GMFG mediates neutrophils' random migration and bacterial chemoattractant fMLP- and chemokine IL-8-induced chemotaxis via effects on F-actin reorganization.
Keywords: migration, fMLF, CXCL8, p38
Abstract
Chemotaxis is fundamental to the directional migration of neutrophils toward endogenous and exogenous chemoattractants. Recent studies have demonstrated that ADF/cofilin superfamily members play important roles in reorganizing the actin cytoskeleton by disassembling actin filaments. GMFG, a novel ADF/cofilin superfamily protein that is expressed in inflammatory cells, has been implicated in regulating actin reorganization in microendothelial cells, but its function in neutrophils remains unclear. Here, we show that GMFG is an important regulator for cell migration and polarity in neutrophils. Knockdown of endogenous GMFG impaired fMLF- and IL-8 (CXCL8)-induced chemotaxis in dHL-60 cells. GMFG knockdown attenuated the formation of lamellipodia at the leading edge of cells exposed to fMLF or CXCL8, as well as the phosphorylation of p38 and PAK1/2 in response to fMLF or CXCL8. Live cell imaging revealed that GMFG was recruited to the leading edge of cells in response to fMLF, as well as CXCL8. Overexpression of GMFG enhanced phosphorylation of p38 but not of PAK1/2 in dHL-60 cells. In addition, we found that GMFG is associated with WAVE2. Taken together, our findings suggest that GMFG is a novel factor in regulating neutrophil chemotaxis by modulating actin cytoskeleton reorganization.
Introduction
Neutrophils play a central role in the innate immune system. In response to chemotactic stimuli, neutrophils rapidly migrate to the sites of infection and provide the first line of host defense against bacteria and other pathogens [1]. Directed migration or chemotaxis of neutrophils occurs in response to a variety of molecules, including fMLF, a bacterial peptide, and chemokines, such as IL-8 (CXCL8), which are released from sites of inflammation or injury. fMLF and CXCL8 bind to their receptors on neutrophils and trigger signaling-pathway activation that induces a series of F-actin-based cytoskeleton rearrangements. This migratory response requires a highly regulated multistep process that includes cell polarization, formation of cell protrusions, directional sensing, and adhesion to the surrounding tissue [2–5]. Failure to regulate any of these events can lead to abnormal immune or inflammatory responses, including immunodeficiency or aberrant inflammatory reactions (e.g., rheumatoid arthritis and inflammatory bowel disease) [6, 7]. Hence, understanding the molecular mechanisms underlying neutrophil migration may reveal effective pharmacologic-targeted therapies for autoinflammatory diseases.
Directional cell migration is accomplished by rearrangement of actin filaments, which protrude to form specific cellular structures called lamellipodia and filopodia at the leading edge of cells to determine the direction of movement [8]. In contrast, actin-based protrusions are inhibited at the trailing edge of cells, thereby generating forces required for movement and regulating adhesion to the ECM and other cells [9, 10]. This asymmetry of cytoskeletal protein activities represents cell polarity. During cell polarization, highly dynamic and rapid assembly and disassembly of actin filaments at the leading edge of cells are essential for continuous migration, whereas cortical actin is oriented toward the trailing edge of cells.
These changes of the actin cytoskeleton are spatially regulated. It is already well known that members of the Rho family of GTPases are crucial regulators of the actin cytoskeleton [11–13]. Moreover, two actin regulatory proteins that have been shown to be important for neutrophil migration—WASP and WAVE—also function by activating Arp2/3-dependent actin polymerization [14–17]. These proteins convert upstream molecular signals into coordinated rearrangements of the actin cytoskeleton by direct involvement in actin polymerization. In addition, other actin-regulatory proteins, including profilin, formins, coronin, and cortactin, have been implicated in controlling the actin-polymerization machinery [18–20].
Recently, ADF/cofilin superfamily members have emerged as important cytoskeletal regulators [21–23]. Among the ADF/cofilin superfamily members, GMFG may play an important role in neutrophil migration, as it is expressed in inflammatory cells and regulates actin-cytoskeletal reorganization through its interaction with the Arp2/3 complex [24–26]. With the use of the techniques of controlled stem cell differentiation, two-dimensional gel electrophoresis-based proteomics, and functional genomics, we reported previously that GMFG is induced by G-CSF and may mediate the pluripotentiality and lineage commitment of human hematopoietic stem cells [24]. GMF is a highly conserved protein throughout the eukaryotes from yeast to mammals, with two isoforms being expressed in mammals—GMFB and GMFG. However, the two GMF isoforms exhibit distinct tissue distribution, suggesting each has a specific function in various actin-related cellular processes in respective tissues. Recent functional studies of Saccharomyces cerevisiae have shown that yeast GMF promotes remodeling and/or disassembly and turnover of branched actin structures through binding of the Arp2/3 complex [25]. Regulation of actin filament branching and/or debranching is important for generating lamellipodial protrusions, which turn over rapidly and exchange actin continuously during chemotaxis. Therefore, GMFG may play a crucial role in chemotaxis by modulating actin-filament debranching. In mammals, GMFG has been found to bind to the Arp2/3 complex and be phosphorylated at the N-terminal serine that is enhanced by Rac1 and Cdc42 [26]. These results imply that GMFG-regulated reorganization of the actin cytoskeleton is likely controlled by its phosphorylation in response to extracellular stimulation.
Although evidence suggests that GMFG interacts with the Arp2/3 complex, its role in actin-cytoskeleton downstream event signaling remains largely unknown. In the present study, we examined the role of GMFG in the directed motility of neutrophils and dHL-60 cells. Our findings indicate that GMFG is an important regulator for cell migration and polarity. Neutrophils in which GMFG has been down-regulated were demonstrated to have an impaired capacity for polarization, as well as impaired directional cell migration in response to the chemoattractants fMLF and CXCL8. These functional defects appear to correlate with reduced phosphorylation of the PAK-p38 MAPK signaling pathway.
MATERIALS AND METHODS
Human neutrophil isolation and culture
Primary human neutrophils were isolated from buffy coats of healthy adult donors according to a protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, NIH (Bethesda, MD, USA), and consistent with federal regulations. Red cells were eliminated by performing a HetaSep sedimentation of the buffy coats (StemCell Technologies, Seattle, WA, USA). Neutrophils were purified from the resulting leukocyte-rich cell suspension by negative selection using the EasySep human neutrophil enrichment cocktail, containing a combination of mAb specific for the CD2, CD3, CD9, CD19, CD36, CD56, and Glycophorin A antigens (StemCell Technologies). This processing yielded >98% purity of neutrophils, as determined by examination of morphology following Giemsa staining [27]. Neutrophils were suspended in HBSS (without Ca2+/Mg2+) on ice until use.
dHL-60 cells
HL-60 cells (American Type Culture Collection, Manassas, VA, USA) were induced to differentiate into human neutrophil-like cells (dHL-60 cells) by the addition of 1.3% (vol/vol) DMSO (Sigma-Aldrich, St. Louis, MO, USA) for 6–7 days [28]. Cell differentiation was assessed using flow cytometric analysis of surface expression of differentiation-related antigens as described previously [29].
RT-PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions. For reverse transcription, 1 μg total RNA/sample was used as a template for cDNA synthesis using Superscript III (Invitrogen, Carlsbad, CA, USA), following the manufacturer's guidelines. RT-PCR reactions (MyiQ Icycler, Bio-Rad, Hercules, CA, USA) were performed using prevalidated TaqMan primer/probe sets for GMFG and the control gene β-actin, purchased as Assays-on-Demand gene expression products (Applied Biosystems, Foster City, CA, USA). Real-time PCR conditions were 5 min at 95°C and 40 cycles of 30 s at 95°C, followed by 1 min at 60°C. The cycle-threshold values were arbitrarily chosen from the linear part of the PCR-amplification curve, where an increase in fluorescence can be detected 10 or more sem above the background signal. RNA copy numbers were calculated by comparison with standard curves, generated from plasmid DNA encoding GMFG and control β-actin templates.
RNA interference and plasmid constructions
Day 4 dHL-60 cells (2×106) or freshly isolated neutrophils (3×106) were transiently transfected with a GMFG siRNA pool, negative-control siRNA, or GFP-GMFG, GFP-actin, or His-tagged GMFG plasmid constructs using the Nucleofector Kit V and Nucleofector I Program T-19 (Amaxa Biosystems, Gaithersburg, MD, USA), according to the manufacturer's protocol. To silence GMFG expression, two different sets of silencer siRNAs from Applied Biosystems that target GMFG on Exons 4 and 2 (Silencer Select siRNA s18303, s18302) were pooled for use. The negative-control siRNA (Neg-siRNA #2) was also obtained from Applied Biosystems. Cells were harvested for use in experiments 24–48 h after transfection.
The expression vector for full-length human GMFG was obtained by RT-PCR using cDNA prepared from HL-60 cells. The forward primer contained the HindIII site and the sequence of the 7 aa of the human GMFG protein, 5′-CCAAGCTTGATGTCTGACTCCCTGGTGGTG-3′. The reverse primer contained the XhoI site and the sequence for the last 6 aa residues of the GMFG protein, 5′-CCCTCGAGCCACGAAAGAAAGACAACTT-3′. To create a His-tagged GMFG expression vector, PCR amplification fragments were excised with HindIII and XhoI, subsequently subcloned into the pcDNA3.1/V5-His vector (Invitrogen), and the nucleotide sequence validated. GFP-GMFG and Myc-tagged human GMFG in pCMV6-Entry plasmid were obtained from Origene (Rockville, MD, USA). A GFP-actin plasmid construct was purchased from Clontech (Mountain View, CA, USA).
Cell migration assay
Neutrophils or dHL-60 cells were resuspended at a density of 5 × 105 cells/mL or 5 × 106 cells/mL, respectively, in chemotaxis buffer (RPMI+0.5% BSA) [30]. Cell suspension (200 μL) was placed in the upper chambers of Transwell plates separated from the lower wells by a 6.5-mm diameter, 3-μm pore Nucleopore polycarbonate membrane (VWR International, West Chester, PA, USA). For the study of chemotaxis, fMLF (1–100 nM) or CXCL8 (1–100 ng/mL) was added separately in 500 μL chemotaxis buffer to the lower wells of the Transwell plates. For the study of random cell migration (chemokinesis), no chemoattractant (vehicle alone) was added to the lower wells. For all assays, the Transwell plates were incubated for 3 h at 37°C under a 5% CO2 atmosphere. The number of cells that migrated to the lower wells was calculated by placing 10-μL aliquots of the media from the lower wells on a hemacytometer and counting four fields in duplicate.
EZ-TAXIScan chemotaxis assay
Chemotaxis experiments were conducted in an EZ-TAXIScan chamber, according to the manufacturer's protocol (Effector Cell Institute, Tokyo, Japan). The EZ-TAXIScan is a visually accessible chemotactic chamber, in which one compartment containing chemoattractant and another compartment containing cells are connected by a microchannel. A stable concentration gradient of chemoattractant can be reproducibly formed and maintained through the channel without medium flow. Phase-contrast images of chemotaxing cells were acquired at 15-s intervals for 30 min. Sequential image data were processed with ImageJ (NIH). Cell migration analysis was conducted with Matlab software. Cell tracks were subsequently used to compute CI and speed for each tracked cell at each time-point, as well as the mean CI and speed for an experiment.
Immunoblotting
Total protein from HL-60 cells, dHL-60 cells, neutrophils, or dHL-60 cells transfected with empty vector or His-tagged GMFG was extracted with the use of the M-PER protein extraction reagent (Pierce Biotechnology, Rockford, IL, USA), as recommended by the manufacturer. dHL-60 cells transiently transfected with negative-control siRNA or GMFG siRNA were stimulated with 100 nM fMLF (Sigma-Aldrich) for 0, 2, 5, or 15 min at 37°C and then lysed in M-PER lysis buffer. Equal amounts of total protein (30 μg/lane) were separated on 4–20% SDS-polyacrylamide gels and transferred to nitrocellulose membrane using standard methods. Blots were probed with primary antibodies for anti-GMFG (Abgent, San Diego, CA, USA); anti-β-actin, antiphospho-PAK1 (Ser144)/PAK2 (Ser141), antiphospho-p38 (Thr180/Tyr182), antiphospho-Erk1/2 (Thr202/Tyr204), or total p38 (Cell Signaling Technology, Danvers, MA, USA); or anti-Myc or anti-His-tag (Origene). HRP-conjugated secondary antibody was used to detect the primary antibodies. Immunoblots were developed using the ECL system (Amersham Biosciences, Piscataway, NJ, USA).
Immunoprecipitation
Twenty-four hours after transfection with Myc-tagged GMFG plasmid, dHL-60 cells were washed once with PBS and lysed in lysis buffer containing 20 mM Tris-base at pH 8.0, 1% Nonidet-40, 10% glycerol, 137 mM NaCl, 100 μM sodium vanadate, and a protease inhibitor mix (Sigma-Aldrich). Equal amounts of protein from cell lysates were precleared with IgG Dynabeads protein A (Invitrogen) for 10 min at 4°C, followed by overnight incubation at 4°C with antimouse IgG or anti-Myc antibody (Origene) using Dynabeads protein A, according to the manufacturer's instructions. The immunoprecipitated Dynabeads complexes were washed three times with washing buffer and then eluted in 30 μL SDS sample buffer. Extracts were resolved on 4–20% gradient SDS-polyacrylamide gels and transferred to nitrocellulose. Anti-Myc (Origene; 1:100) or anti-WAVE2 (Invitrogen; 1:500) antibody was used for protein detection by immunoblotting as described above.
Immunofluorescence microscopy
Neutrophils or dHL-60 cells expressing GFP-GMFG were adhered to glass coverslips, coated with 2.5 μg/mL fibrinogen (Sigma-Aldrich), fixed in 4% paraformaldehyde, permeabilized with 0.2% Triton X-100/PBS, and stained with Alexa Fluor 546 phalloidin (Invitrogen). For stimulation experiments, dHL-60 cells transiently transfected with control siRNA or GMFG siRNA were stimulated with a uniform concentration of 100 nM fMLF at 37°C for 1 and 10 min. Cells were fixed in 4% paraformaldehyde for 10 min at room temperature, washed, permeabilized with 0.2% Triton X-100/PBS for 5 min, washed again, and then blocked with 2% BSA/PBS for 30 min. Cells were costained for 2 h with Alexa Fluor 488 phalloidin (Invitrogen) and anti-CD43 primary antibody (BD Biosciences PharMingen, Franklin Lakes, NJ, USA), followed by detection with Alex Fluor 546-conjugated secondary antibody (Invitrogen). Stained samples were mounted and images collected and analyzed using a Zeiss Axiovert 200 fluorescence microscope and a 40× objective, NA 1.3, with ORCA-ER C4742-95 camera-driven MetaMorph imaging software (Universal Imaging, Downingtown, PA, USA).
Live fluorescence microscopy and chemotaxis
Fluorescence imaging of live cells was performed using time-lapse confocal microscopy. For each experiment, 5 × 105 dHL-60 cells or neutrophils were plated in chemotaxis buffer for 15 min on glass coverslips coated with 2.5 μg/mL fibrinogen. Cells were stimulated with a uniform concentration of chemoattractant or by a point source of chemoattractant emanating from a micropipette tip (Femtotip; Eppendorf, Hamburg, Germany) filled with 100 nM fMLF. Images were recorded every 30 s for 7 min or every 20 s for 3 min at room temperature with a Zeiss LSM 510 META confocal system using 63×/1.3 NA water objective (Carl Zeiss, Jena, Germany). Data are representative of three independent experiments performed in duplicate.
Statistical analyses
Statistical significance for all experiments was determined by Student's paired t test analyses. A P value of <0.05 was considered statistically significant.
RESULTS
GMFG knockdown reduces chemotaxis in neutrophils and dHL-60 cells
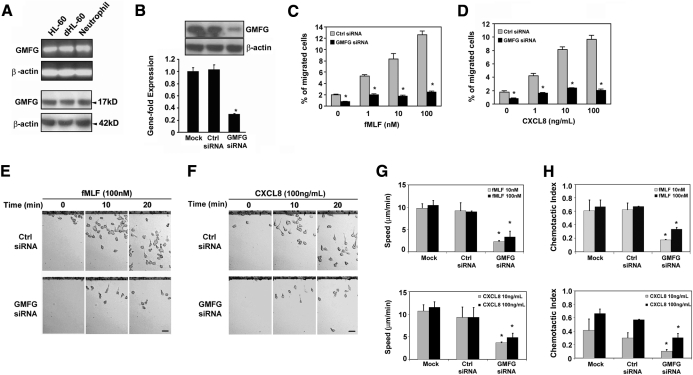
Previous studies have demonstrated that GMFG is expressed in inflammatory cells [24]. To characterize the expression of GMFG in human neutrophils and neutrophil-like dHL-60 cells, we performed RT-PCR and immunoblot analysis. We found that human neutrophils, HL-60 cells, and dHL-60 cells express endogenous GMFG (Fig. 1A). To explore the possible role of GMFG in regulating neutrophil migration, we knocked down endogenous GMFG expression in neutrophil-like dHL-60 cells using a siRNA-based loss-of-function approach. Transfection of dHL-60 cells with GMFG siRNA significantly decreased GMFG mRNA expression by almost 80% compared with mock-transfected cells or cells transfected with an equal concentration of negative-control siRNA (Fig. 1B; lower panel). Immunoblot analysis of transfected dHL-60 cells demonstrated that a mixed GMFG siRNA pool knocked down endogenous protein expression compared with cells transfected with negative-control siRNA. The silencing effect of the GMFG siRNA did not affect the expression level of other proteins, such as β-actin (Fig. 1B; upper panel). Thus, this mixed GMFG siRNA pool was used to assess the effect of GMFG in subsequent experiments.
Figure 1. GMFG mediates cell migration in neutrophils and dHL-60 cells.
(A) RT-PCR (upper panels) and immunoblotting analysis (lower panels) of endogenous GMFG expression in undifferentiated HL-60 and dHL-60 cells and human neutrophils. (B) RT-PCR and immunoblotting analysis of GMFG expression in dHL-60 cells 48 h after mock transfection or transfection with a mixed GMFG siRNA pool or negative-control siRNA. (C and D) Transwell migration assays in response to vehicle alone (chemokinesis) or increasing concentration of fMLF (used as fMLP throughout figures; 1–100 nM) or CXCL8 (used as IL-8 throughout figures; 1–100 ng/mL) in dHL-60 cells expressing control siRNA or GMFG siRNA. The percentage of cells that migrated into the lower well was recorded. (E and F) EZ-TAXIScan assay chemotaxis toward fMLF or CXCL8 in dHL-60 cells expressing control siRNA or GMFG siRNA. Cells were loaded onto the upper chamber, and the lower chamber was filled with 100 nM fMLF or 100 ng/mL CXCL8. Data were collected at 30-s intervals for 30 min. Representative images of migrating cells in a gradient of fMLF or CXCL8 at the indicated time-point are shown. Original scale bars, 20 μm (also see Supplemental Videos 1–8). (G and H) EZ-TAXIScan assay chemotaxis of dHL-60 cells 48 h after mock transfection or transfection with control siRNA or GMFG siRNA in response to increasing concentration of fMLF (1–100 nM) or CXCL8 (1–100 ng/mL). Mean migration speed was quantified from the captured images during the course of the EZ-TAXIScan chemotaxis assay, as described in Materials and Methods (G). The CI, defined as the cosine of the angle between the motion vector of the cells at a given time and the vector pointing to direction of the gradient, was determined from the digital time-lapse movies (H). Data are representative of at least three independent experiments and are expressed as mean ± sd. *P < 0.05 versus control.
To determine the involvement of GMFG in neutrophil migration, we first evaluated the effect of GMFG knockdown on cell migration in response to two well-characterized chemoattractants—fMLF and CXCL8—using Transwell migration assays of dHL-60 cells transfected with GMFG siRNA. GMFG siRNA- or negative-control, siRNA-transfected cells were incubated on top of a permeable Transwell filter and allowed to migrate through 3-μm pores into the lower well that contained varying concentrations of fMLF or CXCL8. The number of cells in the lower well was then used to calculate the percentage of cells migrated. Expression of the GMFG siRNA in dHL-60 cells significantly inhibited cell migration in response to fMLF and CXCL8 over a 100-fold range of concentration when compared with dHL-60 cells expressing negative-control siRNA (Fig. 1C and D). To better study the effects of GMFG knockdown on chemotaxis, we examined the dynamics of directional migration in cells expressing control siRNA or GMFG siRNA using an EZ-TAXIScan chemotaxis chamber. Cell movements toward a series of graded concentrations of fMLF or CXCL8 were time-lapse-recorded such that the path and migration speed of individual cells could be determined. In these assays, the GMFG siRNA-transfected cells exhibited dramatically reduced chemotactic ability toward sources of fMLF or CXCL8 compared with negative-control, siRNA-transfected cells (Fig. 1E and F and Supplemental Videos 1–8). Detailed analysis revealed that GMFG knockdown not only decreased migration speed but also affected the directionality during chemotaxis (Fig. 1G and H). These results suggest that loss of endogenous GMFG inhibits directional migration and point to a role for GMFG in regulating neutrophil chemotaxis.
GMFG regulates polarity during cell chemotaxis in neutrophils or dHL-60 cells
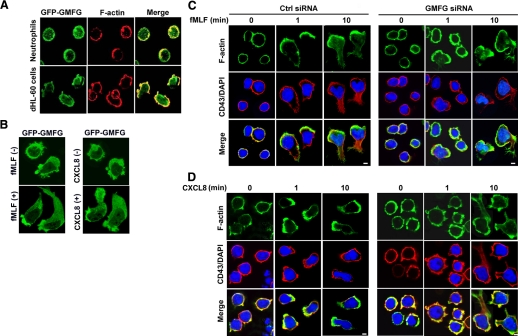
As our data suggest that GMFG regulates neutrophil chemotaxis, and GMFG has been previously reported to regulate actin-cytoskeleton reorganization, we next examined the intracellular localization of GMFG in neutrophils and dHL-60 cells. We transiently transfected cells with GFP-GMFG and then examined GMFG localization using an immunofluorescence assay. We found that GFP-GMFG was distributed uniformly in the cytoplasm around the cell membrane with a small fraction in the nucleus in resting dHL-60 cells, whereas it partially colocalized with F-actin around the cell periphery in resting neutrophils (Fig. 2A). Upon fMLF or CXCL8 stimulation, GFP-GMFG redistributed and accumulated at the leading edge and the uropod of the cells to form a bipolar distribution (Fig. 2B). These results suggest that GMFG may participate in regulating actin turnover in chemotactic dynamic processes. We then tested whether knockdown of GMFG in dHL-60 cells affected F-actin cytoskeleton rearrangement. Confocal microscopic analysis was used to examine the staining patterns of F-actin and CD43 in response to fMLF or CXCL8 in dHL-60 cells. In resting dHL-60 cells, no difference was observed in overall morphology, as well as the distribution of F-actin around the entire cell membrane, between negative control siRNA-expressing cells and GMFG siRNA-expressing cells. After 1 min of chemoattractant (fMLF or CXCL8) exposure, the majority of negative-control siRNA-expressing cells exhibited polarized morphology, with the accumulation of F-actin at the leading edge; this polarity was maintained for more than 10 min (Fig. 2C and D, left panels). In contrast, most of the GMFG knockdown cells were unable to form a well-demarcated leading edge of F-actin in response to fMLF (Fig. 2C, right panel) or CXCL8 (Fig. 2D, right panel); instead, these cells demonstrated irregular, strong F-actin staining throughout their periphery upon stimulation over a similar time period. In addition, the accumulation of the uropod marker CD43 at the trailing edge of cells was markedly impaired in GMFG knockdown cells stimulated by fMLF compared with control siRNA-transfected cells (Fig. 2C, right panel), indicating a failure of tail retraction. Interestingly, we have not observed obvious accumulation of CD43 at the trailing edge in the control siRNA-transfected dHL-60 in response to CXCL8. It seems that CXCL8-induced dHL-60 cell polarity is weaker than fMLF-stimulated. Together, these findings suggest that GMFG may be involved in the regulation of cell polarity in response to fMLF and CXCL8.
Figure 2. GMFG localizes to the leading edge and regulates F-actin polymerization during cell chemotaxis in neutrophils or dHL-60 cells.
(A) Localization of GFP-GMFG in neutrophils or dHL-60 cells. Cells transiently transfected with GFP-tagged GMFG were adhered to glass coverslips and stained for F-actin with phalloidin A-488. Microscopy was used to analyze GMFG and F-actin distribution. (B) Distribution of GFP-GMFG in response to chemoattranctant fMLF or CXCL8 stimulation in dHL-60 cells upon dHL-60 cells expressing GFP-GMFG was plated to glass coverslips and stimulated with 100 nM fMLF or 100 ng/mL CXCL8 for 10 min. (C and D) Distribution of F-actin and CD43 in response to fMLF (C) or CXCL8 (D) in negative-control, siRNA- or GMFG siRNA-expressing dHL-60 cells. Cells transfected with negative-control siRNA or GMFG siRNA were adhered to glass coverslips and stimulated with a uniform concentration of fMLF (100 nM) or CXCL8 (100 ng/mL) for 0, 1, or 10 min. The cells were fixed and stained with F-actin (phalloidin A-488; in green) and anti-CD43 primary antibody (in red) and confocal microscopy used to analyze distribution of staining and morphologic changes. Results shown are representative of three independent experiments. Original scale bar, 5 μm.
GMFG redistributes to the cell leading edge during neutrophil and dHL-60 cell chemotaxis
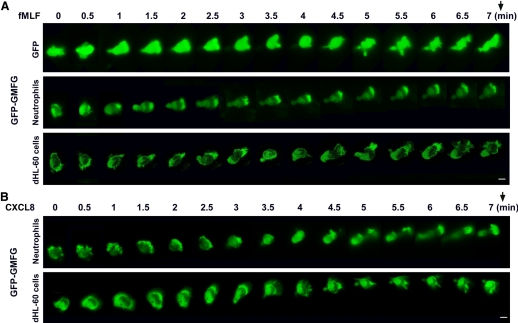
To further define the dynamics of GMFG localization in live cells during cell migration, cells transiently transfected with GFP-GMFG were examined using time-lapse video microscopy in response to fMLF or CXCL8 gradient stimulation. Following exposure to fMLF or CXCL8, dHL-60 cells expressing GFP-GMFG underwent a shape change. The cells became highly polarized, and the GFP-GMFG largely translocated to the leading edge, whereas a partial fraction still remained at the trailing edge. This redistribution was in contrast to that observed in neutrophils that expressed GFP alone, in which GFP was distributed diffusely in the cells during chemotaxis (Fig. 3). However, this redistribution is similar to that of GFP-actin in dHL-60 cells following fMLF stimulation (Fig. 4A). The features of chemoattractant-induced migration of GFP-GMFG-expressing neutrophils displayed the typical behavior of neutrophil movement: pseudopod formation and extension, attachment, contraction of the cell causing the cell body to move forward, and release of rear attachment so the cell itself can move forward (Fig. 3 and Supplemental Videos 9 and 10). Time-lapse imaging revealed the dynamic redistribution of GFP-GMFG toward the leading edge of the cells within 1 min after exposure to fMLF, which occurred before the acquisition of a polarized morphology (Fig. 3). These findings demonstrate that asymmetric distribution of GFP-GMFG occurs rapidly after fMLF exposure and suggest that GMFG is a component of initial signaling events that define polarity. To test this possibility further, we examined whether positional changes in the chemoattractant gradient would alter the localization of GFP-GMFG. We found that moving the position of the fMLF-loaded micropipette resulted in immediate redistribution of GMFG toward the new source of chemoattractant (data not shown). These observations suggest that GMFG is involved in the trafficking of critical membrane components and participates in the formation of protein complexes at the cell leading edge.
Figure 3. GFP-GMFG redistributes to the cell leading edge during neutrophil and dHL-60 cell chemotaxis.
Time-lapse sequences of the migration of neutrophils and dHL-60 cells expressing control GFP vector or GFP-GMFG toward a point source of fMLF or CXCL8. Neutrophils or dHL-60 cells were transfected with GFP vector or GFP-GMFG vector for 24 h, then plated on glass-bottom dishes coated with fibrinogen, and exposed to a chemotactic gradient generated by the slow release of fMLF (100 nM) or CXCL8 (100 ng/mL) from a micropipette. Fluorescence time-lapse images were taken at 30-s intervals for 7 min. The direction of chemoattractant source is indicated by an arrow. Original scale bars, 5 μm (also see Supplemental Videos 9 and 10). Fluorescence images were captured using a Zeiss Axiovert 200 fluorescence microscope and a 40× objective, NA 1.3, with ORCA-ER C4742-95 camera-driven MetaMorph imaging software (Universal Imaging).
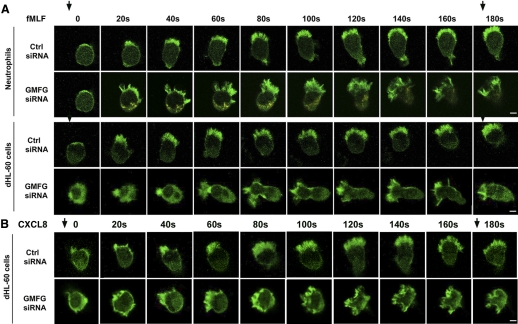
Figure 4. GMFG knockdown induces temporal instability of the leading edge.
Time-lapse sequential images showing the distribution of GFP-actin in negative-control, siRNA- or GMFG siRNA-expressing neutrophils or dHL-60 cells during chemotaxis. GFP-actin-expressing neutrophils or dHL-60 cells cotransfected with control siRNA or GMFG siRNA were plated on glass-bottom dishes coated with fibrinogen and exposed to a chemotactic gradient generated by the slow release of fMLF (100 nM; A) or CXCL8 (100 ng/mL; B) from a micropipette. Fluorescence time-lapse images were recorded in a confocal microscope at 20-s intervals for 3 min. The direction of chemoattractant source is indicated by arrows. Original scale bars, 5 μm.
GMFG knockdown induces temporal instability of the leading edge
To examine GMFG-mediated cell polarity in more detail, actin protrusion dynamics were investigated. The GFP-actin reporter was cotransfected with GMFG siRNA or control siRNA in dHL-60 cells and then these cells exposed to an fMLF or CXCL8 gradient and examined by time-lapse microscopy. Control siRNA-expressing neutrophils and dHL-60 cells rapidly polarized in the presence of fMLF or CXCL8, as identified by their elongated shape and the polarized localization of F-actin at the leading edge, and moved persistently toward the chemoattractant (fMLF or CXCL8). Conversely, GMFG siRNA-expressing neutrophils and dHL-60 cells exhibited rapidly accumulating F-actin at the front of cells oriented toward gradient chemoattractants (fMLF or CXCL8) but induced the formation of several protrusions soon after initial polarity formation; in addition, the cells failed to maintain persistent polarization of F-actin at the leading edge compared with control siRNA-expressing cells in response to fMLF and CXCL8 (Fig. 4A and B). The GMFG knockdown cells with several protrusions developed lateral protrusions that caused deviations in the direction of cell movement and reduced cell motility toward chemoattractant in comparison with control siRNA-transfected cells. Therefore, these data suggest that GMFG regulates migration through maintaining cell polarity. As persistent polarity is essential for directional migration, the impaired migration observed in GMFG knockdown cells was likely a result of a relative inability to become persistently polarized.
GMFG regulates p38 and PAK phosphorylation and interacts with WAVE2
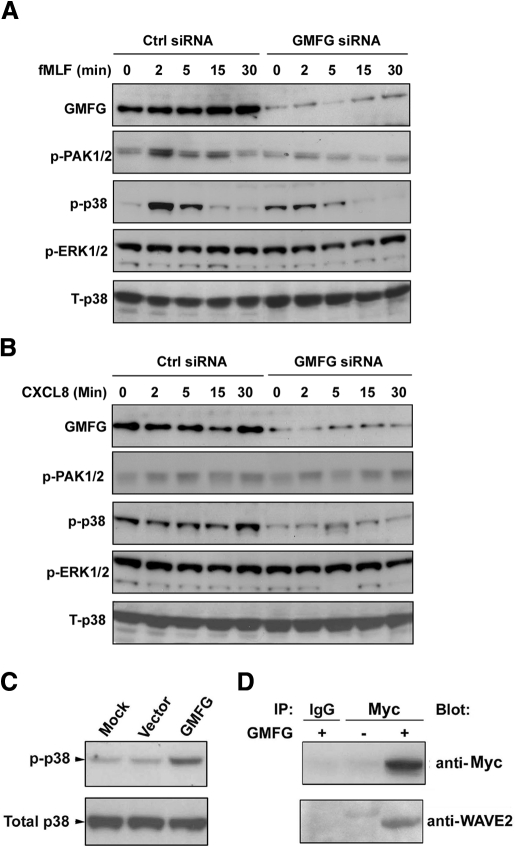
It has been demonstrated that the MAPK family mediates the regulation of cell migration [31]. ERK has been shown to regulate focal adhesion turnover and migration in fibroblasts, whereas p38 inhibits filopodia formation during chemotaxis. In addition, p38 MAPK is known to interact with PAK, and p38 and PAK mediate filopodia formation and stimulate chemotaxis [32]. To examine whether GMFG involves these signaling pathways, we evaluated the activity of these molecules in GMFG knockdown dHL-60 cells after fMLF or CXCL8 stimulation using an immunoblotting assay. We observed a moderate activation of PAK1/2 activation in response to fMLF, as compared with stimulation with CXCL8, in control siRNA-expressing cells (Fig. 5). In control siRNA-expressing cells, stimulation with fMLF resulted in a time-dependent increase in the accumulation of phosphorylated PAK1/2 and p38, which was maximized at 2 min and absent at the 15- to 30-min time-point after stimulation. Conversely, GMFG knockdown cells exhibited a marked reduction of phosphorylated PAK1/2 and p38 MAPK after fMLF stimulation compared with control siRNA-expressing cells (Fig. 5A). Phosphorylation of ERK1/2, however, was not reduced in GMFG knockdown cells compared with control siRNA-expressing cells. The accumulation of phosphorylated p38 increased in a time-dependent manner in response to CXCL8 (Fig. 5B) and was maximized at 2 min after stimulation and persisted to 30 min after stimulation. Similarly, knockdown of GMFG decreased CXCL8-induced phosphorylation of p38 levels compared with levels observed in control siRNA-expressing cells (Fig. 5B). At the same time, overexpression of GMFG induced the phosphorylation of p38 (Fig. 5C) but did not phosphorylate PAK1/2 in dHL-60 cells (data not shown). These observations suggest that GMFG mediates phosphorylation of p38 and PAK1/2, which may be involved in regulation of neutrophil migration.
Figure 5. GMFG regulates p38 and PAK phosphorylation and interacts with WAVE2 in dHL-60 cells.
(A and B) Immunoblot analysis of the phosphorylation (p) of PAK1/2, p38, and ERK1/2 at the indicated times after fMLF (100 nM) or CXCL8 (100 ng/mL) stimulation of dHL-60 cells expressing negative-control siRNA or GMFG siRNA. Equal amounts of total cellular lysates were compared using total p38 antibody (T-p38). (C) dHL-60 cells were mock-transfected or transiently transfected with His-tagged GMFG plasmid or empty vector for 24 h and then lysed. Cell lysates were subjected to immunoblot analysis using antibodies against phosphorylated p38. Equal amounts of total cellular lysates were compared using total p38 antibody. (D) Lysates prepared from dHL-60 cells transfected with Myc-tagged GMFG (+) or empty vector (–) were immunoprecipitated (IP) with anti-Myc antibody or IgG control, and then the immunoprecipitated proteins were subjected to immunoblot analysis with anti-WAVE2 antibody. The same blot was stripped and reprobed with anti-Myc antibody. All figures are representative of at least three independent analyses.
WASP/WAVE family proteins are another class of cytoskeletal regulators that link upstream Rho family GTPases Rac1 and Cdc42 to the Arp2/3 complex. Previous studies have shown that GMFG binds to the Arp2/3 complex [26]. Therefore, we hypothesize that GMFG might interact with WASP or WAVE proteins. To test this, a vector with Myc-tagged GMFG or an empty vector was transfected into dHL-60 cells and proteins immunoprecipitated with anti-Myc antibody, which were then examined for the presence of WAVE. We observed that Myc-tagged GMFG coimmunoprecipitated with WAVE2 in dHL-60 cells (Fig. 5D) but not with WAVE1 or WAVE3 (data not shown). With the use of this same assay, we were unable to detect any interaction between GMFG and WASP proteins (data not shown). These results suggest that GMFG recruits to the leading edge of cells in response to the chemoattractants may through its interaction with WAVE2. However, further analysis is certainly required to conclude its functional relevance with WAVE2.
DISCUSSION
Actin-cytoskeletal remodeling plays a pivotal role in neutrophil chemotactic responses and is tightly regulated by actin regulatory proteins. Although ADF/cofilin superfamily members have emerged as important cytoskeletal regulators, the specific (ADF)/cofilin superfamily proteins that contribute to the asymmetric dynamics of membrane-cytoskeletal interaction during directed neutrophil migration are not fully defined. Here, we show that GMFG, a novel ADF/cofilin family member expressed in human neutrophils and HL-60 cells, regulates the motility of these cells and redistributes to lamellipodia at the leading edge of the migrating cells. Silencing of GMFG significantly inhibited directional cell migration in response to fMLF or CXCL8. Chemoattractant (fMLF or CXCL8)-induced F-actin accumulation and polarized morphology were also found to be defective in migrating GMFG knockdown cells, which developed multiple filopodia-like protrusions rather than sustained lamellipodia at the leading edge of the cells. These data identify an important role for GMFG in neutrophil chemotaxis in response to fMLF or CXCL8 stimulation, which is consistent with a previous study showing that GMFG is involved in actin-based cellular functions of microvascular endothelial cells [26].
Polarization of neutrophils is the morphologic equivalent of intracellular processes, leading to an asymmetric distribution of cytoskeletal components at the front of cells. In this study, we have shown that GMFG undergoes redistribution during the polarization of neutrophils and accumulates at the leading edge, where rapid formation of new actin polymerization and coincident turnover of old branched actin filament networks nucleated by the Arp2/3 complex occur. Previous studies have shown that GMFG associates with Arp2/3, regulating actin-cytoskeleton structure, and that GMFG stimulates disassembly and turnover of branched actin filaments in yeast by inhibiting the activated Arp2/3 complex [25, 26]. Indeed, we show here that GMFG knockdown cells formed transient lamellipodia in response to fMLF or CXCL8, but most were unable to form a single, stable, persistent lamellipodia or to maintain stable polarity. Many of them formed multiple filopodia-like protrusions simultaneously or in rapid succession. This implies that silencing of GMFG leads to lamellipodial instability and diminished persistent polarization, resulting in defective directional migration. Hence, our results indicate that GMFG-mediated cytoskeleton reorganization might occur via disassembly of actin nucleation or debranching filament activity conducted by the Arp2/3 complex.
Activation of the Arp2/3 complex depends on actin-regulatory proteins, such as neuronal WASP and WAVE, which link Rac1 and Cdc42 and converge the stimulatory signals for actin polymerization [14, 33, 34]. The actin-regulatory proteins play a critical role in inducing the actin remodeling underpinning chemotaxis, as these proteins more directly link stimulatory signals to actin polymerization. Accordingly, WASP has been reported to localize to the leading and trailing edge of the cell during polarized cell migration and appears to be required for proper neutrophil polarity and chemotaxis [35]. DCs deficient in WASP show an almost complete lack of migratory capacity [36, 37]. The other WASP protein family member, WAVE/SCAR, mediates Rac-induced actin polymerization and is necessary for actin rearrangements and cell motility [38, 39]. WASP/WAVE also plays a role in preventing lateral membrane protrusions [35]. In this study, we found that GMFG is associated with WAVE2 in dHL-60 cells. Together, these results indicate that GMFG is important for the stabilization of lamellipodia at the leading edge, presumably through its recruitment to this region by interacting with WAVE2 in polarized neutrophils. This importance of GMFG function in neutrophil chemotaxis is demonstrated further by the significantly reduced ability of GMFG knockdown cells to migrate toward fMLF or CXCL8 in Transwell assays and EZ-TAXIScan assays.
Regulation of actin-cytoskeleton dynamics is important for determining the location of protrusions and contractions during cellular polarization. These processes are controlled by localized activation of signaling pathways at the leading edge and the trailing edge of cells. A previous study has indicated that GMFG-mediated cytoskeleton reorganization is presumably regulated by activation of Rac1 and Cdc42 [26]. In this study, we did not see any evidence that knockdown of GMFG expression affected fMLF-induced activation of Rac1 or Cdc42 in dHL-60 cells (as measured by pull-down assays; data not shown). Therefore, we studied the Rac and Cdc42 downstream PAK effectors in GMFG knockdown cells after stimulation with fMLF or CXCL8. Involvement of PAK1 in cytoskeletal rearrangement has been reported [40]. PAK1 also colocalized with actin in activated human neutrophils stimulated with fMLF [41]. Furthermore, PAK interacts with the p38 MAPK to positively regulate its activity [32, 42], and p38 signaling can regulate chemotaxis by restricting filopodia to the leading edge and providing positional information to direct migration [43, 44]. Indeed, our data clearly showed that the phosphorylation of PAK1/2 and p38 MAPK, typically induced by fMLF, is remarkably reduced in GMFG knockdown cells, whereas overexpression of GMFG enhanced phosphorylation of p38 MAPK in dHL-60 cells. Thus, these findings indicated that GMFG-mediated neutrophil chemotaxis in response to fMLF may occur through the PAK1/2-p38 MAPK signaling pathway.
In conclusion, this study provides strong evidence that GMFG is important for chemoattractant-stimulated directional migration in neutrophils, which regulate the polarization underpinning the neutrophil chemotaxis response via stabilizing F-actin filaments in the lamellipodia. Our previous work has identified GMFG as an important G-CSF response protein in myeloid cell development [24]. This study provides new insights into GMFG function in neutrophils, which could be useful for identifying potential targets for therapeutic intervention in neutrophil-mediated diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Christian A. Combs and Daniela A. Malide from the National Heart, Lung, and Blood Institute Light Microscopy Core Facility for their skillful help with confocal microscope imaging and Dr. Daniel Wright for helpful suggestions.
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
- ADF
- actin depolymerization factor
- Arp2/3
- actin-related protein 2/3
- CI
- chemotactic index
- dHL-60
- differentiated HL-60
- GMFG/B
- glia maturation factor-gamma/beta
- M-PER
- mammalian protein extraction reagent
- NA
- numerical aperture
- PAK
- p21-activated kinase
- siRNA
- small interfering RNA
- WASP
- Wiskott-Aldrich syndrome protein
- WAVE
- Wiskott-Aldrich syndrome protein family verprolin-homologous
AUTHORSHIP
W.A. designed and performed experiments and wrote the manuscript; L.L and C.A.P. conducted the EZ-TAXIScan assay and some time-lapse video microscopy experiments; K.C. conducted real-time PCR experiments; J.Z. conducted some of the immunoblot analyses; and G.P.R. designed and supervised the experiments and edited the manuscript.
REFERENCES
- 1. Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 [DOI] [PubMed] [Google Scholar]
- 2. Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Cell migration: integrating signals from front to back. Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 3. Van Haastert P. J., Devreotes P. N. (2004) Chemotaxis: signaling the way forward. Nat. Rev. Mol. Cell Biol. 5, 626–634 [DOI] [PubMed] [Google Scholar]
- 4. Devreotes P., Janetopoulos C. (2003) Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J. Biol. Chem. 278, 20445–20448 [DOI] [PubMed] [Google Scholar]
- 5. Webb D. J., Parsons J. T., Horwitz A. F. (2002) Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat. Cell Biol. 4, E97–E100 [DOI] [PubMed] [Google Scholar]
- 6. Hull K. M., Shoham N., Chae J. J., Aksentijevich I., Kastner D. L. (2003) The expanding spectrum of systemic autoinflammatory disorders and their rheumatic manifestations. Curr. Opin. Rheumatol. 15, 61–69 [DOI] [PubMed] [Google Scholar]
- 7. Weiss S. J. (1989) Tissue destruction by neutrophils. N. Engl. J. Med. 320, 365–376 [DOI] [PubMed] [Google Scholar]
- 8. Le Clainche C., Carlier M. F. (2008) Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 88, 489–513 [DOI] [PubMed] [Google Scholar]
- 9. Niggli V. (1999) Rho-kinase in human neutrophils: a role in signaling for myosin light chain phosphorylation and cell migration. FEBS Lett. 445, 69–72 [DOI] [PubMed] [Google Scholar]
- 10. Eddy R. J., Pierini L. M., Matsumura F., Maxfield F. R. (2000) Ca2+-dependent myosin II activation is required for uropod retraction during neutrophil migration. J. Cell Sci. 113, 1287–1298 [DOI] [PubMed] [Google Scholar]
- 11. Ridley A. J. (2001) Rho GTPases and cell migration. J. Cell Sci. 114, 2713–2722 [DOI] [PubMed] [Google Scholar]
- 12. Katoh H., Hiramoto K., Negishi M. (2006) Activation of Rac1 by RhoG regulates cell migration. J. Cell Sci. 119, 56–65 [DOI] [PubMed] [Google Scholar]
- 13. Raftopoulou M., Hall A. (2004) Cell migration: Rho GTPases lead the way. Dev. Biol. 265, 23–32 [DOI] [PubMed] [Google Scholar]
- 14. Higgs H. N., Pollard T. D. (2001) Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem. 70, 649–676 [DOI] [PubMed] [Google Scholar]
- 15. Rohatgi R., Ho H. Y., Kirschner M. W. (2000) Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J. Cell Biol. 150, 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takenawa T., Miki H. (2001) WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801–1809 [DOI] [PubMed] [Google Scholar]
- 17. Miki H., Suetsugu S., Takenawa T. (1998) WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pollard T. D., Borisy G. G. (2003) Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 [DOI] [PubMed] [Google Scholar]
- 19. dos Remedios C. G., Chhabra D., Kekic M., Dedova I. V., Tsubakihara M., Berry D. A., Nosworthy N. J. (2003) Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol. Rev. 83, 433–473 [DOI] [PubMed] [Google Scholar]
- 20. Welch M. D., Mullins R. D. (2002) Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18, 247–288 [DOI] [PubMed] [Google Scholar]
- 21. Goroncy A. K., Koshiba S., Tochio N., Tomizawa T., Sato M., Inoue M., Watanabe S., Hayashizaki Y., Tanaka A., Kigawa T., Yokoyama S. (2009) NMR solution structures of actin depolymerizing factor homology domains. Protein Sci. 18, 2384–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bernstein B. W., Bamburg J. R. (2010) ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 20, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfaendtner J., De La Cruz E. M., Voth G. A. (2010) Actin filament remodeling by actin depolymerization factor/cofilin. Proc. Natl. Acad. Sci. USA 107, 7299–7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shi Y., Chen L., Liotta L. A., Wan H. H., Rodgers G. P. (2006) Glia maturation factor γ (GMFG): a cytokine-responsive protein during hematopoietic lineage development and its functional genomics analysis. Genomics Proteomics Bioinformatics 4, 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandhi M., Smith B. A., Bovellan M., Paavilainen V., Daugherty-Clarke K., Gelles J., Lappalainen P., Goode B. L. (2010) GMF is a cofilin homolog that binds Arp2/3 complex to stimulate filament debranching and inhibit actin nucleation. Curr. Biol. 20, 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ikeda K., Kundu R. K., Ikeda S., Kobara M., Matsubara H., Quertermous T. (2006) Glia maturation factor-γ is preferentially expressed in microvascular endothelial and inflammatory cells and modulates actin cytoskeleton reorganization. Circ. Res. 99, 424–433 [DOI] [PubMed] [Google Scholar]
- 27. Wei S., Liu J. H., Epling-Burnette P. K., Gamero A. M., Ussery D., Pearson E. W., Elkabani M. E., Diaz J. I., Djeu J. Y. (1996) Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J. Immunol. 157, 5155–5162 [PubMed] [Google Scholar]
- 28. Hauert A. B., Martinelli S., Marone C., Niggli V. (2002) Differentiated HL-60 cells are a valid model system for the analysis of human neutrophil migration and chemotaxis. Int. J. Biochem. Cell Biol. 34, 838–854 [DOI] [PubMed] [Google Scholar]
- 29. Lehman J. A., Paul C. C., Baumann M. A., Gomez-Cambronero J. (2001) MAP kinase upregulation after hematopoietic differentiation: role of chemotaxis. Am. J. Physiol. Cell Physiol. 280, C183–C191 [DOI] [PubMed] [Google Scholar]
- 30. Weiner O. D., Servant G., Welch M. D., Mitchison T. J., Sedat J. W., Bourne H. R. (1999) Spatial control of actin polymerization during neutrophil chemotaxis. Nat. Cell Biol. 1, 75–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C., Jacobson K., Schaller M. D. (2004) MAP kinases and cell migration. J. Cell Sci. 117, 4619–4628 [DOI] [PubMed] [Google Scholar]
- 32. Coles L. C., Shaw P. E. (2002) PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 21, 2236–2244 [DOI] [PubMed] [Google Scholar]
- 33. Miki H., Suetsugul S., Takenawa T. (1998) WAVE, a novel WASP-family protein involved in actin reorganization induced by Rac. EMBO J. 17, 6932–6941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goley E. D., Welch M. D. (2006) The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell Biol. 7, 713–726 [DOI] [PubMed] [Google Scholar]
- 35. Myers S. A., Han J. W., Lee Y., Firtel R. A., Chung C. Y. (2005) A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis. Mol. Biol. Cell 16, 2191–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Burns S., Thrasher A. J., Blundell M. P., Machesky L., Jones G. E. (2001) Configuration of human dendritic cell cytoskeleton by Rho GTPases, the WAS protein, and differentiation. Blood 98, 1142–1149 [DOI] [PubMed] [Google Scholar]
- 37. De Noronha S., Hardy S., Sinclair J., Blundell M. P., Strid J., Schulz O., Zwirner J., Jones G. E., Katz D. R., Kinnon C., Thrasher A. J. (2005) Impaired dendritic-cell homing in vivo in the absence of Wiskott-Aldrich syndrome protein. Blood 105, 1590–1597 [DOI] [PubMed] [Google Scholar]
- 38. Yamazaki D., Suetsugu S., Miki H., Kataoka Y., Nishikawa S., Fujiwara T., Yoshida N., Takenawa T. (2003) WAVE2 is required for directed cell migration and cardiovascular development. Nature 424, 452–456 [DOI] [PubMed] [Google Scholar]
- 39. Beli P., Mascheroni D., Xu D., Innocenti M. (2008) WAVE and Arp2/3 jointly inhibit filopodium formation by entering into a complex with mDia2. Nat. Cell Biol. 10, 849–857 [DOI] [PubMed] [Google Scholar]
- 40. Bokoch G. M. (2003) Biology of the p21-activated kinases. Annu. Rev. Biochem. 72, 743–781 [DOI] [PubMed] [Google Scholar]
- 41. Dharmawardhane S., Brownson D., Lennartz M., Bokoch G. M. (1999) Localization of p21-activated kinase 1 (PAK1) to pseudopodia, membrane ruffles, and phagocytic cups in activated human neutrophils. J. Leukoc. Biol. 66, 521–527 [DOI] [PubMed] [Google Scholar]
- 42. Zhang S., Han J., Sells M. A., Chernoff J., Knaus U. G., Ulevitch R. J., Bokoch G. M. (1995) Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J. Biol. Chem. 270, 23934–23936 [DOI] [PubMed] [Google Scholar]
- 43. Affolter M., Weijer C. J. (2005) Signaling to cytoskeletal dynamics during chemotaxis. Dev. Cell 9, 19–34 [DOI] [PubMed] [Google Scholar]
- 44. Heid P. J., Geiger J., Wessels D., Voss E., Soll D. R. (2005) Computer-assisted analysis of filopod formation and the role of myosin II heavy chain phosphorylation in Dictyostelium. J. Cell Sci. 118, 2225–2237 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.