Abstract
Global left ventricular ejection fraction (LVEF) has been used as a measure of improvement in LV function following cell therapy. Although the impact of cell therapy on LVEF in short and long-term follow-up has been generally positive, there is concern that research evaluating regional therapeutics (e.g., cell or gene therapy) may require analysis of regional LV function localized to the site of intervention. Regional LV assessment is traditionally performed with qualitative or quantitative analysis of wall thickening within 16 myocardial segments, but advances in noninvasive imaging permit an increasingly more detailed and accurate evaluation of LV function. Wall thickness measurements can now include evaluation of over 1,000 myocardial segments. In addition to higher resolution measures of wall thickening, automated assessments of myocardial segment deformation, like strain imaging, exist. Strain imaging allows for direct evaluation of the mechanical properties that may improve following regional therapeutic intervention. Improvements in regional LV function may also be assessed by determining regional ejection fraction (EF). Regional EF offers the advantage of summarizing the end result of all of the complex deformations in the adjacent myocardial segments. While regional EF and strain imaging, as compared to wall thickening, enhance detection of improvement in complex measures of regional myocardial function, it remains unclear whether such measures are better able to predict meaningful improvement in clinical outcomes.
Keywords: Left Ventricular Function, Regional Ventricular Function, Regional Ejection Fraction, Strain Imaging
INTRODUCTION
Global left ventricular ejection fraction (LVEF) has been used as the predominant measure to assess LV function following cell therapy. However, the impact of cell therapy on LVEF in short and long-term follow-up has been variable. The National Institutes of Health identified clinical aspects of cell-based therapy as an unmet area of need and established the Cardiovascular Cell Therapy Research Network (CCTRN). The primary goal of the CCTRN is to conduct multiple collaborative cell therapy clinical protocols. CCTRN investigators recognized that many imaging based parameters of LV function are available to quantify the effect of regional cell therapy but the basis for choosing one over another had not been critically examined. Accordingly, the uniqueness of this document is to review the advantages and limitations of various noninvasive imaging modalities available to quantify global and regional LV mechanical function. From this information, we articulate why the CCTRN investigators reached a consensus on which measures of LV function have the potential to most successfully predict clinically meaningful improvement in patient outcomes after regional therapeutic intervention. CCTRN protocols include acquisition of cardiac MRI and echo images with response variables quantified at dedicated echo and MRI core laboratories with results submitted to a central data coordinating center. Because the CCTRN will collect many variables provided by multiple modalities, its work should help answer which measures of regional LV function are most clinically relevant.
GLOBAL LEFT VENTRICULAR ASSESSMENT
Myocardial contraction is a complex process that occurs in four dimensions in both a global and segmental domain. Since segmental myocardial contraction has been difficult to measure in patients, global measures to assess LV performance have often been used. It remains unclear whether measures of global LV pump performance or regional LV function are most predictive of cardiovascular (CV) outcomes.
Methods of Global LV Assessment
Global LV function is most frequently reported as LVEF, which refers to the percentage of the LV end-diastolic volume ejected with each contraction. LVEF can be determined by multiple imaging modalities, including echocardiography, ventriculography, magnetic resonance imaging (MRI), computed tomography (CT), and radionuclide angiography. Although MRI is widely regarded as the reference standard for measurement of LVEF, echocardiography is most frequently used, being inexpensive, portable, and without contraindications. However, there are limitations with echocardiographic assessment of LVEF. Foreshortening of the apical views will result in an underestimate of LV volumes and overestimate of LVEF, while the presence of regional wall motion abnormalities can lead to errors when limited imaging planes are used to assess LVEF. Additionally, there is more inter-study variability of LVEF with echocardiography compared with MRI (1). Due to the different handling of endocardial trabeculations, echocardiography typically results in lower volumes than those determined with MRI. Use of microbubbles for contrast can significantly improve endocardial visualization by echocardiography and provide volume estimates that more closely approximate those determined with CT (2). 3D echocardiography can also improve LVEF assessment, particularly in the presence of regional dyskinesis (3). However, endocardial definition is sometimes suboptimal with 3D acquisition. Determination of LVEF with MRI may overcome the challenge of regional wall motion abnormalities by utilizing a series of short axis imaging planes that incorporate the entire LV. However, MRI is expensive, less widely available, and at the present time prohibited in patients with electronic devices. This may limit its usefulness among patients with advanced heart failure enrolled in cell therapy trials who have pacemakers or defibrillators.
Applications of Global LV Assessment
Assessment of global LVEF provides a robust predictor of risk for CV outcomes with the potential to significantly influence patient management. For example, in heart failure patients the risk for all-cause mortality is increased by 39% for every 10% reduction in LVEF below 45% (4). Similarly, CV death and heart failure hospitalization rates declined with increasing LVEF up to 45%. The discriminatory effect of LVEF for prediction of outcomes is limited in patients with a LVEF >45%. This lack of discrimination may be due to inability to detect subtle abnormalities of segmental LV systolic function not contained within the imaging planes used to assess LVEF. An assessment of global LV function based on the composite of function in each segment has the potential to overcome these limitations and is superior to LVEF in predicting risk for adverse outcome (5). It is noteworthy that the LVEF in many patients enrolled in CV cell therapy trials exceeds this 45% threshold (6).
Although global LVEF may improve after intervention, such improvements may be subtle. LVEF improved only 1–5% in patients with otherwise evidence-documented lifesaving therapies (e.g., coronary revascularization, angiotensin-converting enzyme inhibitors, angiotensin receptor blockade, or beta blockade) after acute myocardial infarction (7). Thus, small changes in LV function may translate to meaningful improvement in clinical outcomes, but global LVEF may be an insensitive measure of this change in outcome. This is supported by the findings in the REPAIR-AMI Study which demonstrated that adverse outcomes were significantly reduced among patients receiving cell therapy compared with placebo, despite only a small improvement in LVEF (6).
As compared with revascularization alone, additional improvements in LVEF after cell therapy and revascularization have ranged from 0% to as much as a 6% (Table 1) (8–13). If global LVEF is to be used as the measure of interest in cell therapy trials, use of an imaging modality that limits need for geometrical or 3D assumptions, such as with MRI or 3D echocardiography, would be preferred. However, important, but subtle, changes in LV function after cell therapy may not be detected with global assessment of LV function, whereas regional assessment of LV function may enhance detection of these important changes.
Table 1.
Cell Therapy Trials Evaluating Changes in both Global and Regional LV Function*.
| Trial [ref.] | No. Patients Randomized | Imaging Variable | Follow-Up Duration | Change in Imaging Variable in Cell Treated Patients vs. Change in Imaging Variable in Controls “Treatment Effect” |
|---|---|---|---|---|
| BOOST, Wollert et al., 2004(13) and Meyer et al., 2006 (12) | 60 | MRI Global EF | 6 months | Improved 6 % (P 0.0026) |
| 18 months | Improved 2.8 % (P 0.27) | |||
| MRI Regional EF | 6 months | Improved 5.7% (P 0.04) | ||
| ASTAMI, Lunde et al., 2006 (10) and Beitnes et al., 2010 (8) | 100 | Echo (Simpson) Global EF | 6months | Improved 0.6% (P 0.7) |
| MRI Global EF | 6 months | Worsened 3% (P 0.054) | ||
| Speckle Echo Long. Strain | 3 years | Improved 0.4% (P 0.45) | ||
| Meluzin et al., 2006 (11) | 66 | SPECT Global EF | 3 months | Improved 3% (P 0.041) |
| Long. Strain Rate | 3 months | Improved 0.9 cm/sec (P 0.008) | ||
| Herbots et al. 2009 (9) | 67 | Echo (Simpson) Global EF | 4 months | Worsened 1.5% (P 0.84) |
| TDI Echo Long. Strain | 4 months | Improved 3.7 % (P<0.01) |
All studies included in this table were in the setting of acute ischemia with intra-coronary delivery of cells.
REGIONAL LEFT VENTRICULAR ASSESSMENT
Regional LV assessment can include evaluation of mechanical function, segmental perfusion, and tissue characterization. Segmental perfusion and tissue characteristics are important components of regional myocardial function after acute ischemic injury, as the presence of microvascular obstruction and the, ratio of edematous to infarcted tissue predict improvement in contractile function and future CV events (14–16). However, due to space limitations, the current review focuses on measures of mechanical function. Assessment of regional LV mechanical function can be performed with quantification of both regional EF or segmental myocardial contraction. Regional EF offers the advantage of summarizing the end effect of the complex deformations in adjacent myocardial segments, while quantification of segmental myocardial contraction allows for direct evaluation of mechanical properties of selected myocardial segments that may be improved following cell therapy.
Regional EF
Regional EF has been determined with nuclear, MRI, and echocardiographic techniques (17, 18), and with each imaging modality, has been defined by different methods. For example, regional EF measured with MRI can be as simple as the comparison of the end-diastolic and end-systolic volumes constrained by the endocardial border in a single short axis MRI image. This method was employed in the BOOST trial as regional EF was determined only in slices that contained late contrast enhancement (13). Other studies have reported regional EF using the fixed centerpoint method. Briefly, the ventricle is divided into a number of wedge-shaped subvolumes radiating from the LV long axis, and the proportional change in each subvolume from diastole to systole is reported as the regional EF. Finally, the CCTRN MRI Core Laboratory divides the LV volume into thirds and measures the end-diastolic and end-systolic volume within each of the apical, mid and basal regions.
Regional EF strongly correlates with the function of adjacent myocardial segments as determined by wall motion, wall thickening, and infarct transmurality. Thus, regional EF measured by MRI in acute myocardial infarction patients was significantly lower in regions with infarction vs. regions with no infarction in adjacent myocardial segments (17).
Regional EF measured with radionuclide ventriculography has been shown to improve with revasularization (19). Additionally, regional EF measured by MRI did improve after revascularization for acute myocardial infarction, whereas wall thickening did not (17). Regional EF may also be a sensitive marker of improvement after cell therapy. In the BOOST Trial, patients who received intra-coronary bone marrow cells had significant improvement in regional EF at 6 months compared with those who did not receive cells (13). However, this difference was not present at 18 months, largely due to delayed improvement in regional EF in the control group (12). In BOOST, regional EF and global EF improved in a similar degree in patients with cell therapy. Therefore, while regional EF appears more sensitive than wall thickening in detection of improved LV function after traditional revascularization, it remains unclear if regional EF will advance detection of improved LV function following cell therapy.
Segmental Myocardial Contraction
Assessment of regional myocardial function can be improved by characterization of segmental deformation throughout the heart. This is complicated because the LV has a complex architecture with a left-handed helix in the epicardium, a right-handed helix in the endocardium, and nearly circumferential fibers in the midwall (20). This architecture leads to four contraction patterns during systole: radial wall thickening; circumferential contraction; descent of the base of the heart toward the apex with longitudinal shortening; and torsion, as epicardial helical fibers rotate the apex counterclockwise (as viewed from the apex) relative to the base (21). There are numerous methods for quantifying each of these, but it is unclear which will prove most accurate for characterizing the response to cell therapy.
Among the oldest methods to quantify radial wall thickening is the centerline method, which measures myocardial thickening relative to the center of the wall (22). Wall thickening is traditionally reported as average thickening within each of the American Heart Association 16 or 17 segments. However, the higher resolution afforded by advanced noninvasive imaging techniques, such as MRI, coupled with increased processing power for image reconstruction and analysis, provide new opportunities. For example, the CCTRN MRI Core Laboratory has chosen to analyze 8 to 12 short axis slices with 100 chords per slice. As a result, approximately 1000 chords are analyzed for each study, with wall thickening and motion calculated for each chord using the centerline method.
Methods of Strain Imaging
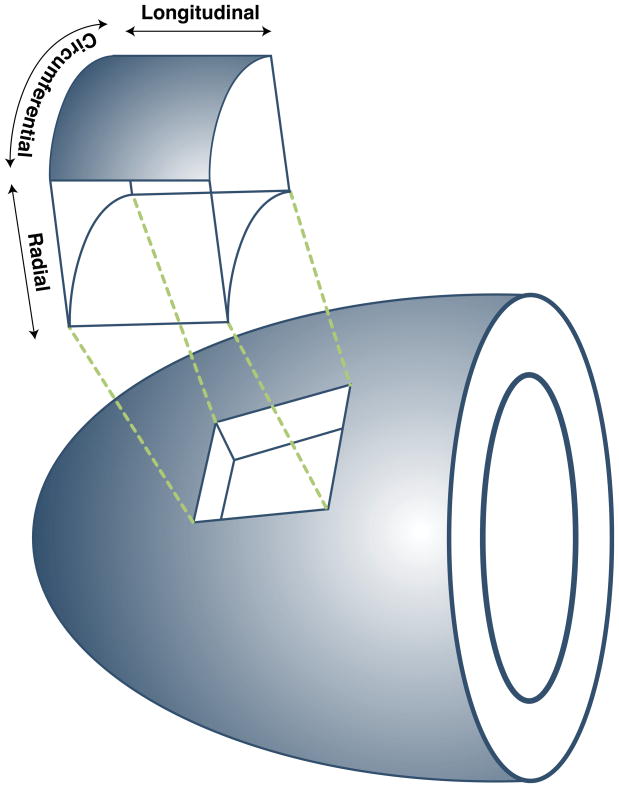
A more advanced approach to describe regional contraction is through use of strain imaging, which characterizes myocardial tissue deformation. Mathematically, strain is a 3×3 tensor, which captures both linear deformation (stretching or compression along the three principal axes) and shear (sliding of one tissue plane against another). In the heart, strain is typically referenced to the coordinate system of the LV, yielding radial, circumferential, and longitudinal strain components (23) (Figure 1). In addition to these three components of myocardial segment deformation, strain imaging allows for quantification of LV torsion, which is an important component of normal LV mechanics (24). Regional EF correlates strongest with circumferential and longitudinal strain compared with wall thickening, suggesting that these parameters may have a greater impact on stroke volume and cardiac output than wall thickening alone (25).
Figure 1. The Three Orthogonal Axes of the Left Ventricle.
Diagram demonstrates the orientation of the three types of myocardial segmental deformation most commonly reported with strain imaging.
Magnetic Resonance Imaging
The most frequent approach to MRI strain imaging is by tracking a grid of magnetic tags formed by inverting protons in specific locations throughout the image (26). Radiofrequency tagging is accomplished by altering the net magnetization of the tissue with carefully designed radiofrequency pulses (27). Each tag is created as a 3D plane that extends through the tissue, and it is seen as a dark line (Figure 2). Because these tags result from alterations of magnetization of tissue itself, the motion of tags matches motion of underlying tissue which can be tracked over time to quantify myocardial deformation. Wall thickening determined by tagged MRI correlates with wall thickening measures by sonomicrometry, although systolic thickening appears to be systematically higher with tagged MRI vs. sonomicrometry (Table 2) (17, 18, 28–34).
Figure 2. Example of MRI Tagging.
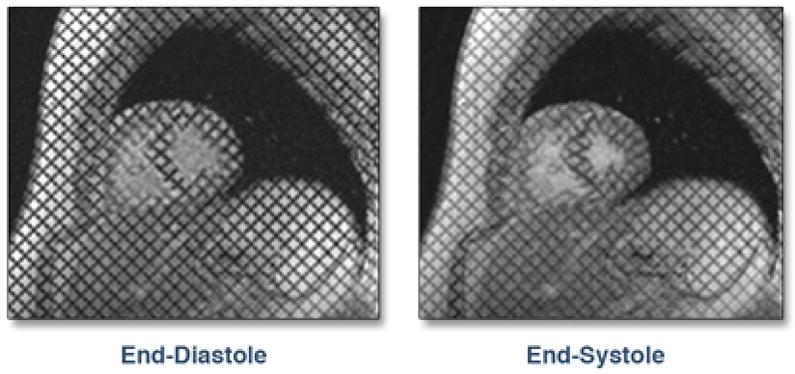
The tags are seen as dark lines. The deformation of the tag lines can be seen at end-systole.
Table 2.
Accuracy and Precision of Measures of Global and Regional LV Function
| Reference Standard | Correlation with Reference Standard | Intra- Observer Reliability | Inter- Observer Reliability | |
|---|---|---|---|---|
| Estimate of Global EF | ||||
| Echocardiography(29, 33) MRI (29, 34) Ventriculography (33) RNA (33) |
MRI | r=0.41 | 4.4% mean diff. | 6.1% mean diff. |
| Echo | r=0.41 | Not Available | Not Available | |
| Echo | r=0.85 | 4.3% mean diff. | 6.7% mean diff. | |
| Echo | r=0.86 | 2.5% mean diff. | 6.8% mean diff. | |
| Estimate of Regional EF | ||||
| Echocardiography (31) MRI (17) RNA (18) |
Not Available | 4.9% mean diff. | 5.8% mean diff. | |
| Not Available | 1.6% mean diff. | 1.9% mean diff. | ||
| Not Available | r=0.63–0.93 | r=0.6–0.98 | ||
| Estimate of Segmental Contraction | ||||
| MRI-Radial Strain (30, 32) | Sonomicrometry | r=0.87 | r=0.69–0.77 | r=0.57–0.71 |
| Sonomicrometry | r=0.79 | COR 4.6% | COR 7% | |
Bland-Altman limits of agreement were not available for all modalities and are therefore not included.
Echocardiography
Unfortunately, MRI strain measurements are rarely made in clinical practice, due to cost, lengthy time of analysis, and contraindications for patients with implanted devices. Echocardiography can be used to calculate regional myocardial strain by two basic techniques: analysis of velocity from tissue Doppler and tracking of myocardial speckles. Tissue Doppler maps the component of velocity directed toward the transducer throughout the image. The spatial derivative of velocity along the scan line yields the strain rate, the temporal integral of which yields strain (35). However, this technique is dependent on alignment of the ultrasound beam in the appropriate region of interest. Therefore, only certain axes of deformation can be determined in a limited number of myocardial segments.
Speckle tracking echocardiography (STE) overcomes many limitations of tissue Doppler techniques. STE is based on tracking fairly constant speckle patterns created by interference of the ultrasound beam with microscopic structures within myocardium (28). Because STE analyzes routinely acquired grayscale images, it is, unlike tissue Doppler, angle independent and can be quickly and reproducibly performed offline after study completion. STE allows strain and strain rate measurement along all three orthogonal axes of ventricular deformation for all LV segments. Both tissue Doppler and STE modestly correlate with MRI tagging and sonomicrometry (28, 36). However, image quality, frame rate, depth and direction of movement of the tracked speckles can impact fidelity of the analysis. At times as much as 10–20%of data in a single study may be “too noisy” for appropriate analysis (37, 38). Recently, STE has been applied to 3D echocardiograms (39). Whether the advantage of being able to track speckles wherever they move in 3D space outweighs the much lower frame rate of 3D echo and associated further impairment in the fidelity of the analysis, remains to be determined.
A limitation of both 2D and 3D STE is that it is very vendor specific, typically applied to images stored in proprietary ultrasound scan line formats. There are limited data demonstrating equivalence between strain measurements obtained by different vendors. The American Society of Echocardiography and the European Association of Echocardiography have formed a task force with industry to address these concerns and hopefully this will make STE more widely applicable to images stored in standard DICOM format.
Applications of Strain Imaging in Ischemic Heart Disease
These newer measures of segmental myocardial contraction have been used in many different ways to evaluate patients with ischemic heart disease. Echocardiographic and MRI strain imaging may assist with identification of ischemic tissue (Table 3) (37, 40–43). Additionally, strain imaging provides estimation of segmental myocardial viability and likelihood of improvement with revascularization (37). Furthermore, strain imaging has demonstrated the ability to predict final infarct size and LV remodeling after infarction (42, 43). Therefore, strain analysis may assist in identifying those patients who would benefit from an early, invasive strategy that includes revascularization and/or cell therapy. Importantly, strain imaging may increase the sensitivity to detect improvement of LV function after regional therapy and may enhance localization of infarcted tissue that might benefit from such therapy.
Table 3.
Operating Characteristics of Different Imaging Techniques for Various Applications in Patients with IHD
| APPLICATION/Technique | REFERENCE STANDARD | SENSITIVITY | SPECIFICITY |
|---|---|---|---|
| Prediction of CAD
| |||
| Dobutamine MRI-Qualitative Wall Motion (40) | >50% stenosis on angiography | 83% | 83% |
|
|
|||
| Dobutamine Echo-Qualitative Wall Motion (41) | >50% stenosis on angiography | 76% | 93% |
|
|
|||
| Dobutamine STE-Longitudinal Strain (41) | >50% stenosis on angiography | 84% | 88% |
|
| |||
| Viability/Prediction of Recovery after Revascularization
| |||
| MRI-Gadolinium Hyperenhancement (37) | Visually determined improvement in segmental contraction after revasc. | 72% | 92% |
|
|
|||
| Resting STE-Radial Strain (37) | Visually determined improvement in segmental contraction after revasc. | 70% | 85% |
|
| |||
| Prediction of Infarct Size after Acute Injury
| |||
| LVEF (43) | MRI determined infract size greater than 20% of LV mass | 80% | 55% |
|
|
|||
| STE-LV Global Strain (43) | MRI determined infarct size greater than 20% of LV mass | 90% | 86% |
|
| |||
| Prediction of LV Remodeling after Acute Injury
| |||
| STE-Longitudinal Strain (42) | Increase in LV end-diastolic volume of ≥ 15% 3 months after acute MI | 91% | 86% |
Markers of Improved Function after Cell Therapy
Assessment of improvement in LV function after cell therapy may be a more difficult task than simply assessing a change in overall LVEF. This may be particularly true in the setting of acute myocardial infarction where hyperdynamic contraction of remote regions may elevate LVEF. Quantified measures of LV segmental myocardial strain may allow for better recognition of these early and subtle improvements in myocardial function in the peri-infarct region following cell therapy. For example, early after reperfusion for acute myocardial infarction, there was no significant difference in the improvement in LVEF comparing patients treated with cells and controls (9). However, strain of infarcted segments improved significantly more in the cell-treated group. In another pilot study, 12 patients who received intramyocardial autologous cells during coronary artery bypass surgery were evaluated with echocardiography before and 1 year after injection (44). On average, longitudinal strain increased 40% in segments that underwent revascularization without cell therapy, but increased 93% in segments that underwent revascularization with cell therapy (P=0.002). In cell treated segments, visual estimates of segmental myocardial contractility increased 5%, whereas longitudinal strain increased 159% (P=0.0001). While these exploratory studies suggest that strain imaging may be more sensitive in detecting improvement in LV function after cell therapy, not all studies have demonstrated strain to be superior to LVEF as a marker of improvement after cell therapy. In the ASTAMI trial, improvements in LVEF and longitudinal strain were no different in cell treated patients compared with those who received placebo (8). In this study, strain measurements may not have improved because of the lack of true improvement in myocardial function. Future studies comparing the ability of strain and LVEF to detect improvement following cell therapy are clearly needed.
As reported above, regional EF may also be a sensitive marker of improvement in LV function after cell therapy. Because comparative studies are lacking, it remains unclear whether regional EF or strain will be the most sensitive measure of improved LV function after cell therapy. Also unknown are the amounts and types of improvements in these regional measures of LV function that might predict improvements in morbidity and mortality. For example, is improvement in apical regional EF or strain more relevant than improvement in basal regional LVEF or strain? Data from the CCTRN core labs could help answer these questions.
CONCLUSIONS AND FUTURE DIRECTIONS
Although the impact of cell therapy on global LVEF in short and long-term follow-up has been variable, overall the results show modest improvement. To better assess the effect of regional therapeutics (such as cell or gene therapy) we propose that future research include analysis of regional LV function localized to the site of intervention (Table 4). Regional LV mechanical function can be quantified with regional EF or measures of segmental myocardial contraction. Segmental contraction can be evaluated by indices of wall thickening or with MRI or echocardiographic strain imaging.
Table 4.
Goals for Imaging Variables in Future Cell Therapy Trials.
|
Changes in regional EF and myocardial segmental strain appear to offer an enhanced ability to detect subtle improvements in LV function after intervention. While regional EF and strain, compared with wall thickening, may enhance sensitivity for detecting improvement in LV function, it remains unclear whether such measures are better able to predict improvement in clinical outcomes. Accordingly, the CCTRN will implement use of high definition wall thickening measurements in addition to regional EF measurements. The CCTRN will consider incorporating strain imaging in future projects, and will aim to determine which measures of regional LV mechanical function best link with clinical outcomes. Further, assessment of LV function after cell therapy may not be appropriately addressed with a single measure of a single region. Assimilation of all of the regional information provided to the CCTRN central data coordinating center should allow for the investigation of new indices or computational models to help better understand the impact of cell therapy on LV function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90:29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 2.Thomson HL, Basmadjian AJ, Rainbird AJ, et al. Contrast echocardiography improves the accuracy and reproducibility of left ventricular remodeling measurements: a prospective, randomly assigned, blinded study. J Am Coll Cardiol. 2001;38:867–75. doi: 10.1016/s0735-1097(01)01416-4. [DOI] [PubMed] [Google Scholar]
- 3.Thomas JD, Popovic ZB. Assessment of left ventricular function by cardiac ultrasound. J Am Coll Cardiol. 2006;48:2012–25. doi: 10.1016/j.jacc.2006.06.071. [DOI] [PubMed] [Google Scholar]
- 4.Solomon SD, Anavekar N, Skali H, et al. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112:3738–44. doi: 10.1161/CIRCULATIONAHA.105.561423. [DOI] [PubMed] [Google Scholar]
- 5.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–64. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 6.Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 7.Reffelmann T, Konemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–8. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 8.Beitnes JO, Gjesdal O, Lunde K, et al. Left ventricular systolic and diastolic function improve after acute myocardial infarction treated with acute percutaneous coronary intervention, but are not influenced by intracoronary injection of autologous mononuclear bone marrow cells: a 3 year serial echocardiographic sub-study of the randomized-controlled ASTAMI study. Eur J Echocardiogr. 2010 Sep 17; doi: 10.1093/ejechocard/jeq116. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Herbots L, D’Hooge J, Eroglu E, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: a randomized, double-blind strain rate imaging study. Eur Heart J. 2009;30:662–70. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 10.Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 11.Meluzin J, Mayer J, Groch L, et al. Autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction: the effect of the dose of transplanted cells on myocardial function. Am Heart J. 2006;152:975, e9–15. doi: 10.1016/j.ahj.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 13.Wollert KC, Meyer GP, Lotz J, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 14.Aletras AH, Tilak GS, Natanzon A, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 15.Eitel I, Desch S, Fuernau G, et al. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:2470–9. doi: 10.1016/j.jacc.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 16.Nijveldt R, Beek AM, Hirsch A, et al. Functional recovery after acute myocardial infarction: comparison between angiography, electrocardiography, and cardiovascular magnetic resonance measures of microvascular injury. J Am Coll Cardiol. 2008;52:181–9. doi: 10.1016/j.jacc.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Masci PG, Dymarkowski S, Rademakers FE, Bogaert J. Determination of regional ejection fraction in patients with myocardial infarction by using merged late gadolinium enhancement and cine MR: feasibility study. Radiology. 2009;250:50–60. doi: 10.1148/radiol.2493080340. [DOI] [PubMed] [Google Scholar]
- 18.Papapietro SE, Yester MV, Logic JR, et al. Method for quantitative analysis of regional left ventricular function with first pass and gated blood pool scintigraphy. Am J Cardiol. 1981;47:618–25. doi: 10.1016/0002-9149(81)90546-4. [DOI] [PubMed] [Google Scholar]
- 19.Wong CK, Freedman SB, Bautovich G, Hutton BF. Correlation between post-ejection shortening and improvement in regional wall motion after revascularization in patients with coronary artery disease. Int J Cardiol. 1996;54:61–7. doi: 10.1016/0167-5273(96)02564-8. [DOI] [PubMed] [Google Scholar]
- 20.Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–47. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 21.Notomi Y, Martin-Miklovic MG, Oryszak SJ, et al. Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging. Circulation. 2006;113:2524–33. doi: 10.1161/CIRCULATIONAHA.105.596502. [DOI] [PubMed] [Google Scholar]
- 22.Sheehan FH, Bolson EL, Dodge HT, Mathey DG, Schofer J, Woo HW. Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation. 1986;74:293–305. doi: 10.1161/01.cir.74.2.293. [DOI] [PubMed] [Google Scholar]
- 23.D’Hooge J, Heimdal A, Jamal F, et al. Regional strain and strain rate measurements by cardiac ultrasound: principles, implementation and limitations. Eur J Echocardiogr. 2000;1:154–70. doi: 10.1053/euje.2000.0031. [DOI] [PubMed] [Google Scholar]
- 24.Opdahl A, Helle-Valle T, Remme EW, et al. Apical rotation by speckle tracking echocardiography: a simplified bedside index of left ventricular twist. J Am Soc Echocardiogr. 2008;21:1121–8. doi: 10.1016/j.echo.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Bogaert J, Rademakers FE. Regional nonuniformity of normal adult human left ventricle. Am J Physiol Heart Circ Physiol. 2001;280:H610–20. doi: 10.1152/ajpheart.2001.280.2.H610. [DOI] [PubMed] [Google Scholar]
- 26.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55. doi: 10.1186/1532-429X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59–63. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 28.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47:789–93. doi: 10.1016/j.jacc.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 29.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable? Eur Heart J. 2000;21:1387–96. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 30.Castillo E, Osman NF, Rosen BD, et al. Quantitative assessment of regional myocardial function with MR-tagging in a multi-center study: interobserver and intraobserver agreement of fast strain analysis with Harmonic Phase (HARP) MRI. J Cardiovasc Magn Reson. 2005;7:783–91. doi: 10.1080/10976640500295417. [DOI] [PubMed] [Google Scholar]
- 31.Li XC, Yao GH, Zhang C, et al. Quantification of regional volume and systolic function of the left ventricle by real-time three-dimensional echocardiography. Ultrasound Med Biol. 2008;34:379–84. doi: 10.1016/j.ultrasmedbio.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Lima JA, Jeremy R, Guier W, et al. Accurate systolic wall thickening by nuclear magnetic resonance imaging with tissue tagging: correlation with sonomicrometers in normal and ischemic myocardium. J Am Coll Cardiol. 1993;21:1741–51. doi: 10.1016/0735-1097(93)90397-j. [DOI] [PubMed] [Google Scholar]
- 33.Naik MM, Diamond GA, Pai T, Soffer A, Siegel RJ. Correspondence of left ventricular ejection fraction determinations from two-dimensional echocardiography, radionuclide angiography and contrast cineangiography. J Am Coll Cardiol. 1995;25:937–42. doi: 10.1016/0735-1097(94)00506-L. [DOI] [PubMed] [Google Scholar]
- 34.Pattynama PM, Lamb HJ, van der Velde EA, van der Wall EE, de Roos A. Left ventricular measurements with cine and spin-echo MR imaging: a study of reproducibility with variance component analysis. Radiology. 1993;187:261–8. doi: 10.1148/radiology.187.1.8451425. [DOI] [PubMed] [Google Scholar]
- 35.Heimdal A, Stoylen A, Torp H, Skjaerpe T. Real-time strain rate imaging of the left ventricle by ultrasound. J Am Soc Echocardiogr. 1998;11:1013–9. doi: 10.1016/s0894-7317(98)70151-8. [DOI] [PubMed] [Google Scholar]
- 36.Cho GY, Chan J, Leano R, Strudwick M, Marwick TH. Comparison of two-dimensional speckle and tissue velocity based strain and validation with harmonic phase magnetic resonance imaging. Am J Cardiol. 2006;97:1661–6. doi: 10.1016/j.amjcard.2005.12.063. [DOI] [PubMed] [Google Scholar]
- 37.Becker M, Lenzen A, Ocklenburg C, et al. Myocardial deformation imaging based on ultrasonic pixel tracking to identify reversible myocardial dysfunction. J Am Coll Cardiol. 2008;51:1473–81. doi: 10.1016/j.jacc.2007.10.066. [DOI] [PubMed] [Google Scholar]
- 38.Delgado V, Ypenburg C, van Bommel RJ, et al. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–52. doi: 10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 39.Saito K, Okura H, Watanabe N, et al. Comprehensive evaluation of left ventricular strain using speckle tracking echocardiography in normal adults: comparison of three-dimensional and two-dimensional approaches. J Am Soc Echocardiogr. 2009;22:1025–30. doi: 10.1016/j.echo.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 40.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100:1697–702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 41.Ng AC, Sitges M, Pham PN, et al. Incremental value of 2-dimensional speckle tracking strain imaging to wall motion analysis for detection of coronary artery disease in patients undergoing dobutamine stress echocardiography. Am Heart J. 2009;158:836–44. doi: 10.1016/j.ahj.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Park YH, Kang SJ, Song JK, et al. Prognostic value of longitudinal strain after primary reperfusion therapy in patients with anterior-wall acute myocardial infarction. J Am Soc Echocardiogr. 2008;21:262–7. doi: 10.1016/j.echo.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Sjoli B, Orn S, Grenne B, et al. Comparison of left ventricular ejection fraction and left ventricular global strain as determinants of infarct size in patients with acute myocardial infarction. J Am Soc Echocardiogr. 2009;22:1232–8. doi: 10.1016/j.echo.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Nasseri BA, Kukucka M, Dandel M, et al. Two-dimensional speckle tracking strain analysis for efficacy assessment of myocardial cell therapy. Cell Transplant. 2009;18:361–70. doi: 10.3727/096368909788534924. [DOI] [PubMed] [Google Scholar]