Abstract
Cells have evolved sophisticated molecular machinery, such as kinesin motor proteins and microtubule filaments, to support active intracellular transport of cargo. While kinesins tail domain binds to a variety of cargoes, kinesins head domains utilize the chemical energy stored in ATP molecules to step along the microtubule lattice. The long, stiff microtubules serve as tracks for long-distance intracellular transport.
These motors and filaments can also be employed in microfabricated synthetic environments as components of molecular shuttles 1. In a frequently used design, kinesin motors are anchored to the track surface through their tails, and functionalized microtubules serve as cargo carrying elements, which are propelled by these motors. These shuttles can be loaded with cargo by utilizing the strong and selective binding between biotin and streptavidin. The key components (biotinylated tubulin, streptavidin, and biotinylated cargo) are commercially available.
Building on the classic inverted motility assay 2, the construction of molecular shuttles is detailed here. Kinesin motor proteins are adsorbed to a surface precoated with casein; microtubules are polymerized from biotinylated tubulin, adhered to the kinesin and subsequently coated with rhodamine-labeled streptavidin. The ATP concentration is maintained at subsaturating concentration to achieve a microtubule gliding velocity optimal for loading cargo 3. Finally, biotinylated fluorescein-labeled nanospheres are added as cargo. Nanospheres attach to microtubules as a result of collisions between gliding microtubules and nanospheres adhering to the surface.
The protocol can be readily modified to load a variety of cargoes such as biotinylated DNA4, quantum dots 5 or a wide variety of antigens via biotinylated antibodies 4-6.
Protocol
1.) Buffers and Reagents
These solutions should be prepared in advance and stored in conveniently sized aliquots. An aliquot should contain sufficient solution for a typical experiment and a fresh aliquot should be used for each motility assay. The storage conditions and typical aliquot sizes are also mentioned in the following protocols.
1. BRB80 buffer, (80 mM PIPES, 1 mM MgCl2, 1 mM EGTA in deionized distilled (dd) water, pH adjusted to 6.9 by KOH)
Make up a 100 mL stock solution of 0.5 M EGTA in dd water. Adjust pH to 7.0 using 2 M NaOH solution.
Make up a 100 mL stock solution of 1 M MgCl2 in dd water. Autoclave the solution.
Add 24.2 g of PIPES and 3.1 g KOH pellets in approximately 800 mL of dd water and stir to dissolve. Adjust pH to 6.9 using 1 M KOH solution. Add 2 mL of 0.5 M EGTA stock solution and 1 mL of 1 M MgCl2 stock solution. Bring up the volume to 1000 mL with dd water.
Aliquot into 50 mL falcon tubes and freeze at -20°C for future use. The BRB80 tube currently being used can be stored at 4°C or at room temperature.
2. Magnesium Chloride, MgCl2 (100 mM in dd water)
Dilute in dd water to achieve a final concentration of 100 mM.
Aliquot (10 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use
3. Guanosine-5'-triphosphate, disodium salt, GTP (25 mM in dd water, pH adjusted to 7 by NaOH)
Weigh out and dissolve in dd water and adjust the pH to 7 by 2 M NaOH solution.
Verify concentration by measuring UV absorbance at 260 nm. (Use an extinction coefficient of 11.7 x 103 M−1cm−1).
Aliquot (10 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
4. Dimethyl sulfoxide, DMSO
Aliquot (10 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
5. Taxol (1 mM in DMSO)
Weigh out and dissolve in DMSO under fume hood to achieve a final concentration of 1 mM.
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
6. D-(+)-Glucose, (2 M in dd water)
Weigh out and dissolve in dd water to achieve a final concentration of 2 M.
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
7. Glucose Oxidase, (2 mg/mL in BRB80)
Dissolve in BRB80 to achieve a final concentration of 2 mg/mL.
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
8. Dithiothreitol, DTT (1 M in dd water)
Dissolve in dd water under fume hood to achieve a final concentration of 1 M.
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
9. Catalase, (0.8 mg/mL in BRB80)
Dissolve in BRB80 in at least 2 stages to achieve a final concentration of 0.8 mg/mL. Determine the concentration at each stage by measuring UV absorbance at 276 nm and 406 nm (Use an extinction coefficient of 3.1 x 105 M-1 cm-1 at 276 nm and 2.2 x 105 M-1 cm-1 at 406 nm and the equation A=cL).
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
10. Adenosine-5'-triphosphate, ATP (100 mM in 100mM MgCl2)
Prepare a stock solution of 100 mM MgCl2 in dd water. Weigh out dry powder and dissolve in this stock solution to achieve a final concentration of 100mM.
Verify concentration by measuring UV absorbance at 260 nm. (Use an extinction coefficient of 15.4 x 103 M−1cm−1).
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20°C for future use.
11. Casein solution (20 mg/mL casein in BRB80)
Add approximately 3 g casein to 30 mL dd water in a 50 mL falcon tube. Vortex for approximately 1 hour until solution develops thick consistency.
Centrifuge at approximately 15000 g for 30 minutes. Filter the supernatant through 0.5 μm and 0.2 μm syringe filters.
Determine the concentration of the supernatant by measuring UV absorbance at 280 nm (Use an extinction coefficient of 0.67 mL mg-1 cm-1). Dilute it to 20 mg/mL in BRB80.
Aliquot (20 μL volume) into 0.5 mL microcentrifuge tubes and store at -20 °C for future use.
2.) Standard Solutions
These are prepared on the day of the experiment and should be discarded after the experiment is over. Prepare 1 mL of each.
1. BRB80CS0.5
Dilute casein solution in BRB80 to a final concentration of 0.5 mg/mL and store over ice. This solution is introduced into the flow cell prior to kinesin and helps retain kinesin activity after surface adsorption.
2. BRB80CA
Prepare 0.2 mg/mL casein and 1 mM ATP in BRB80 and store over ice. Kinesin is further diluted using this solution before introduction into the flow cell.
3. BRB80T
Dilute taxol solution in BRB80 to a final concentration of 10 μM and store at room temperature. This solution is used to stabilize microtubules.
4. BRB80CT
Prepare 10 μM taxol and 0.2 mg/mL casein in BRB80 and store at room temperature. This is used to further prepare the antifade and microtubule solutions.
5. BRB80AF
Prepare 20 mM D-glucose, 20 μg/mL glucose oxidase, 8 μg/mL catalase, 10 mM DTT, and 20 μM ATP in BRB80CT and store at room temperature. This solution is used to dilute streptavidin and nanospheres and "wash out" the excess streptavidin in the flow cell. The kinesin speed can be controlled by adjusting the ATP concentration in this solution.
3.) Kinesin Preparation
Express a kinesin construct consisting of the wild-type, full-length Drosophila melanogaster kinesin heavy chain and a C-terminal His-tag in Escherichia coli and purify using a Ni-NTA column as described in 6.
Make aliquots (10 μL each) in 0.5 mL microcentrifuge tubes and store at -80°C for future use. The concentration of active kinesin in these aliquots is approximately 200 nM.7
For a typical experiment, dilute the kinesin solution 20 fold in BRB80CA. Label the solution KIN20 and store over ice.
4.) Microtubule Preparation
In a 0.5 mL microcentrifuge tube, prepare 25 μL of growth solution: 4 mM MgCl2, 1 mM GTP and 5% DMSO (v/v) in BRB80 buffer.
Add 6.25 μL of this solution to a 20 μg aliquot of lyophilized biotinylated tubulin.
Vortex, then place in a heat bath at 37°C for 30 minutes to polymerize. Dilute 100-fold in BRB80T and vortex gently. Label the solution MT100 and store at room temperature.
Make a 10 fold dilution of MT100 in BRB80AF. Label the solution MT1000.
5.) Streptavidin and Nanosphere Solution
Prepare AlexaFluor568-labeled streptavidin at a concentration of 100 nM in BRB80AF. Label it STV100 and store over ice. Similarly, dilute nanospheres 5000 fold in BRB80AF solution. Label it NS5000 and store over ice.
6.) Flow Cell Construction
Construct a flowcell using two glass coverslips separated by double-sided tape. This flow cell is approximately 2 cm long, 1 cm wide and 100 μm high, and has a volume of approximately 20 μL. Solutions are introduced into the flow cell from one side using a pipette and wicked out from the other using filter paper.
7.) Inverted Assay Assembly
Glass surface is first coated with casein which allows kinesin to retain its functionality upon adsorption. After kinesin is adsorbed, microtubules are introduced, which are held by kinesin. Microtubules are then coated with fluorescent streptavidin. After washing out the excess streptavidin, biotinylated polystyrene fluorescein nanospheres (40 nm diameter) are introduced. Surface adsorbed stationary nanospheres collide with the moving microtubules and get loaded onto them (Figure 1).
The order of flow of solutions and time allowed before the introduction of the next solution are listed below.
BRB80CS0.5, 5 minutes
KIN20, 5 minutes
MT1000, 5 minutes
STV100, 5 minutes
BRB80AF, 3x
NS5000
8.) Microscopy
Mount the flow cell on the microscope stage immediately after nanosphere introduction. In this experiment, an Eclipse TE2000-U fluorescence microscope (Nikon, Melville, NY) equipped with a 100X oil objective (N.A. 1.45), an X-cite 120 lamp (EXFO, Ontario, Canada) and an iXon EMCCD camera (ANDOR, South Windsor, CT) was used. A FITC filter cube (#48001) and a TRITC filter cube (#48002, Chroma Technologies, Rockingham, VT) were used to image nanospheres and microtubules respectively on the bottom surface of flow cells. The exposure time was 0.2s, while the time between exposures was 2 s.
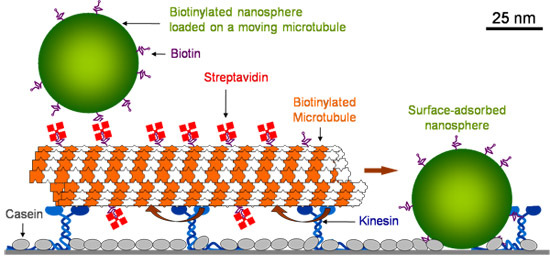 Figure 1. Schematic drawing of the molecular shuttles.
Figure 1. Schematic drawing of the molecular shuttles.
Discussion
With minor modifications, this protocol has been successfully used by a variety of groups to assemble kinesin-microtubule based motility assays. 10 mM DTT in the final motility solution can be replaced with 0.5% β-mercaptoethanol. Standard solutions (BRB80AF, KIN20 and MT1000) more than 2 hours old should not used. Any solution containing taxol and especially microtubules should never be placed on ice. Excessive exposure of the flow cell to UV excitation light results in photodamage to the functional components: microtubules and kinesin.8 This effect is even more pronounced if the flow cell contains polymers with high oxygen diffusivity such as the polystyrene nanospheres.9
Disclosures
No conflicts of interest declared.
Acknowledgments
We are heavily indebted to Jonathon Howard, whose group developed the basic protocol for a gliding motility assay which was subsequently adapted by us. Financial support from NSF grant DMR0645023 is gratefully acknowledged.
References
- Agarwal A, Hess H. Biomolecular motors at the intersection of nanotechnology and polymer science. Progress in Polymer Science. 2010;35(1-2):252–252. [Google Scholar]
- Howard J, Hunt AJ, Baek S. Assay of microtubule movement driven by single kinesin molecules. Methods Cell Biol. 1993;39:137–137. doi: 10.1016/s0091-679x(08)60167-3. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Katira P, Hess H. Millisecond curing time of a molecular adhesive causes velocity-dependent cargo-loading of molecular shuttles. Nano Lett. 2009;9(3):1170–1170. doi: 10.1021/nl803831y. [DOI] [PubMed] [Google Scholar]
- Diez S, Reuther C, Dinu C, Seidel R, Mertig M, Pompe W, Howard J. Stretching and Transporting DNA Molecules Using Motor Proteins. Nano Lett. 2003;3(9):1251–1251. [Google Scholar]
- Bachand GD, Rivera SB, Boal AK, Gaudioso J, Liu J, Bunker BC. Assembly and transport of nanocrystal CdSe quantum dot nanocomposites using microtubules and kinesin motor proteins. Nano Lett. 2004;4(5):817–817. [Google Scholar]
- Coy DL, Wagenbach M, Howard J. Kinesin takes one 8-nm step for each ATP that it hydrolyzes. J. Biol. Chem. 1999;274(6):3667–3667. doi: 10.1074/jbc.274.6.3667. [DOI] [PubMed] [Google Scholar]
- Katira P, Agarwal A, Fischer T, Chen H-Y, Jiang X, Lahann J, Hess H. Quantifying the performance of protein-resisting surfaces at ultra-low protein coverages using kinesin motor proteins as probes. Advanced Materials. 2007;19:3171–3171. [Google Scholar]
- Vigers GPA, Coue M, McIntosh JR. Fluorescent Microtubules Break Up Under Illumination. J. Cell Biol. 1988;107:1011–1011. doi: 10.1083/jcb.107.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner C, Hess H, Ernst K-H, Vogel V. Lifetime of biomolecules in hybrid nanodevices. Nanotechnology. 2004;15(10):S540–S540. [Google Scholar]


