidelines for the development of teleoncology programs in low- and middle-income countries.
Keywords: teleoncology, telemedicine, pediatric oncology, telepathology, developing countries, underserved patients
CANCER CARE DISPARITY: A GLOBAL PROBLEM
There are gaps in cancer care globally. The inadequacies in low- and middle-income countries (LMC) are most widely recognized. The World Health Organization (WHO) recently reported that the further development of LMC is hindered by the significant morbidity and mortality of chronic disease.1 An estimated 80% of chronic disease deaths occur in LMC,1,2 which lose more lives each year to a single chronic disease—cancer—than to HIV/AIDS.2
National economic status is an important element in access to modern cancer care. The World Bank classifies nations by gross annual per-capita income as low-income countries (LIC, US $935 or less), middle-income countries (MIC, $936–11,455), and high-income countries (HIC, $11,456 or more). MIC are further divided into lower ($936–3,705) and upper ($3,706–11,455) MIC.
The global incidence of cancer is projected to increase by 50% over the next 20 years,3 and most cases will occur in LMC,2 which possess only 5% of the world’s resources. Further, children are not spared; for example, cancer is the second most common cause of death in children in many Latin American countries,4 although as many as 70% of pediatric cancers are curable with the appropriate diagnosis and treatment.5
HIC have disparities in cancer care as well, usually involving the unavailability of specific specialties, diagnostic facilities, and treatment infrastructure in remote or rural areas.6 These disparities are likely to increase; in the United States (US), a shortage of approximately 3800 oncologists is projected by the year 2020.7
THE POTENTIAL OF TELEONCOLOGY
Systematic and effective communication between individuals at advanced oncology centers and those at remote or resource-poor centers can improve cancer care and enhance opportunities for continuing clinical education. Therefore, disparities in cancer care can be reduced by the development of resources—human capital and telecommunication infrastructure—that link institutions with different levels of funding and expertise (Figure 1).
Figure 1.
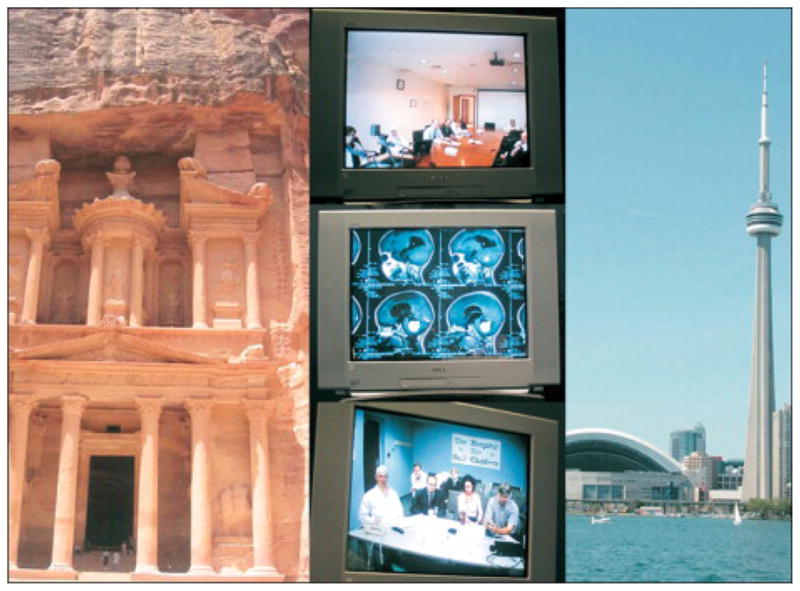
Teleoncology links ancient and new worlds. Left, the ancient city of Petra, Jordan. Right, the CN Tower, Toronto, Canada. Central images show a monthly videoconference that allows the neuro-oncology teams of King Hussein Cancer Center (Amman, Jordan; top) and Hospital for Sick Children (Toronto, Canada; bottom) to view and discuss complex brain tumor cases (center). The ongoing videoconference series began in 2004.
Telemedicine is defined variously, but the definitions used by WHO, the European Commission, and the American Telemedicine Association center on the “use of telecommunication to advance health”. Teleoncology is the application of telemedicine to oncology, including diagnostics (laboratory, radiology, pathology), treatment (surgery, radiation oncology, medical oncology), and supportive (rehabilitation and palliative) care. Therefore, teleoncology includes any telemedicine application used to advance cancer care.8 Data derived from telemedicine in general will be provided where it informs potential teleoncology efforts.
TELECOMMUNICATION TECHNOLOGIES
Several communication technologies can support effective teleoncology. Synchronous (real-time) interactive videoconferencing is one of most common.9 A fully equipped videoconferencing unit with six integrated-services digital network (ISDN) transmission channels (384 kb/s) is costly and requires technical support that is not available in many LMC.9 However, more affordable systems may be feasible. In Ecuador, a videoconference unit using a modem for transmission (56 kb/s) was recently installed for less than US$1000,10 although it has not been assessed for teleoncology applications.
Many collaborative internet protocols that allow synchronous interaction among participants have recently emerged. Some of these, collectively termed web conferencing protocols, are very robust and support voice and visual teaching applications such as slide presentations. The main advantages of these systems are their low cost and minimal technical maintenance requirements. A web conferencing initiative hosted by the St. Jude Children’s Research Hospital (SJCRH) site Cure4Kids (www.cure4kids.org) has been successfully used for 6 years to support the hospital’s International Outreach Program partners.11
The high-end synchronous systems, such as telesynergy systems,12 robotic telesurgery,13 and virtual microscopy,14 are likely to be used only in resource-rich countries. Because they can transmit high-resolution images for clinical, pathological, and radiological diagnosis, many hospitals in the US and Europe use them to overcome local lack of expertise. Table 1 describes the advantages and disadvantages of current methods of synchronous telecommunication.
Table 1.
Advantages and disadvantages of synchronous technologies applicable to teleoncology
| Technology | Advantages | Disadvantages |
|---|---|---|
| Web conferencing | Low cost Wide availability |
Limited resolution of images Images cannot be manipulated Participants may not see each othera |
| Videoconferencing | Good image resolution Images can be manipulated Participants can see each other Readily available Can present/interview patients Supports image-intensive clinical case collaborations (diagnosis, radiation/surgery planning, disease monitoring) |
Expensive Requires maintenance |
| Telesynergy ® | A multimedia workstation integrates all components for collaborative multidisciplinary teleoncology High image resolution Transmits images from their primary sources Allows image manipulation Supports comprehensive multidisciplinary case review and discussion Supports collaborative planning of radiation and surgery |
Very expensive Requires ~ 20 ISDN channels Requires many peripheral components Difficult to install Requires intensive maintenance Requires dedicated storage space |
| Virtual telemicroscope | Operator can control microscope without special hardware or software Good image resolution |
Limited to pathology Expensive Performance depends on the user’s computer |
| Robotic telesurgery | Circumvents hand tremors Supports fine surgical movements |
Bulky equipment Very expensive Requires special training |
A significant increase in bandwidth and expense would be required.
Asynchronous interaction, also known as the store-and-forward method, uses software applications to transmit, store, and retrieve data or digital images.15 Store-and-forward communication is practical in fields that require imaging. As an example, the nonprofit organization ORBIS (www.orbis.org) links clinicians in developing countries with mentors in developed countries to improve the diagnosis and management of ocular diseases, including cancer.16 Retinal images obtained via fundus or retinal camera can be uploaded to the ORBIS site, which also supports related MR, CT, and ultrasound images, allowing full consideration of specific case details by the mentor (Figure 2). Telepathology frequently uses store-and-forward methods.17 One of the earliest non–real time telemedicine initiatives, SateLife, began in 1991 and continues to support e-mail consultations, teleconferencing, and online educational content via a low-orbit satellite.18 Finally, e-mail is a widely used but under-reported method of teleoncology.19,20
Figure 2.
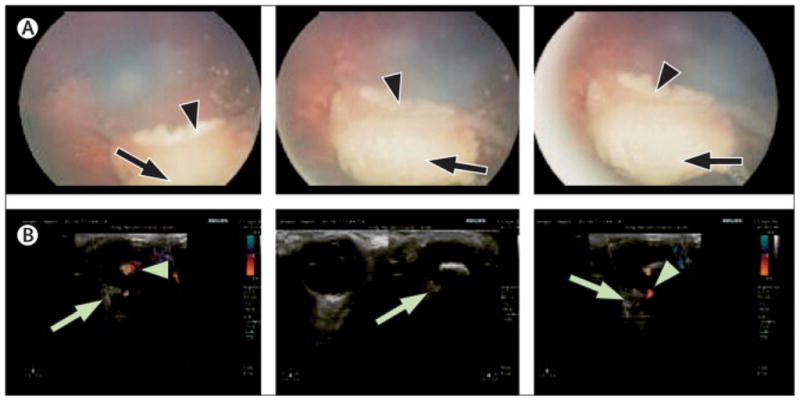
Presurgical ocular teleoncology consultation. A complex case of bilateral retinoblastoma was treated in Jordan. The policy at the Jordanian center required that cases be discussed with the mentoring St. Jude Children’s Research Hospital ocular oncology team before any major intervention. Images show the right eye after chemotherapy. (A) Retinal photographs show tumor (arrows) and retinal folds (arrowheads). (B) Doppler ultrasound images show tumor (arrows) and active blood flow (arrowheads). Because blood flow suggested viable tumor, enucleation was initially considered. The mentoring team recommended observation, as blood flow appeared to be localized in the retinal fold. Although the left eye required enucleation, the right eye was salvaged and the young patient retained useful vision.
TELEONCOLOGY IN HIGH-INCOME COUNTRIES
Improvement of outcomes in underserved areas and dispersed populations
Europe
The regions and countries of Europe are heterogeneous in their resources, populations, and needs, and cancer outcomes can vary accordingly.21 Table 2 provides several examples of successful teleoncology initiatives launched at the continental or national level.
Table 2.
Summary of teleoncology initiatives in high-income countries
| Initiative | Service area | Field(s)/Disease | Goal(s) | Method/Technology | Reference(s) |
|---|---|---|---|---|---|
| CONQUEST | Europe | RT Oncology |
QA Improve CT Access to MR |
Videoconference | 21 |
| TENPET | Europe | Radiology | PET scans QA |
Interactive Store and forward |
23 |
| UICC-TCCa | Europe | Pathology | Consultation | Web-based | 17 |
| i-Patha | Europe | Pathology | Consultation | Web-based Email-based |
24,48 |
| QA for RT | Norway | RT | Discuss RT planning | Videoconference | 25 |
| TELEMAM | Scotland | Breast cancer | Randomized trialb | Videoconference | 27 |
| TeleRT network | Germany | RT Lymphoma |
Centralized RT review QA Improve CT Education |
Videoconference Store and forward |
28 |
| VISN service area 20 | US military (AK, ID, OR, WA) | Oncology | MDTB for dispersed populations Improve referrals |
Videoconference | 30 |
| Kansas University Medical Center | US (rural KS) | Oncology Hospice |
Serve rural populations | Videoconference | 31,32 |
| Cancer genetic counseling | US (rural NC) | Genetic counseling | Serve rural areas | Videoconference | 33 |
| NCC network | Japan | Oncology Radiology Pathology |
MDTB Education |
Videoconference | 34 |
| IPHECA | Belarus, Kazakhstan | Thyroid cancer Radiology Cytology |
Assist affected populations | Communication satellite | 35,36 |
| Teledermatology | New Zealand | Skin cancer | Serve rural populations | Videoconference | 37 |
| Teledermatology | Australia | Skin cancer | Serve rural populations | Email (digital images) | 38 |
RT, radiotherapy; QA, quality assurance; CT, clinical trials; MR, medical records; TENPET, Trans-European Network for Positron Emission Tomography; UICC-TCC, International Union Against Cancer Telepathology Consultation Center; NA, not available; VISN, Veterans Integrated Service Network; AK, Alaska; ID, Idaho; OR, Oregon; WA, Washington; MDTB, multidisciplinary tumor board; KS, Kansas; NC, North Carolina; NCC, National Cancer Center; IPHECA, International Program on the Health Effects of the Chernobyl Accident
European-based telepathology initiatives that are also used widely in LMC, especially Asia and Africa.
A randomized trial of consultation in person vs. via videoconference, showing no difference in clinical effectiveness
In response to widely disparate rates of breast cancer recurrence at European hospitals (10.5%–36% after breast-conserving therapy and 4.6%–21.3% after mastectomy),22 the Clinical Oncology Network for Quality in European Standards of Treatment (CONQUEST) initiative was launched.21 Another continent-wide project is the Trans-European Network for Positron Emission Tomography (TENPET), which supports teleconsultation for the performance and interpretation of PET scans.23 The International Union Against Cancer’s Telepathology Consultation Center17 and the i-Path24 system are widely used to support pathology consultation in Europe. At the national level, Norway25 in 1996 became the first country to reimburse providers for telemedicine services.26 Scotland 27 and Germany28 have implemented teleoncology systems for treatment planning for breast cancer and Hodgkin lymphoma, respectively.
The United States
Like Europe, the US has underserved populations. There is substantial evidence that cancer outcomes are worse in rural or remote areas.6,29 Onega et al6 proposed that specialty cancer care be delivered via teleoncology in the US to decrease the travel burden and improve access. The many successful and sustainable teleoncology initiatives in the US include cancer care for the widely dispersed US military population30 and a well known teleoncology application developed by Kansas University Medical Center to serve its large rural patient base31,32 (Table 2). Gaps in care are greater in rare oncology subspecialties such as cancer genetics.33
Other high-income countries
Japan’s teleoncology cancer center network34 conducts approximately 130 teleconferences per year attended by 16,000 people and hosts regular telepathology and teleradiology meetings. A program developed by the WHO to meet the surging need for oncology care after the Chernobyl catastrophe35 links Nagasaki University to two hospitals in Belarus and Kazakhstan.36 Both New Zealand37 and Australia38 have active teleoncology services for skin cancer (Table 2).
Improvement of outcome in clinical trials
The negative impact of errors in diagnosis,39 staging,40 and treatment delivery41 is well documented by retrospective studies. For example, 30% of patients who had a diagnosis of high-grade glioma were subsequently found to have had low-grade glioma.39 These patients underwent unnecessarily aggressive therapy that could have been prevented by protocol-directed prospective (i.e., before treatment) telepathology review. Packer et al found, at the end of a medulloblastoma study, that patients who had received inadequate radiological staging were less likely to survive.40 Donaldson et al found that the 5-year local control of Ewing’s sarcoma was 80% with the correct dose and volume of radiotherapy41; however, in the same cohort, patients with minor and major treatment deviations had 5-year local control rates of only 48% and 16%, respectively. Rapid expert opinions at the time of staging and treatment planning can improve patient outcomes, improve the integrity of clinical trials data, and build the expertise of local cancer teams.
TELEONCOLOGY IN LOW- AND MIDDLE-INCOME COUNTRIES
Limiting factors
Teleoncology is less available in LMC than elsewhere. However, internet access is now readily available in all major cities of Africa,42 and wireless high-speed internet service (using less costly medium-orbit satellites) is being introduced in LMC by commercial providers. Desktop computers can be purchased for less than $200, and laptops with wireless connectivity have been produced for less than US$100 by the nonprofit organization One Laptop Per Child. China and India have almost 37% of the world population, have their own space programs and high-speed internet service, and manufacture all equipment required for teleoncology. However, despite these resources, official telemedicine activity began in China only in 1995.43 We believe that human factors, rather than lack of resources and technology, is often the main obstacle to teleoncology in LMC.44 As Ganapathy stated elegantly, “what is required is not implementing better technology and getting funds, but changing the mindset of the people involved”.45 In China there has recently been a proliferation of telemedicine units, that has not been matched by a similar increase in human resources, leaving many such units underutilized.46 In India, a fully equipped videoconference connection was established between two centers 1500 kilometers apart to help in neurology, a rare specialty in India, in which the whole country has only 750 neurologist.47 Over a 4-year period only 22 successful sessions were held.47
In many LMC,44 conflicts over professional and political power, fear of change, reluctance to seek a second opinion, and other human factors have obstructed the optimal use of this system.
Successful teleoncology initiatives in LMC
There have been several successful teleoncology initiatives in LMC, although more are needed. India has one of the largest telemedicine operations in the developing world, with participation by both private and public sectors.3,45,47,48 Other experiences from Cambodia,49 Solomon Islands,50 Brazil,51 and Jordan.9,16,20 are summarized in Table 3. We will emphasize the Cure4Kids experience as an example of a successful sustained international teleoncology experience.
Table 3.
Summary of teleoncology initiatives in low- and middle-income countries
| LMC | Mentoring HIC | Field | Technology | Goal(s) | Ref |
|---|---|---|---|---|---|
| India | None a | Radiation oncology Cancer detection, treatment, and pain relief |
Videoconference Videoconference |
Establish a 3-tier radiation oncology network Decrease referrals Education |
3 48 |
| Cambodia | USA | Consultation | Second opinion | 49 | |
| Solomon Islands |
Europe (i-Path) | Pathology | Second opinion | 50 | |
| Brazil | Nonea | Pediatric oncology | Videoconference | Establish a cancer network | 51 |
| International (Cure4Kids)b | USA | Pediatric oncology | Web conferencing | Twinning Specific diseases Data management |
11 52 53 |
| Jordan | Canada | Pediatric neuro- oncology | Videoconference E-mails |
Second opinion Build local expertise |
9 20 |
| USA | Pediatric ocular oncolgy | Web-based (Orbis) | Team structure | 16 |
These initiatives in India and Brazil are designed to link resource-rich with resource-poor institutions or networking in each country and not for twinning with centers in HIC.
Cure4Kids is used to facilitate twinning and telemedicine between centers in HIC and LMC.
The Cure4Kids website (see above) is an excellent, sustained teleoncology service linking providers in LMC with experts in HIC.11 The site regularly hosts synchronous discussions of specific diseases,52 data management,53 and other oncology issues54 by staff at St. Jude and its partner sites. More than 4,000 oncology professionals attended the online live meetings hosted by Cure4Kids from 2002 through 2008.55 The site also offers extensive multilingual educational material that has been accessed more than 2.5 million times. The pediatric oncology nursing course alone has been viewed more than 120,000 times).55 The site was created by the St. Jude International Outreach Program (IOP), established in 1994 to improve the survival rates of children with catastrophic illness worldwide through the transfer of knowledge, technology, and organizational skills.56 A detailed analysis of success factors during the first decade of IOP experience56 revealed the importance of sustained efforts and an emphasis on the human factor.52,56 Teleoncology in retinoblastoma through ORBIS, Cure4kids, videoconferencing, or e-mail was a major component in IOP initiatives in Central America52 and Jordan.16 Such long-term involvement with the partner sites helped to build trust and contributed to the “change in mind set” that facilitated the rapid acceptance of teleoncology.
Finally, teleoncology experiences reported in languages other than English57 or in publications not available through PubMed may be underrepresented here. For example, the Mexican National Center for Health Technology Excellence (www.cenetec.salud.gob.mx) provides detailed guidelines in Spanish for use of the Mexican telemedicine program (which includes teleoncology).
GUIDELINES FOR PLANNING TELEONCOLOGY PROGRAMS LINKING INSTITUTIONS IN HIC AND LMC
LMC are heterogeneous in their needs, communication infrastructure, and resources. We believe that teleoncology programs that are customized to these features have the greatest potential to improve cancer care. We offer the following guidelines to those planning to develop teleoncology initiatives in such countries.
Focus on the human factor
There is little available information about the attributes necessary to ensure the success and sustainability of telemedicine programs.58,59 Many of those identified are human factors that suggest that a telemedicine program must have grass-roots, “bottom up” support.58 The available reports also stress that clinicians, not politicians, should be the decision makers58 and that efforts should focus on solutions to current health problems.58,59 Otherwise, telecommunications equipment may be purchased but languish in disuse due to political conflict, competing priorities, or miscommunication.59 It is also important that clinicians be trained to use the equipment58 and that local technical staff be instructed in updating antivirus software and maintaining the equipment.60 A study comparing private- and public-sector telemedicine in India found that the former is more successful in improving clinical medical services.48 The authors attributed this finding to the needs-based approach of the private sector as compared to the top-down approach characteristic of public programs.
Build on twinning programs
Teleoncology enhances and builds on established programs. Teleoncology initiatives in LMC often work best in the context of a “twinning program” linking existing local cancer centers61 to cancer centers in HIC. Evidence to date suggests that twinning improves cancer survival in LMC,52,56,63 and the integration of teleoncology into twinning programs maximizes clinical benefits and the effective use of resources.16,19,52 To ensure broader benefits, the partner sites in LMC should be encouraged to establish local, regional, or national networks. Further, cooperation among multiple cancer centers within a single LIC or MIC, and between those in different LIC and MIC, should be promoted. Figure 3 illustrates the proposed model. Such multi-tier telemedicine projects have been suggested3 or piloted51,57,64 in LMC but to date have lacked the element of twinning with HIC cancer centers.
Figure 3.
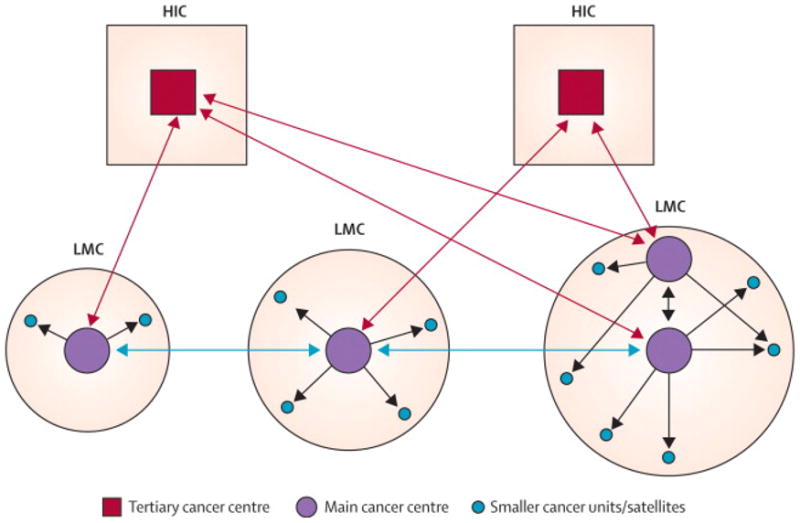
Proposed multi-tier collaborative teleoncology scheme linking cancer centers. Teleoncology would be implemented at the international level between cancer centers in HIC and those in LIC/MIC (red arrows) and between cancer centers in different LIC/MIC (orange arrows). The scale of the programs would depend on the population and number of cancer centers; a more extensive program would be appropriate for a large LIC/MIC with more than one cancer center (example at lower right). The level of technology used would depend on needs and resources. The main centers within each country would communicate with each other via advanced teleoncology such as videoconferencing and (using less expensive technology) would mentor smaller cancer units (gray arrows).
Tailor the approach to the country and targeted diseases
The first principle of a successful teleoncology system is “pragmatic selection” of goals and methods on the basis of needs and resources rather than politics and publicity.58 Therefore, teleoncology programs in lower-MICs (e.g., Jordan9,16) or upper-MICs (e.g., South Africa65) cannot realistically be used as models for programs in LICs (e.g., Yemen or Nigeria). Even within the MIC group, a lower-MIC (per capita income, US$936) is not comparable to an upper-MIC (per capita income, $11,456). To ensure an optimal clinical cost-benefit ratio, the needs and available resources of a specific country should dictate the objectives and the approach used for teleoncology within that country. The cancers to be targeted must be chosen similarly, on the basis of need, existing infrastructure, resources, and the complexity of the required treatment. For example, it would be a misuse of resources for a country like the Solomon Islands, which has no pathologist and whose internet bandwidth connections are limited,50 to invest in videoconference units or to start a retinoblastoma eye salvage program. Instead, this nation, with the help of cancer centers in HICs (Switzerland, Germany, and Australia) developed a practical, effective, and economical system that utilized an existing telepathology resource (iPath) based at Basel University (Switzerland). Further, an e-mail interface was added to overcome the limited internet connections in the Solomon Islands.
Involve allied health professionals
Nurses, technicians, and even medical and nursing students. The lack of specialty physicians can hinder teleoncology initiatives in many LMC. We propose that nurses and other allied health professionals can help to fill the gap by taking on additional training and responsibility. In both of the teleoncology initiatives in Jordan, the specialized nursing staffs of the neuro-oncology and ocular oncology services66 provided indispensable support,9,16 and their disease-specific skills greatly improved clinical care.66
In Brazil, a 150-minute training session on melanoma allowed first-year medical students to accurately diagnose melanoma via a telemedicine model.67 In another pilot project, a medical student from Emory University (US) spent 6 weeks in the Solomon Islands and was able to conduct 8 teleconsultations with his mentors in the US after 1 hour of training in the use of the system.68 After the student departed, the local Solomon Islands team used this pilot telemedicine system to conduct 60 more teleconsultations.
Avoid sophisticated technologies
Advanced methods such as Tesynergy,12 virtual microscopy,13 and robotic telesurgery14 are unlikely to significantly improve health care in LMC.69 Expensive technologies invite the mismanagement of funds and may actually increase the gap in cancer care if access is available only to the wealthy.69 As the costs of these approaches decrease in the future, they may become more accessible; however, in general they should be discouraged if they do not offer a clear benefit. Less expensive options, as discussed above, should be selected as appropriate for local needs and resources.
Expensive technologies that offer a clear advantage, such as videoconferencing, may already be in use for other purposes in LMC. We suggest that the use of existing equipment and resources be explored as an alternative to purchasing new units for teleoncology. For example, the nongovernmental organization Medical Missions for Children, with support from commercial corporations, has established active videoconferencing capabilities for pediatrics in 58 LMC.70 In addition, many banks and other institutions in HIC and LMC acquire videoconference units to unify their organizations and expedite communication (www.tandberg.com). Such institutions may readily lend their units to local health care providers for a few hours per month as a goodwill or public relations gesture. Finally, many private hospitals in LMC (as in India48) own telemedicine units that could be used for health care in the public sector if appropriate private- and public-sector cooperation is established.
OTHER POTENTIAL APPLICATIONS OF TELEONCOLOGY IN LMC
Linkage of resource-rich and resource-poor institutions within countries
Many large LMC, such as India, China, Russia, and Brazil, have tertiary cancer centers in their major cities that can serve as regional hubs, extending resources and expertise to peripheral hospitals (see model, Figure 3). India’s OncoNET48 project for public hospitals is one such initiative that has reduced the burden of referrals to tertiary centers and improved cancer care and education in peripheral hospitals. Datta and Rajasekar proposed a three-tier model for radiation therapy facilities in India that depends on resources available at each level of therapy.3 Similar initiatives exist for pathology in Russia57 and for pediatric oncology in Brazil.51 A similar telemedicine program (applicable to teleoncology) is being developed in Argentina to connect hospitals in rural areas with tertiary centers.64
Support of clinical investigation
Many of the advanced cancer centers in upper-MIC are equipped to participate in international clinical trials, although other centers may benefit from assistance in developing the regulatory and clinical best practices necessary to support such trials. Programmatic telecommunications offers a feasible approach to the training and mentoring of health care professionals in establishing and overseeing these important elements of human research. The integration of teleoncology into international clinical trials will also help to ensure data integrity and patient safety in both LMC71 and HIC39–41 and, most importantly, will create local capacity for clinical investigation. It may also allow more cancer centers in MIC to participate in clinical trials, thus expediting accrual—an especially important consideration in rare cancers— and benefiting all participants.
Improvement of quality of life of cancer patients
Cancer is commonly accompanied by suffering (pain, dyspnea, and other discomfort). However, resources and expertise in palliation are least likely to be available, and suffering is least likely to be adequately addressed, in LMC,72 especially in rural areas.73 Telemedicine has been utilized in hospice care,32 and similar uses should be explored in LMC. Telemedicine links between HIC and LMC can be established to improve palliative care in major centers, and links between resource-rich and resource-poor institutions within LMC can then improve palliative care in remote or rural areas.73 Such links should also be explored to provide or improve ancillary services such as rehabilitation, social work, child life, and others needed to optimize quality of life in cancer patients.
CONCLUSION
Teleoncology is not a panacea for global oncology problems. If it is not used wisely, or if the “human factor” is not addressed, it can even exacerbate existing problems. Implementation of teleoncology should be guided by local communities’ needs and introduced to potential stakeholders as a pragmatic means of enhancing access to oncology care. Local professionals should be recruited as stakeholders and provided with thorough training. When done well, teleoncology is, as Furtado commented, the “next-best thing to being there.”74
Acknowledgments
This work was supported in part by grant CA21765 from the U.S. Public Health Service and by the American Lebanese Syrian Associated Charities (ALSAC). The manuscript was edited by Sharon Naron. The artwork was supported by Klo Spelshouse and Elizabeth Stevens. The authors thank Raul Ribeiro, MD, for critical review, information, and advice throughout the project; Eric Bouffet, MD, for helpful review; and Yuri Quintana, PhD, for technical advice.
Funding: None other than that reported above.
Footnotes
Disclosure: The authors have no financial or other conflict of interest related to the contents of this article. Since some of the teleoncology programs mentioned are conducted at King Hussein Cancer Center (KHCC) and the International Outreach Program (IOP), it should be noted that Dr. Qaddoumi worked at KHCC in Amman, Jordan, from October 2002 until September 2007 and has been with the IOP since October 2007.
CONTRIBUTORS
Ribhi Hazin: Conception, design, literature search, data collection, data analysis, manuscript writing, and final approval of manuscript.
Ibrahim Qaddoumi: Conception, design, literature search, data collection, data analysis, figures design, manuscript writing, and final approval of manuscript.
Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE and PubMed using the search terms “teleoncology”, “telemedicine”, and “cancer” or “chemotherapy”. Abstracts and meeting reports were excluded. Only reports published in English between 1993 and 2009 were included. Useful websites for further information include the World Bank (www.worldbank.org), the United Nations (www.un.org), and the world fact book at the Central Intelligence Agency website (www.cia.gov).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. [Accessed February 12, 2009];Preventing chronic diseases: a vital investment. 2008 December; Available http:/www.who.int/chp/chronic_disease_report/en/
- 2.Sloan FA, Gelband VH, editors. Committee on Cancer Control in Low- and Middle-Income Countries, Board on Global Health, Institute of Medicine. Washington, D.C: The National Academics Press; 2007. Cancer Control Opportunities in Low- and Middle-Income Countries. [PubMed] [Google Scholar]
- 3.Datta NR, Rajasekar D. Improvement of radiotherapy facilities in developing countries: a three-tier system with a teleradiotherapy network. Lancet Oncol. 2004;5:695–8. doi: 10.1016/S1470-2045(04)01613-4. [DOI] [PubMed] [Google Scholar]
- 4.Parkin DM, Whelan SL, Ferlay J, Raymond L, editors. Cancer Incidence in Five Continents. VII. Lyon: IARC Scientific Publications No 143; 1997. [Google Scholar]
- 5.Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. 5. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 6.Onega T, Duell EJ, Shi X, Wang D, Demidenko E, Goodman D. Geographic access to cancer care in the U. S. Cancer. 2008;112:909–18. doi: 10.1002/cncr.23229. [DOI] [PubMed] [Google Scholar]
- 7.Hortobagyi GN American Society of Clinical Oncology. A shortage of oncologists? The American Society of Clinical Oncology workforce study. J Clin Oncol. 2007;25:1468–9. doi: 10.1200/JCO.2007.10.9397. [DOI] [PubMed] [Google Scholar]
- 8.Wysocki WM, Komorowski AL, Aapro MS. The new dimension of oncology. Teleoncology ante portas. Crit Rev Oncol Hematol. 2005;53:95–100. doi: 10.1016/j.critrevonc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Qaddoumi I, Mansour A, Musharbash A, et al. Impact of telemedicine on pediatric neuro-oncology in a developing country: the Jordanian-Canadian experience. Pediatr Blood Cancer. 2007;48:39–43. doi: 10.1002/pbc.21085. [DOI] [PubMed] [Google Scholar]
- 10.Cone SW, Hummel R, León J, Merrell RC. Implementation and evaluation of a low-cost telemedicine station in the remote Ecuadorian rainforest. J Telemed Telecare. 2007;13:31–4. doi: 10.1258/135763307779701220. [DOI] [PubMed] [Google Scholar]
- 11.Quintana Y, O’Brien R, Patel A, et al. Cure4Kids: Research challenges in the design of a website for global education and collaboration. Inform Desig Jour. 2008;16:243–249. [Google Scholar]
- 12.McAleer JJ, O’Loan D, Hollywood DP. Broadcast quality teleconferencing for oncology. Oncologist. 2001;6:459–62. doi: 10.1634/theoncologist.6-5-459. [DOI] [PubMed] [Google Scholar]
- 13.Nezhat F. Minimally invasive surgery in gynecologic oncology: laparoscopy versus robotics. Gynecol Oncol. 2008;111:S29–32. doi: 10.1016/j.ygyno.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Wolf G, Petersen D, Dietel M, Petersen I. Telemicroscopy via the Internet. Nature. 1998;391:613–4. doi: 10.1038/35429. [DOI] [PubMed] [Google Scholar]
- 15.Callahan CW, Malone F, Estroff D, Person DA. Effectiveness of an Internet-based store-and-forward telemedicine system for pediatric subspecialty consultation. Arch Pediatr Adolesc Med. 2005;159:389–93. doi: 10.1001/archpedi.159.4.389. [DOI] [PubMed] [Google Scholar]
- 16.Qaddoumi I, Nawaiseh I, Mehyar M, et al. Team management, twinning, and telemedicine in retinoblastoma: a 3-tier approach implemented in the first eye salvage program in Jordan. Pediatr Blood Cancer. 2008;51:241–4. doi: 10.1002/pbc.21489. [DOI] [PubMed] [Google Scholar]
- 17.Dietel M, Nguyen-Dobinsky TN, Hufnagl P The UICC Telepathology Consultation Center. International Union Against Cancer. A global approach to improving consultation for pathologists in cancer diagnosis. Cancer. 2000;89:187–91. doi: 10.1002/1097-0142(20000701)89:1<187::aid-cncr25>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Mullaney J. SatelLife: pioneering the path for electronic communication and health information in the developing world. Clin Perform Qual Health Care. 1997;5:38–44. [PubMed] [Google Scholar]
- 19.Swinfen R, Swinfen P. Low-cost telemedicine in the developing world. J Telemed Telecare. 2002;8:63–5. [PubMed] [Google Scholar]
- 20.Qaddoumi I, Bouffet E. Supplementation of a successful pediatric neuro-oncology telemedicine-based twinning program by e-mails. Telemed J E Health. 2009 doi: 10.1089/tmj.2009.0043. in press. [DOI] [PubMed] [Google Scholar]
- 21.Ricke J, Bartelink H. Telemedicine and its impact on cancer management. Eur J Cancer. 2000;36:826–33. doi: 10.1016/s0959-8049(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 22.van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92:1143–50. doi: 10.1093/jnci/92.14.1143. [DOI] [PubMed] [Google Scholar]
- 23.Kontaxakis G, Visvikis D, Ohl R, et al. Integrated telemedicine applications and services for oncological positron emission tomography. Oncol Rep. 2006;15:1091–100. doi: 10.3892/or.15.4.1091. [DOI] [PubMed] [Google Scholar]
- 24.Brauchli K, Oberli H, Hurwitz N, et al. Diagnostic telepathology: long-term experience of a single institution. Virchows Arch. 2004;444:403–9. doi: 10.1007/s00428-004-0980-x. [DOI] [PubMed] [Google Scholar]
- 25.Olsen DR, Bruland S, Davis BJ. Telemedicine in radiotherapy treatment planning: requirements and applications. Radiother Oncol. 2000;54:255–9. doi: 10.1016/s0167-8140(99)00185-1. [DOI] [PubMed] [Google Scholar]
- 26.Hartvigsen G, Johansen MA, Hasvold P, et al. Challenges in telemedicine and eHealth: lessons learned from 20 years with telemedicine in Tromsø. Stud Health Technol Inform. 2007;129:82–6. [PubMed] [Google Scholar]
- 27.Kunkler IH, Prescott RJ, Lee RJ, et al. TELEMAM: a cluster randomised trial to assess the use of telemedicine in multi-disciplinary breast cancer decision making. Eur J Cancer. 2007;43:2506–14. doi: 10.1016/j.ejca.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Eich HT, Müller RP, Schneeweiss A, et al. Initiation of a teleradiotherapeutic network for patients in German lymphoma studies. Int J Radiat Oncol Biol Phys. 2004;58:805–8. doi: 10.1016/S0360-3016(03)01565-7. [DOI] [PubMed] [Google Scholar]
- 29.Campbell NC, Ritchie LD, Cassidy J, Little J. Systematic review of cancer treatment programmes in remote and rural areas. Br J Cancer. 1999;80:1275–80. doi: 10.1038/sj.bjc.6690498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billingsley KG, Schwartz DL, Lentz S, et al. The development of a telemedical cancer center within the Veterans Affairs Health Care System: a report of preliminary clinical results. Telemed J E Health. 2002;8:123–30. doi: 10.1089/15305620252933464. [DOI] [PubMed] [Google Scholar]
- 31.Doolittle GC, Allen A. Practising oncology via telemedicine. J Telemed Telecare. 1997;3:63–70. doi: 10.1258/1357633971930869. [DOI] [PubMed] [Google Scholar]
- 32.Doolittle GC, Yaezel A, Otto F, Clemens C. Hospice care using home-based telemedicine systems. J Telemed Telecare. 1998;4:58–9. doi: 10.1258/1357633981931470. [DOI] [PubMed] [Google Scholar]
- 33.Current trials list, Trial NCT00609505. U.S. National Institutes of Health; [Accessed January 16, 2009]. Telemedicine vs. Face-to-Face Cancer Genetic Counseling in Rural Oncology Clinics. Adams MB, Principal Investigator. Available http://clinicaltrials.gov/ct2/show/NCT00609505. [Google Scholar]
- 34.Mizushima H, Uchiyama E, Nagata H, et al. Japanese experience of telemedicine in oncology. Int J Med Inform. 2001;61:207–15. doi: 10.1016/s1386-5056(01)00142-3. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita S, Carr Z, Repacholi M. Long-term health implications of the Chernobyl accident and relevant projects of the World Health Organization. Health Phys. 2007;93:538–41. doi: 10.1097/01.HP.0000281686.14210.4f. [DOI] [PubMed] [Google Scholar]
- 36.Yokota K, Takamura N, Shibata Y, Yamashita S, Mine M, Tomonaga M. Evaluation of a telemedicine system for supporting thyroid disease diagnosis. Stud Health Technol Inform. 2001;84:866–9. [PubMed] [Google Scholar]
- 37.Oakley A, Duffill M, Astwood D, Reeve P. Melanoma-diagnosis by telemedicine. J Telemed Telecare. 1996;2:174–5. doi: 10.1258/1357633961929916. [DOI] [PubMed] [Google Scholar]
- 38.See A, Lim AC, Le K, See JA, Shumack SP. Operational teledermatology in Broken Hill, rural Australia. Australas J Dermatol. 2005;46:144–9. doi: 10.1111/j.1440-0960.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 39.Fouladi M, Hunt DL, Pollack IF, et al. Outcome of children with centrally reviewed low-grade gliomas treated with chemotherapy with or without radiotherapy on Children’s Cancer Group high-grade glioma study CCG-945. Cancer. 2003;98:1243–52. doi: 10.1002/cncr.11637. [DOI] [PubMed] [Google Scholar]
- 40.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–8. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 41.Donaldson SS, Torrey M, Link MP, et al. A multidisciplinary study investigating radiotherapy in Ewing’s sarcoma: end results of POG #8346. Pediatric Oncology Group. Int J Radiat Oncol Biol Phys. 1998;42:125–35. doi: 10.1016/s0360-3016(98)00191-6. [DOI] [PubMed] [Google Scholar]
- 42.Bukachi F, Pakenham-Walsh N. Information technology for health in developing countries. Chest. 2007;132:1624–30. doi: 10.1378/chest.07-1760. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Wang L. Telemedicine in border area of China. Telemed J. 1998S;4:39–41. doi: 10.1089/tmj.1.1998.4.39. [DOI] [PubMed] [Google Scholar]
- 44.Wootton R. Telemedicine support for the developing world. J Telemed Telecare. 2008;14:109–14. doi: 10.1258/jtt.2008.003001. [DOI] [PubMed] [Google Scholar]
- 45.Ganapathy K. Telemedicine and neurosciences in developing countries. Surg Neurol. 2002;58:388–94. doi: 10.1016/s0090-3019(02)00924-2. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh RK, Hjelm NM, Lee JC, Aldis JW. Telemedicine in China. Int J Med Inform. 2001;61:139–46. doi: 10.1016/s1386-5056(01)00136-8. [DOI] [PubMed] [Google Scholar]
- 47.Misra UK, Kalita J, Mishra SK, Yadav RK. Telemedicine in neurology: underutilized potential. Neurol India. 2005;53:27–31. doi: 10.4103/0028-3886.15047. [DOI] [PubMed] [Google Scholar]
- 48.Sood SP, Negash S, Mbarika VW, Kifle M, Prakash N. Differences in public and private sector adoption of telemedicine: Indian case study for sectoral adoption. Stud Health Technol Inform. 2007;130:257–68. [PubMed] [Google Scholar]
- 49.Kvedar J, Heinzelmann PJ, Jacques G. Cancer diagnosis and telemedicine: a case study from Cambodia. Ann Oncol. 2006;17:37–42. doi: 10.1093/annonc/mdl986. [DOI] [PubMed] [Google Scholar]
- 50.Brauchli K, Jagilly R, Oberli H, et al. Telepathology on the Solomon Islands--two years’ experience with a hybrid Web- and email-based telepathology system. J Telemed Telecare. 2004;10:14–7. doi: 10.1258/1357633042614249. [DOI] [PubMed] [Google Scholar]
- 51.Hira AY, Nebel de Mello A, Faria RA, Odone Filho V, Lopes RD, Zuffo MK. Development of a telemedicine model for emerging countries: a case study on pediatric oncology in Brazil. Conf Proc IEEE Eng Med Biol Soc. 2006;1:5252–6. doi: 10.1109/IEMBS.2006.259380. [DOI] [PubMed] [Google Scholar]
- 52.Wilimas JA, Wilson MW, Haik BG, et al. Development of retinoblastoma programs in Central America. Pediatr Blood Cancer. 2009;53:42–6. doi: 10.1002/pbc.21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ayoub L, Fú L, Peña A, et al. Implementation of a data management program in a pediatric cancer unit in a low income country. Pediatr Blood Cancer. 2007;49:23–7. doi: 10.1002/pbc.20966. [DOI] [PubMed] [Google Scholar]
- 54.Howard SC, Metzger ML, Wilimas JA, et al. Childhood cancer epidemiology in low-income countries. Cancer. 2008;112:461–72. doi: 10.1002/cncr.23205. [DOI] [PubMed] [Google Scholar]
- 55.Quintana Y, editor. Cure4Kids 2008 Annual Report. Memphis, Tenn: St. Jude Children’s Research Hospital; 2009. [Google Scholar]
- 56.Ribeiro RC, Howard SC, Pui CH. Acute leukemia in countries with limited resources. In: Pui CH, editor. Childhood Leukemias. 2. New York: Cambridge University Press; 2006. pp. 625–638. [Google Scholar]
- 57.Perov IuL, Frank GA, Gribunov IP, Khodasevich LS. Using telepathology for remote pathoanatomical diagnostics. Arkh Patol. 2003;65:32–6. [Russian] [PubMed] [Google Scholar]
- 58.Yellowlees P. Successful development of telemedicine systems--seven core principles. J Telemed Telecare. 1997;3:215–22. doi: 10.1258/1357633971931192. [DOI] [PubMed] [Google Scholar]
- 59.Obstfelder A, Engeseth KH, Wynn R. Characteristics of successfully implemented telemedical applications. Implement Sci. 2007;2:25. doi: 10.1186/1748-5908-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krupinski EA, Charness N, Demiris G, et al. Human factors in telemedicine. Telemed J E Health. 2008;14:1024–30. doi: 10.1089/tmj.2008.8471. [DOI] [PubMed] [Google Scholar]
- 61.Ribeiro RC, Steliarova-Foucher E, Magrath I, et al. Baseline status of paediatric oncology care in ten low-income or mid-income countries receiving My Child Matters support: a descriptive study. Lancet Oncol. 2008;8:721–9. doi: 10.1016/S1470-2045(08)70194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howard SC, Pedrosa M, Lins M, et al. Establishment of a pediatric oncology program and outcomes of childhood acute lymphoblastic leukemia in a resource- poor area. JAMA. 2004;291:2471–5. doi: 10.1001/jama.291.20.2471. [DOI] [PubMed] [Google Scholar]
- 63.Masera G, Baez F, Biondi A, et al. North-South twinning in paediatric haemato- oncology: the La Mascota programme, Nicaragua. Lancet. 1998;352:1923–6. doi: 10.1016/s0140-6736(98)07077-9. [DOI] [PubMed] [Google Scholar]
- 64.Sistero RF, Scavuzzo CM, Lamfri MA, Albornoz CH, Sistero RI. A telemedicine program suitable for developing countries. Telemed Today. 1999;7:24–6. [PubMed] [Google Scholar]
- 65.Jithoo R, Govender PV, Corr P, Nathoo N. Telemedicine and neurosurgery: experience of a regional unit based in South Africa. J Telemed Telecare. 2003;9:63–6. doi: 10.1258/135763303321327894. [DOI] [PubMed] [Google Scholar]
- 66.Al-Qudimat MR, Day S, Almomani T, Odeh D, Qaddoumi I. Clinical nurse coordinators: a new generation of highly specialized oncology nursing in Jordan. J Pediatr Hematol Oncol. 2009;3:38–41. doi: 10.1097/MPH.0b013e31818b3536. [DOI] [PubMed] [Google Scholar]
- 67.Chao LW, Enokihara MY, Silveira PS, Gomes SR, Böhm GM. Telemedicine model for training non-medical persons in the early recognition of melanoma. J Telemed Telecare. 2003;9:S4–7. doi: 10.1258/135763303322196141. [DOI] [PubMed] [Google Scholar]
- 68.Mukundan S, Jr, Vydareny K, Vassallo DJ, Irving S, Ogaoga D. Trial telemedicine system for supporting medical students on elective in the developing world. Acad Radiol. 2003;10:794–7. doi: 10.1016/s1076-6332(03)80125-3. [DOI] [PubMed] [Google Scholar]
- 69.Mullaney J. SatelLife: pioneering the path for electronic communication and health information in the developing world. Clin Perform Qual Health Care. 1997;5:38–44. [PubMed] [Google Scholar]
- 70.Ozuah PO, Reznik M. The role of telemedicine in the care of children in under- served communities. J Telemed Telecare. 2004;10:78–80. doi: 10.1258/1357633042614294. [DOI] [PubMed] [Google Scholar]
- 71.Rivera GK, Quintana J, Villarroel M, et al. Transfer of complex frontline anticancer therapy to a developing country: the St. Jude osteosarcoma experience in Chile. Pediatr Blood Cancer. 2008;50:1143–6. doi: 10.1002/pbc.21444. [DOI] [PubMed] [Google Scholar]
- 72.Shaikh AJ, Khokhar NA, Raza S, et al. Knowledge, attitude and practices of non-oncologist physicians regarding cancer and palliative care: a multi-center study from Pakistan. Asian Pac J Cancer Prev. 2008;9:581–4. [PubMed] [Google Scholar]
- 73.Maserat E. Information communication technology: new approach for rural cancer care improvement. Asian Pac J Cancer Prev. 2008;9:811–4. [PubMed] [Google Scholar]
- 74.Furtado R. Telemedicine: the next-best thing to being there. Dimens Health Serv. 1982;59:10–2. [PubMed] [Google Scholar]


