Systemic mast cells degranulate during sepsis and mast cell-derived products, including IL-6, increase mortality during cecal ligation and puncture induced septic peritonitis in mice.
Keywords: IL-6, innate immunity, TLR4, infection
Abstract
MCs are required for an effective host response during septic peritonitis. Local MC degranulation facilitates neutrophil recruitment, activation, and bacterial killing. However, the role of MCs located distant from the site of infection is unknown. We studied the temporal and spacial degranulation of MCs following CLP-induced septic peritonitis. The functional importance of systemic MC degranulation during infection was evaluated by compartment-specific MC reconstitution. Serum histamine, reflecting MC degranulation, was elevated 4 h after onset of septic peritonitis. Histologic examination revealed progressive MC degranulation in select tissues during the first 24 h of infection. MC-deficient Wsh mice, reconstituted only in the peritoneal compartment, had improved survival after CLP compared with controls. However, reconstitution in peritoneal plus systemic compartments worsened survival after CLP. IL-6 contributed to the detrimental effects of systemic MCs on survival, as mice systemically reconstituted with IL-6−/− MCs were more likely to survive than control mice. These results indicate that in contrast to the benefits of local MC activation during infection, systemic MC activation worsens survival during CLP-induced sepsis.
Introduction
Sepsis is a complex syndrome with a high mortality [1, 2]. The temporal importance of different molecular and cellular components of the immune response during sepsis remains poorly defined. There is mounting evidence that immune mechanisms required for the initiation of host defense against local infections may also contribute to multisystem organ injury and death [3]. MCs are key early initiators of the immune response to infection [4, 5]. They line interfaces between the host and the environment, including the epidermis and luminal surfaces of the airways and gut. MCs improve survival during experimental mouse sepsis by secreting TNF, tryptase [6], and IL-6, which recruit and activate bactericidal neutrophils [4, 5]. MCs also improve survival during sepsis by degrading shock-promoting peptides, including endothelin-1 [7] and neurotensin [8]. In contrast, other studies show that some MC-derived products negatively affect survival during infection. For example, mice lacking dipeptidyl peptidase I [9] or IL-15 only in MCs [10] have improved survival during infection. Thus, MCs produce mediators that can improve or worsen survival after severe bacterial infections.
The importance of MCs at the site of bacterial infection is well established [4, 5, 11]. However, it is not known if MCs distant from the site of infection contribute to the host response during sepsis. To define the response of systemic MCs during infection, MC degranulation was evaluated by measuring serum histamine and examining histologic sections during CLP-induced sepsis. In addition, the contribution of systemic MC degranulation to circulating inflammatory cytokines was assessed using MC-deficient mice and cromolyn, a MC stabilizer. Lastly, to test the importance of local and systemic MCs during infection, MC-deficient Wsh mice were selectively reconstituted with BMMCs in the local or the local plus the systemic compartments and studied using the CLP model of sepsis.
MATERIALS AND METHODS
Materials
All chemicals were from Sigma-Aldrich (St. Louis, MO, USA) unless noted otherwise.
Experimental animals
C57BL/6 IL-6+/+ (WT) and C57BL/6 IL-6−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). C57BL/6 Wsh mice [12] were initially provided by Peter Besmer (Memorial Sloan-Kettering Institute, New York, NY, USA). Compared with control mice, C57BL/6 Wsh mice have a severe reduction in total MC number. Eight-week-old Wsh mice have a 90% reduction in tissue MCs compared with controls, and MCs are undetectable in 10- to 12-week-old Wsh mice [13, 14]. All experimental procedures were performed on 8- to 12-week-old mice, and protocols were approved by the University of California San Francisco (USA) Committee on Animal Research.
CLP
A 1-cm midline incision was made in the abdominal wall of anesthetized mice and the cecum identified. The distal 50% of exposed cecum was ligated with 3-0 silk suture and punctured once with a 22-gauge needle. The cecum was replaced into the abdomen, the incision closed with 3-0 suture, and the mouse recovered with a 0.5-ml i.p. injection of sterile 0.9% NaCl. Mice were monitored three times daily and survival recorded. Moribund mice were killed by CO2 inhalation and cervical dislocation.
MC culture from BM
Mouse BMMCs were grown and differentiated in rmIL-3 and rmSCF (PeproTech, Rocky Hill, NJ, USA), as described [15]. Cells were used after 5 weeks in culture, at which time, the cell populations consisted of >95% MCs (identified by metachromatic granules in toluidine blue-stained cells). BMMC cultured from IL-6+/+ and IL-6−/− BM showed similar granular morphology, levels of active tryptase, and expression of FcεR-Ia and CD117, indicating that they have similar maturation when cultured in the presence of IL-3 and SCF [16]. IL-6−/− BMMC reconstituted tissues with similar numbers of MCs to mice reconstituted with IL-6+/+ BMMC [4, 17].
MC reconstitution of Wsh mice
We used Wsh MC-deficient mice [18] for these studies, as they have the same genetic background as the IL-6−/− mice and have no MCs detectable by metachromatic staining [13]. To reconstitute i.p. MCs, 4 × 106 IL-6+/+ or IL-6−/− BMMCs suspended in 500 μl sterile PBS were injected into the peritoneum of 5-week-old Wsh mice. After allowing 5 weeks for MCs to differentiate within the peritoneum [11, 19], reconstituted mice were used in experiments. This method selectively reconstitutes peritoneal MCs to similar levels in the mesentery or peritoneum (3.6×105±1.2×105 vs. 3.4×105±2.3×105 MCs/peritoneum, respectively) using IL-6+/+ or IL-6−/− BMMCs.
To reconstitute the systemic compartment of Wsh mice, 107 BMMCs suspended in 200 μl PBS were injected into the tail vein of 5-week-old Wsh mice. After allowing 12 weeks for MCs to migrate to and differentiate in tissues [13, 14], the reconstituted mice were used in experiments. This method reconstitutes MCs in tissues to similar levels using IL-6+/+ or IL-6−/− BMMCs [13, 16]. In addition, as i.v. administration of BMMCs does not adequately reconstitute the skin to WT mouse levels [14], 107 BMMCs from WT or IL 6−/− mice were injected s.d. into the back of systemically reconstituted Wsh mice.
Peritoneal lavage
To recover i.p. inflammatory fluid, anesthetized mice were killed by cervical dislocation and the abdominal skin cleansed with 70% ethanol. Sterile 0.9% NaCl (4 ml) was then instilled into the peritoneum. The abdomen was massaged gently for 1 min, opened with sterile scissors, and lavage fluid reclaimed.
Quantification of bacterial CFUs
After sterile collection, 10 μl blood or peritoneal lavage fluid was plated onto nutrient agar plates and incubated for 24 h at 37°C under aerobic conditions. Bacterial colonies were then counted.
Preparation of histologic specimens
Mice were killed at baseline, 4 h, and 24 h after CLP. The trachea, stomach, and skin at the nape of the neck were removed gently, fixed with 4% PFA, and then embedded in paraffin. Specimens were then sectioned and stained with toluidine blue. Cytospins of peritoneal lavage fluid were stained with toluidine blue to quantify peritoneal MC degranulation.
Quantification of MC degranulation
Histologic sections were stained with toluidine blue and degranulated MCs identified by the presence of diffusely exocytosed granules [7, 20, 21]. In each tissue section, the total number of MCs was counted, and the number of degranulated MCs was quantified as a percentage of total MCs in a blinded manner.
Cytokine analysis
IL-6 (R&D Systems, Minneapolis, MN, USA) and histamine (Beckman Coulter, Miami, FL, USA) concentrations were measured in serum using ELISA kits, according to the manufacturers′ protocols.
In vivo studies of MC stabilization
Eight- to 12-week-old WT mice were treated with an i.p. injection of 100 mg/kg cromolyn dissolved in sterile PBS. Injections were given 16 h and 90 min before i.p. injection of 3 mg/kg LPS (Escherichia coli 0111:B4). Mice were anesthetized and blood harvested by cardiac puncture 90 min after LPS injection.
Measurement of LPS
LPS was measured in sterilely obtained serum using the ToxinSensor gel clot endotoxin assay kit. The sensitivity of this assay is 0.25 endotoxin units LPS/ml serum.
Statistical analysis
Survival curves were analyzed using the log-rank (Mantel-Cox) test or the Gehan-Breslow-Wilcoxon test if the majority of mouse deaths occurred in the first 2 days after CLP. Normally, distributed data were compared using the Student′s t test. Non-normally distributed data were compared using the Mann-Whitney test. Comparisons of three or more datasets were performed by one-way ANOVA with a Dunnett's post hoc test. All statistical tests were performed using GraphPad Prism 5.0 software (GraphPad, La Jolla, CA, USA). Data are displayed as mean ± sem. Significance was assigned to P values <0.05.
RESULTS AND DISCUSSION
Serum histamine rises during infection and is a biological marker of MC degranulation
To investigate whether MCs are systemically activated and degranulate during systemic infection, we measured serum histamine at baseline and then 4, 12, and 24 h after CLP-induced peritonitis. Serum histamine increased significantly 4 h after CLP (Fig. 1A) and remained significantly elevated 12 h after CLP and then decreased between 12 h and 24 h (Fig. 1A).
Figure 1. Systemic histamine levels increase rapidly after CLP.
(A) WT mice were subjected to CLP (ligation at 50% of cecum; single puncture with 22-gauge needle), and serum histamine levels were measured at baseline (0 h) and 4, 12, and 24 h after CLP (n=4–5/group). (B) WT and Wsh mice were subjected to CLP, and histamine was measured in serum obtained 8 h later. Elevated histamine levels in WT mice indicate MC degranulation, as serum histamine was not elevated to the same degree in Wsh mice. (C) IL-6 increases after CLP. Error bars indicate sem; *P < 0.05; #P < 0.01.
Although MCs are the main physiologic source of histamine in mice, other cells, including basophils and neutrophils, may release histamine during infection [22]. To confirm that the observed increase in serum histamine depicted in Fig. 1A reflects the activation and degranulation of MCs, histamine levels in MC-deficient Wsh mice were compared with histamine levels in WT mice (Fig. 1B). Serum histamine levels were determined 8 h after infection, as this time interval is close to the peak of histamine release in WT mice (Fig. 1A). Although serum histamine levels rose slightly in Wsh mice (Fig. 1B, black bars), the absolute level of histamine was significantly higher in WT mice when compared with Wsh mice (Fig. 1B). Collectively, these data support the hypothesis that MCs are activated and degranulate during CLP-induced infection. In addition, as the half-life of histamine in serum is only 1–2 min [23], these data suggest that there is ongoing MC degranulation throughout the first 12 h of infection. Serum IL-6 was measured at baseline and 4, 12, and 24 h after CLP in WT mice (Fig. 1C) to illustrate the magnitude of the inflammatory response in mice following CLP and the temporal relationship between serum histamine and IL-6 after infection. The peak and decline of histamine are similar to the peak and decline of IL-6.
MCs degranulate in tissues distant from the nidus of infection
To confirm that the rise in serum histamine is a marker of systemic MC degranulation, the percent of degranulated MCs was quantified histologically in several tissues after CLP (Fig. 2). Histologic specimens were obtained from skin, trachea, and stomach at baseline and then 4 h and 24 h after CLP. MC degranulation was also quantified in peritoneal lavage fluid at baseline, 4 h, and 12 h after CLP. There was a trend toward increased MC degranulation in the skin, trachea, and stomach 4 h after CLP (Fig. 2A–C). MC degranulation increased further between 4 h and 24 h after CLP, and this increase was statistically significant in skin (Fig. 2A, 35.1% vs. 62.1%; P<0.05) and trachea (Fig. 2B, 25.8% vs. 66.0%; P<0.05) but not in stomach. There was also a significant increase in peritoneal MC degranulation 4 h after CLP (Fig. 2D). These results indicate that MCs degranulate locally within the first 4 h of CLP and systemically in locations distant from the initial site of infection within 24 h of CLP. The magnitude of degranulation is greater in the peritoneal compartment compared with tissues distant to the infection site 4 h after CLP. This illustrates that there are tissue-specific differences in the timing and degree of MC degranulation during the host response to infection. The observed differences in percent degranulation between MCs in mucosal tissues (stomach) and connective tissues (skin, trachea) may reflect important functional differences between these distinct MC populations. The baseline degranulation between 25% and 40% in various tissues is consistent with prior reports [24] and may reflect basal MC degranulation in unstimulated mice.
Figure 2. MCs in tissues distant from the nidus of infection degranulate after CLP.
Percentage of degranulated MCs in selected tissues (A–D) determined by morphologic appearance (E, inset) of toluidine blue-stained MCs identified in sections of skin, trachea, stomach, and peritoneal lavage fluid in uninfected mice and then 4 h and 12 h or 24 h after CLP-induced infection (n=5–9 mice/group). (E) Photomicrograph of a representative section of skin 24 h after CLP stained with toluidine blue. Thick arrows indicate degranulated MCs, and thin arrows represent nondegranulated MCs, which show tightly packed cytoplasmic granules after toluidine blue staining (Non-degran), whereas degranulated MCs have less tightly packed granules, which can be seen spilling into the surrounding tissue (Degran). (F) Bacterial loads in the blood and peritoneum [peritoneal lavage (PL)] of mice, 4 h and 24 h after CLP. Error bars indicate sem; *P < 0.05.
Timing of systemic MC degranulation in relation to bacteremia
The molecular signals that lead to systemic MC degranulation during infection are unknown. MCs express PRRs, including TLR2 and -4, and signaling through these receptors can lead to MC degranulation and cytokine release [25]. To examine whether circulating bacteria are the cause of systemic MC degranulation during infection, blood and peritoneal bacterial loads were measured 4 h and 24 h after CLP. As shown in Fig. 2E, small numbers of bacteria (7.2±7.1 CFU/10 μl blood) were cultured from blood in a minority of mice (two of 20), 4 h after infection, despite the presence of bacteria in the peritoneum of these mice (16.1±10.1 CFU/10 μl lavage fluid). Bacterial loads were higher 24 h after infection in the blood (105.1±72.5 CFU/10 μl blood) and the peritoneum (287.9±98.7 CFU/10 μl lavage fluid). However, a minority of mice (three of 20) had positive blood cultures 24 h after infection. To evaluate whether circulating LPS could lead to MC degranulation, LPS was measured in serum 4 h and 24 h after CLP. LPS was not detected in serum 4 h after CLP and was only detected in one out of 14 samples, 24 h after CLP. These results suggest that circulating bacteria or LPS are unlikely the primary stimulus for systemic MC degranulation after CLP.
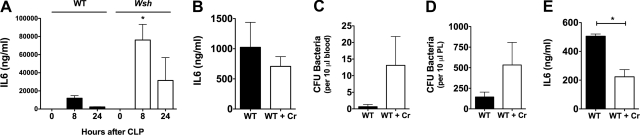
MCs contribute to the circulating pool of IL-6 during infection and inflammation
MCs are an important local source of IL-6 during CLP-induced peritonitis [4]. Several approaches were used to examine whether MCs similarly contribute to circulating IL-6 levels during infection and inflammation. First, serum IL-6 levels were measured in WT and Wsh mice, 8 h and 24 h after infection (Fig. 3A). Wsh mice had higher circulating IL-6 levels 8 h and 24 h after infection. This is likely a result of the poor bacterial clearance and higher bacterial loads of Wsh mice after CLP [26]. To further assess the contribution of systemically activated MCs to serum IL-6, cromolyn, a stabilizer of MCs and to a lesser degree, eosinophils and basophils [27], was used to stabilize systemic MCs during CLP. Twenty-four hours after infection, IL-6 levels were lower in mice treated with cromolyn (Fig. 3B). These levels were again confounded by the higher bacterial loads in the serum (Fig. 3C) and peritoneum (Fig. 3D) of mice treated with cromolyn. In a final attempt to assess the contribution of MCs to circulating IL-6 levels during inflammation, LPS was administered as an acute, noninfectious model of systemic inflammation to allow for equal comparisons between experimental groups without confounding differences in bacterial loads, which can alter systemic IL-6 levels [28]. IL-6 was measured 90 min after LPS, as previous experiments showed that this time interval captures the peak of serum IL-6 levels after LPS injection. Importantly, mice treated with cromolyn (100 mg/kg) before LPS injection (3 mg/kg) had significantly reduced circulating IL-6 levels (Fig. 3\E), suggesting that MCs contribute substantially to total serum IL-6 levels during systemic inflammation.
Figure 3. MC stabilization decreases systemic IL-6 levels after infection or inflammation.
(A) IL-6 was measured in the serum of WT and Wsh mice at baseline, 8 h, and 24 h after infection. Wsh mice had higher serum IL-6 8 h after infection. (B) Cromolyn (Cr) leads to a nonstatistically significant decrease in IL-6, which is likely confounded by higher serum (C) and peritoneal (PL; D) bacterial loads. (E) Serum IL-6 levels were determined 90 min after LPS administration (3 mg/kg) in WT and cromolyn-treated WT mice. Mice treated with cromolyn had lower serum IL-6 levels, indicating that MCs contribute to the total amount of circulating IL-6 during systemic inflammation. Error bars indicate sem; *P < 0.05.
Systemic MC degranulation increases mortality in sepsis, and MC-derived IL-6 may mediate increased mortality
Based on the findings that MCs degranulate in several different tissues during the course of infection and that MC stabilization reduces circulating IL-6 during systemic inflammation, we hypothesized that systemic MC degranulation, through the release of IL-6, is detrimental during CLP-induced peritonitis. To test this hypothesis, survival during CLP was measured in six experimental groups of reconstituted Wsh mice (Fig. 4A–C). The goal of these experimental groups was to illustrate the effects of systemic MCs on survival during CLP (Fig. 4A) and to delineate the role of MC-derived IL-6 from local or systemically activated MCs during CLP (Fig. 4B).
Figure 4. The systemic activation of tissue and subdermal MCs worsens survival during sepsis, and IL-6 derived from systemically activated MCs contributes to sepsis mortality.
Survival curves of Wsh mice during CLP after compartment-specific MC reconstitution. (A) Survival curves of Wsh mice without reconstitution (●), with WT local (i.p.) BMMC (○), and with WT local (i.p.) BMMC plus WT systemic (i.v./s.d.) BMMC (□). The absence of WT systemic BMMC (i.v./s.d.) improves survival. (B) Survival curves of Wsh selectively reconstituted with BMMC lacking IL-6−/−. Wsh mice reconstituted with local (i.p.) IL-6−/− BMMC and WT systemic (i.v./s.d.) BMMCs (▵), local (i.p.) IL-6−/− BMMC and systemic IL-6 −/− BMMC (i.v./ s.d.; ▴), and WT local (i.p.) BMMCs and systemic (i.v./s.d.) IL-6 −/− BMMCs (■). i.p. reconstitution with WT BMMC provided the largest survival benefit, whereas Wsh mice without constitution or Wsh with i.p./i.v./s.c. reconstitution had nearly identical survival after CLP. The survival detriment of full reconstitution is partially abrogated when systemic MCs lack IL-6 (n=15–38 mice/group). CLP was performed with 50% ligation and a single puncture with a 22-gauge needle. (C) Table of overall survival of all six groups of reconstituted mice; *P < 0.05.
The survival detriment of systemic MC degranulation is illustrated in Fig. 4A. MC-deficient Wsh mice had the highest mortality, with only 29% surviving 7 days after CLP. Wsh mice selectively reconstituted in the peritoneal compartment had the best survival (66% at 7 days after CLP), illustrating the protective role of i.p. MCs, described previously, during CLP [5, 11]. Most importantly, the survival of Wsh mice that were reconstituted in the peritoneal compartment and the systemic compartment (i.v. and s.d.) had a significantly lower survival when compared with Wsh mice, reconstituted with only peritoneal MCs. Notably, survival of fully reconstituted Wsh mice was nearly identical to that of unreconstituted Wsh mice. These results illustrate that the presence of systemic MCs, which undergo systemic activation and degranulation during infection, worsens survival during CLP.
To assess the importance of MC-derived IL-6 from the local or systemic compartments during CLP, three experimental groups were created: Wsh mice reconstituted with IL-6−/− BMMC in the local (i.p.) and systemic compartment (i.v./s.d.); Wsh mice reconstituted with WT BMMC in the local (i.p.) compartment and IL-6−/− MCs (i.v./s.d.) in the systemic compartment; and lastly, Wsh mice reconstituted with IL-6−/− MCs in the local compartment (i.p.) and WT MCs in the systemic compartment (i.v./s.d.). Wsh mice reconstituted with IL-6−/− BMMC in the local compartment (i.p.) and WT BMMC in the systemic compartment (i.v./s.d.) had the lowest survival (26.3%). Importantly, Wsh mice reconstituted with WT BMMC locally (i.p.) and with IL-6−/− MCs in the systemic compartment (i.v./s.d.) showed improved survival compared with mice reconstituted locally (i.p.) and systemically (i.v./s.d.) with WT MCs. Collectively, these data support the finding observed previously that local MCs are important for host defense during bacterial infection and that the activation of local MCs improves survival. However, these data also illustrate the novel finding that the activation of MCs distant from the original nidus of infection worsens survival during CLP-induced infection. The mechanism of increased mortality is, at least in part, dependent on MC IL-6, as the selective absence of IL-6 from MCs in the systemic compartment was associated with better survival after CLP-induced sepsis.
To dissect the mechanism through which IL-6 released by systemically activated MCs worsens survival during CLP-induced sepsis, peritoneal bacterial loads were measured in four sets of reconstituted mice, 24 h after CLP (Fig. 5A). Congruent with the survival curves in Fig. 4, Wsh mice reconstituted with WT MCs in the peritoneum have lower bacterial loads than Wsh mice without reconstitution. In addition, peritoneal bacterial loads were lower in Wsh mice reconstituted with WT MCs in the peritoneum and systemic IL-6−/− MCs (i.v./s.d.) than Wsh mice reconstituted with WT MCs systemically or Wsh mice without reconstitution. These results suggest that IL-6 released from systemically activated MCs impairs the clearance of i.p. bacteria. The mechanism for this difference, however, requires further investigation. Notably, there was no difference in absolute peritoneal cell counts and differentials 24 h after CLP (data not shown). Circulating IL-6 was also measured in all four groups of reconstituted mice, 24 h after CLP (Fig. 5B). A trend toward lower IL-6 in Wsh mice reconstituted with IL-6−/− BMMC in the systemic compartment is consistent with the idea that systemically activated BMMCs contribute to circulating IL-6 levels.
Figure 5. Peritoneal bacterial loads and serum IL-6 are lower in Wsh mice reconstituted with IL-6−/− MCs in the systemic compartment.
(A) Peritoneal bacterial loads were measured in reconstituted mice, 24 h after CLP. Concordant with the survival data in Fig. 4, nonreconstituted Wsh mice have statistically higher bacterial loads than Wsh mice reconstituted with WT MCs in the peritoneal compartment and systemically with IL-6−/− MCs. (B) IL-6 was measured by ELISA, 24 h after CLP in differentially reconstituted mice. Mice with lower survival in Fig. 4 have trends toward higher serum IL-6. In addition, there is a trend toward lower serum IL-6 in Wsh mice systemically reconstituted IL-6−/− MCs in the systemic compartment. Error bars indicate sem; *P < 0.05.
Several studies have shown that local MC activation plays pleiotropic roles in regulating survival from sepsis. MCs are required for effective neutrophil recruitment and bacterial killing [4, 5, 11, 29]. In contrast, MCs release products such as dipeptidyl peptidase I or IL-15, which hasten death during sepsis [9, 10]. This is the first study to show that MCs distant from the nidus of infection degranulate and release IL-6, which worsens survival during CLP-induced infection. This is in contrast to prior reports showing that release of IL-6 by MCs at the site of infection improves bacterial clearance and survival [4]. The mechanism by which IL-6 released from systemically activated MCs increases mortality is uncertain. However, the data presented in this manuscript suggest that systemic IL-6 may impair bacterial clearance, as evidenced by higher bacterial loads in the peritoneums of mice reconstituted in the systemic compartment with WT MCs (Fig. 5A and B). Other investigators have found that circulating IL-6 can worsen survival by increasing expression of C5aR [30]. Reducing the level of circulating IL-6 through antibody blockade reduces C5aR expression in the hearts and lungs of septic mice and may be the mechanism for improved survival after moderate-dose IL-6-blocking antibodies [31]. Alternatively, circulating IL-6 could worsen survival by contributing to cardiac dysfunction during sepsis [32].
It is possible that the release of other mediators from systemically activated MCs contributes to death from sepsis as well. For example, Ramos et al. [33], using a LPS model of inflammation and an antibiotic-treated CLP model, showed that MC stabilization with cromolyn and other MC stabilizers decreased systemic TNF and high-mobility group protein 1 levels and improved survival. They also found that MC stabilization decreased apoptosis in the spleen during CLP-induced sepsis; however, the mechanisms for decreased apoptosis were not established. These results are consistent with our findings that systemic MC degranulation worsens survival during sepsis and suggest other MC-derived mediators that may regulate survival during sepsis.
The mechanisms that lead to systemic MC activation and degranulation during infection remain unclear. Circulating bacteria and LPS could only be detected in a minority of mice (Fig. 2), 24 h after CLP. This suggests that circulating bacteria or LPS are not the major cause of MC activation and degranulation during the first 24 h after CLP. Other candidate mediators include complement [34], endothelin [7], IgG-FcγR [35], or neuroimmune interactions [36]. Future studies should focus on the molecular mediators that lead to systemic MC activation and degranulation during sepsis.
In summary, this study shows for the first time that MCs are systemically activated and degranulate during infection and that this process increases mortality during sepsis. These data reinforce the paradigm that immune mechanisms essential for the control of local infections may also play a role in organ injury and death when activated systemically during severe bacterial infections. This work suggests that inhibiting systemic MC activation with MC stabilizers has the potential to be therapeutically beneficial to patients with sepsis.
ACKNOWLEDGMENTS
This work was supported by NIH grant HL075026. We thank Michael Matthay and George Caughey for their insightful comments.
Footnotes
- BM
- bone marrow
- CLP
- cecal ligation and puncture
- MC
- mast cell
- rm
- recombinant mouse
- SCF
- stem cell factor
- s.d.
- subdermally
- Wsh
- KitWsh/KitWsh
AUTHORSHIP
P.W. conceived of the original concepts for this study, E.S., S.K., R.S., and P.W. performed the research; E.S. and P.W. wrote the manuscript.
DISCLOSURE
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Martin G. S., Mannino D. M., Eaton S., Moss M. (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 [DOI] [PubMed] [Google Scholar]
- 2. Angus D. C., Wax R. S. (2001) Epidemiology of sepsis: an update. Crit. Care Med. 29, S109–S116 [DOI] [PubMed] [Google Scholar]
- 3. Fuchs T. A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D. D., Jr., Wrobleski S. K., Wakefield T. W., Hartwig J. H., Wagner D. D. (2010) Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 107, 15880–15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sutherland R. E., Olsen J. S., McKinstry A., Villalta S. A., Wolters P. J. (2008) Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J. Immunol. 181, 5598–5605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malaviya R., Ikeda T., Ross E., Abraham S. N. (1996) Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 [DOI] [PubMed] [Google Scholar]
- 6. Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J. Biol. Chem. 282, 20809–20815 [DOI] [PubMed] [Google Scholar]
- 7. Maurer M., Wedemeyer J., Metz M., Piliponsky A. M., Weller K., Chatterjea D., Clouthier D. E., Yanagisawa M. M., Tsai M., Galli S. J. (2004) Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 432, 512–516 [DOI] [PubMed] [Google Scholar]
- 8. Piliponsky A. M., Chen C. C., Nishimura T., Metz M., Rios E. J., Dobner P. R., Wada E., Wada K., Zacharias S., Mohanasundaram U. M., Faix J. D., Abrink M., Pejler G., Pearl R. G., Tsai M., Galli S. J. (2008) Neurotensin increases mortality and mast cells reduce neurotensin levels in a mouse model of sepsis. Nat. Med. 14, 392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallen-St Clair J., Pham C. T., Villalta S. A., Caughey G. H., Wolters P. J. (2004) Mast cell dipeptidyl peptidase I mediates survival from sepsis. J. Clin. Invest. 113, 628–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orinska Z., Maurer M., Mirghomizadeh F., Bulanova E., Metz M., Nashkevich N., Schiemann F., Schulmistrat J., Budagian V., Giron-Michel J., Brandt E., Paus R., Bulfone-Paus S. (2007) IL-15 constrains mast cell-dependent antibacterial defenses by suppressing chymase activities. Nat. Med. 13, 927–934 [DOI] [PubMed] [Google Scholar]
- 11. Echtenacher B., Mannel D. N., Hultner L. (1996) Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 [DOI] [PubMed] [Google Scholar]
- 12. Duttlinger R., Manova K., Berrozpe G., Chu T. Y., DeLeon V., Timokhina I., Chaganti R. S., Zelenetz A. D., Bachvarova R. F., Besmer P. (1995) The Wsh and Ph mutations affect the c-kit expression profile: c-kit misexpression in embryogenesis impairs melanogenesis in Wsh and Ph mutant mice. Proc. Natl. Acad. Sci. USA 92, 3754–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wolters P. J., Mallen-St Clair J., Lewis C. C., Villalta S. A., Baluk P., Erle D. J., Caughey G. H. (2005) Tissue-selective mast cell reconstitution and differential lung gene expression in mast cell-deficient Kit(W-sh)/Kit(W-sh) sash mice. Clin. Exp. Allergy 35, 82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimbaldeston M. A., Chen C. C., Piliponsky A. M., Tsai M., Tam S. Y., Galli S. J. (2005) Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am. J. Pathol. 167, 835–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. (1984) Interleukin 3: a differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J. Immunol. 132, 1479–1486 [PubMed] [Google Scholar]
- 16. Sun J., Sukhova G. K., Wolters P. J., Yang M., Kitamoto S., Libby P., MacFarlane L. A., Mallen-St Clair J., Shi G. P. (2007) Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat. Med. 13, 719–724 [DOI] [PubMed] [Google Scholar]
- 17. Liu J., Divoux A., Sun J., Zhang J., Clement K., Glickman J. N., Sukhova G. K., Wolters P. J., Du J., Gorgun C. Z., Doria A., Libby P., Blumberg R. S., Kahn B. B., Hotamisligil G. S., Shi G. P. (2009) Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15, 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tono T., Tsujimura T., Koshimizu U., Kasugai T., Adachi S., Isozaki K., Nishikawa S., Morimoto M., Nishimune Y., Nomura S., et al. (1992) c-kit gene was not transcribed in cultured mast cells of mast cell-deficient Wsh/Wsh mice that have a normal number of erythrocytes and a normal c-kit coding region. Blood 80, 1448–1453 [PubMed] [Google Scholar]
- 19. Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. (1985) Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J. Exp. Med. 162, 1025–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carroll N. G., Mutavdzic S., James A. L. (2002) Distribution and degranulation of airway mast cells in normal and asthmatic subjects. Eur. Respir. J. 19, 879–885 [DOI] [PubMed] [Google Scholar]
- 21. Kunder C. A., St John A. L., Li G., Leong K. W., Berwin B., Staats H. F., Abraham S. N. (2009) Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 206, 2455–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu X., Zhang D., Zhang H., Wolters P. J., Killeen N. P., Sullivan B. M., Locksley R. M., Lowell C. A., Caughey G. H. (2006) Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J. Exp. Med. 203, 2907–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ind P. W., Brown M. J., Lhoste F. J., Macquin I., Dollery C. T. (1982) Concentration effect relationships of infused histamine in normal volunteers. Agents Actions 12, 12–16 [DOI] [PubMed] [Google Scholar]
- 24. Gagari E., Tsai M., Lantz C. S., Fox L. G., Galli S. J. (1997) Differential release of mast cell interleukin-6 via c-kit. Blood 89, 2654–2663 [PubMed] [Google Scholar]
- 25. Supajatura V., Ushio H., Nakao A., Akira S., Okumura K., Ra C., Ogawa H. (2002) Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J. Clin. Invest. 109, 1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piliponsky A. M., Chen C. C., Grimbaldeston M. A., Burns-Guydish S. M., Hardy J., Kalesnikoff J., Contag C. H., Tsai M., Galli S. J. (2010) Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 176, 926–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Y., Lu J. Y., Wu X., Summer S., Whoriskey J., Saris C., Reagan J. D. (2010) G-protein-coupled receptor 35 is a target of the asthma drugs cromolyn disodium and nedocromil sodium. Pharmacology 86, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Remick D. G., Bolgos G. R., Siddiqui J., Shin J., Nemzek J. A. (2002) Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock 17, 463–467 [DOI] [PubMed] [Google Scholar]
- 29. Gordon J. R., Galli S. J. (1991) Release of both preformed and newly synthesized tumor necrosis factor α (TNF-α)/cachectin by mouse mast cells stimulated via the Fc ε RI. A mechanism for the sustained action of mast cell-derived TNF-α during IgE-dependent biological responses. J. Exp. Med. 174, 103–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Riedemann N. C., Neff T. A., Guo R. F., Bernacki K. D., Laudes I. J., Sarma J. V., Lambris J. D., Ward P. A. (2003) Protective effects of IL-6 blockade in sepsis are linked to reduced C5a receptor expression. J. Immunol. 170, 503–507 [DOI] [PubMed] [Google Scholar]
- 31. Riedemann N. C., Guo R. F., Neff T. A., Laudes I. J., Keller K. A., Sarma V. J., Markiewski M. M., Mastellos D., Strey C. W., Pierson C. L., Lambris J. D., Zetoune F. S., Ward P. A. (2002) Increased C5a receptor expression in sepsis. J. Clin. Invest. 110, 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang H., Wang H. Y., Bassel-Duby R., Maass D. L., Johnston W. E., Horton J. W., Tao W. (2007) Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am. J. Physiol. Heart Circ. Physiol. 292, H2408–H2416 [DOI] [PubMed] [Google Scholar]
- 33. Ramos L., Pena G., Cai B., Deitch E. A., Ulloa L. (2010) Mast cell stabilization improves survival by preventing apoptosis in sepsis. J. Immunol. 185, 709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gommerman J. L., Oh D. Y., Zhou X., Tedder T. F., Maurer M., Galli S. J., Carroll M. C. (2000) A role for CD21/CD35 and CD19 in responses to acute septic peritonitis: a potential mechanism for mast cell activation. J. Immunol. 165, 6915–6921 [DOI] [PubMed] [Google Scholar]
- 35. Karlberg M., Xiang Z., Nilsson G. (2008) Fc γ RI-mediated activation of human mast cells promotes survival and induction of the pro-survival gene Bfl-1. J. Clin. Immunol. 28, 250–255 [DOI] [PubMed] [Google Scholar]
- 36. Tore F., Tuncel N. (2009) Mast cells: target and source of neuropeptides. Curr. Pharm. Des. 15, 3433–3445 [DOI] [PubMed] [Google Scholar]