Abstract
Objective
The effects of sigma receptor antagonists on methamphetamine (METH)-induced stereotypy have not been examined. We examined the effects of sigma antagonists on METH-induced stereotypy in mice.
Results
The administration of METH (10 mg/kg) to male ddY mice induced stereotyped behavior consisting of biting (90.1%), sniffing (4.2%), head bobbing (4.1%), and circling (1.7%) during an observation period of 1 h. Pretreatment of the mice with BMY 14802 (α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol; 1, 5, and 10 mg/kg), a non-specific sigma receptor antagonist, significantly increased METH-induced sniffing (19.2, 30.5, and 43.8% of total stereotypical behavior) but decreased biting (76.6, 66.9, and 49.3% of total stereotypical behavior) in a dose-dependent manner. This response was completely abolished by (+)-SKF 10,047 ([2S-(2α,6α,11R)]-1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(2-propenyl)-2,6-methano-3-benzazocin-8-ol; 4 and 10 mg/kg), a putative sigma1 receptor agonist, and partially by PB 28 (1-cyclohexyl-4-[3-(1,2,3,4-tetrahydro-5-methoxy-1-naphthalen-1-yl)-n-propyl]piperazine; 1 and 10 mg/kg), a putative sigma2 receptor agonist. The BMY 14802 action on METH-induced stereotypy was mimicked by BD 1047 (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine; 10 mg/kg), a putative sigma1 receptor antagonist, but not by SM-21 ((±)-tropanyl 2-(4-chlorophenoxy)butanoate; 1 mg/kg), a putative sigma2 receptor antagonist. The BD 1047 effect on METH-induced stereotypy was also abolished completely by (+)-SKF 10,047 and partially by PB 28. The overall frequency of METH-induced stereotypical behavior was unchanged with these sigma receptor ligands, despite the alteration in particular behavioral patterns. The BMY 14802 action on METH-induced stereotypy was unaffected by pretreatment with centrally acting histamine H1 receptor antagonists (pyrilamine or ketotifen, 10 mg/kg), suggesting that these effects are independent of histamine H1 receptor signaling systems.
Conclusion
In summary, modulation of central sigma1 receptors alters the pattern of METH-induced stereotypy, producing a shift from stereotypical biting to stereotypical sniffing, without affecting the overall frequency of stereotypical behavior.
Keywords: methamphetamine, stereotypy, sniffing, biting, BMY14802, sigma ligand, sigma receptor
Introduction
The systemic administration of amphetamines to rodents induces increased motor activity that is replaced by repetitive and compulsive behaviors called stereotypies at higher doses (Randrup and Munkvad, 1967; Rebec and Bashore, 1984; Segal and Kuczenski, 1997; Berridge, 2006). Initial descriptions of amphetamine-induced behavior found that locomotor activity is mediated by the ventral striatum, whereas stereotypical behavior is mediated by the dorsal striatum (Kelly et al., 1975; Kelly and Iversen, 1976). Psychostimulant-induced stereotyped behavior in rodents can be characterized as several specific behaviors including vigorous grooming, sniffing, biting, licking, head-bobbing, and circling. Individual behavioral components of amphetamine-induced stereotypies can also be dissociated anatomically, particularly with regard to striatal subregions. Stereotyped biting is associated with central and anterior portions of the caudate-putamen, while stereotyped sniffing is associated with the nucleus accumbens (Costall et al., 1977). Other orofacial behaviors appear to involve the ventrolateral striatum (Dickson et al., 1994). Some of these behaviors may be more specifically related to particular pathologies, such as biting to self-injurious behavior, which would potentially implicate particular striatal subregions in these pathologies.
Overall, mesolimbic dopaminergic mechanisms have been proposed to play a critical role in the expression of stereotypy after acute psychostimulant administration (Robinson and Becker, 1986; Lieberman et al., 1990; Budygin, 2007). Since some of the specific behaviors produced in these models closely resemble that of humans abusing amphetamines, animals that display stereotypy have been considered as an animal model for amphetamine psychosis and are considered to be particularly relevant to schizophrenia (Randrup and Munkvad, 1967; Kramer et al., 1967; Rebec and Bashore, 1984; Lieberman et al., 1990; Segal and Kuczenski, 1997), but because of the compulsive and repetitive nature of the behavior, amphetamine-induced stereotypies have also been considered as potential animal models of obsessive compulsive disorder (Woods-Kettelberger et al., 1997) and autism (Aman, 1982; Moy et al., 2008).
Once initiated, methamphetamine (METH)-induced stereotypy persists for several hours in rodents, and the abnormal behavior is diminished by dopamine antagonists (e.g. Hamamura et al., 1991; Okuyama et al., 1999), but less so by other agents (Tatsuta et al., 2005, 2006, 2007; Kitanaka et al., 2007). Of the agents examined in those studies, lobeline, an alkaloid extracted from the plant Lobelia inflata, attenuated METH-induced stereotypy in a dose-dependent manner (Tatsuta et al., 2006) but did not alter the pattern of specific METH-induced behaviors in mice (Kitanaka et al., 2008). In contrast to these observations, metoprine and SKF 91488, specific inhibitors of the histamine degrading enzyme histamine N-methyltransferase (HMT), altered the levels of specific METH-induced stereotyped behaviors, reducing biting but increasing sniffing, without affecting the overall frequency of stereotypical behavior (Kitanaka et al., 2007). These findings provided us with an approach to evaluate agents by their ability to modulate two independent components of psychostimulant-induced stereotypy: the overall frequency of stereotyped behavior and the distribution of distinct behavioral subcomponents.
The sigma receptor signaling system is an interesting novel therapeutic target for treatment and prevention of amphetamine abuse. Although the endogenous ligands specific for the sigma receptors are still unknown, some synthetic sigma receptor antagonists possess inhibitory effects on psychostimulant actions, including hyperactivity, behavioral sensitization, striatal dopamine release, and neurotoxicity (Ujike et al., 1992, 1994; Terleckyj and Sonsalla, 1994; Izenwasser et al., 1998; Takahashi et al., 2000; Nguyen et al., 2005; Matsumoto et al., 2007). The molecular basis of action of sigma antagonists on psychostimulant-induced behaviors is uncertain, but sigma receptor antagonists may attenuate extracellular dopamine release in specific brain regions because some sigma receptor agonists increase extracellular dopamine levels in the nigrostriatal system (Goldstein et al., 1989; Gudelsky, 1999; Moison et al., 2003).
The effects of sigma receptor antagonists on psychostimulant-induced stereotypy have not been examined. Initially we examined the effects of a non-selective sigma antagonist on METH-induced stereotypy. However, since two sigma receptor subtypes (i.e. sigma1 and sigma2) have been found in the brain (for reviews see Walker et al, 1990; Guitart et al., 2004; Hayashi and Su, 2004), we further examined the effects of selective sigma1 and sigma2 receptor agents on METH-induced stereotypy in mice in terms of the overall frequency of stereotypy as well as the pattern of specific behaviors. In addition, we investigated whether actions of sigma receptor antagonists on METH-induced stereotypy were mediated by H1 histaminergic neurotransmission, which is known to alter the expression pattern of METH-induced stereotypy (Kitanaka et al., 2007).
Materials and Methods
Subjects
Male ddY mice (10 weeks old; Japan SLC, Shizuoka, Japan) were housed in groups of eight (cage size, 37 × 22 × 15 cm) in a temperature- (22 ± 2°C) and humidity- (50 ± 10%) controlled environment under a 12 h light/dark cycle (lights on at 07:00) with food and water available ad libitum except during testing. Observation of stereotyped behavior was made by trained observers, while measurements of locomotor activity were made using the Animex apparatus, as described below. Animal handling and care were conducted according to the Guide for the Care and Use of Laboratory Animals (7th edition, Institute of Laboratory Animal Resources-National Research Council, National Academy Press 1996) and all experiments were reviewed and approved by our Institutional Animal Research Committee. Mice were used only once (11-12 weeks old, 37-53 g) after at least one-week habituation in the facility.
Reagents
METH hydrochloride was purchased from Dainippon Pharmaceutical Co. (Osaka, Japan). BMY 14802 hydrochloride (α-(4-fluorophenyl)-4-(5-fluoro-2-pyrimidinyl)-1-piperazinebutanol hydrochloride, a non-specific sigma receptor antagonist), BD 1047 dihydrobromide (N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine dihydrobromide, a putative sigma1 receptor antagonist), SM-21 maleate ((±)-tropanyl 2-(4-chlorophenoxy)butanoate maleate, a putative sigma2 receptor antagonist), (+)-SKF 10,047 hydrochloride ([2S-(2α,6α,11R)]-1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(2-propenyl)-2,6-metha no-3-benzazocin-8-ol hydrochloride, a putative sigma1 receptor agonist), and PB 28 dihydrochloride (1-cyclohexyl-4-[3-(1,2,3,4-tetrahydro-5-methoxy-1-naphthalen-1-yl)-n-propyl]pi perazine dihydrochloride, a putative sigma2 receptor agonist) were purchased from Tocris Cookson, Inc. (Ellisville, MO, USA). Pyrilamine maleate (N-[4-methoxyphenyl]methyl-N’,N’-dimethyl-N-[2-pyridinyl]-1,2-ethanediamine, also known as mepyramine, a histamine H1 receptor antagonist) and ketotifen fumarate (4-(1-methyl-4-piperidylidene)-4H-benzo[4,5]cyclohepta[1,2-b]thiophen-10(9H)-one fumarate, a histamine H1 receptor antagonist) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of the highest purity commercially available. The doses of drugs refer to the weight of salt (base equivalent is presented below under Treatment Protocol). All reagents were dissolved in sterile saline. Drug solutions were prepared in such a way that the necessary dose could be injected in a volume of 0.1 ml/10 g of body weight by an i.p. route (METH, BMY 14802, BD 1047, SM-21, and SKF 10,047) or in a volume of 0.025 ml/10 g of body weight by i.v. tail injection (PB 28).
Treatment protocol
Effect of BMY 14802 on METH-induced stereotypy
Mice were weighed, divided randomly into eight groups (n = 8 per group), and treated with 10 mg/kg of METH or saline (vehicle) 30 min after indicated doses of BMY 14802 injection (0, 1, 5, and 10 mg/kg). After the challenge injection, all mice were placed in the test apparatus for measurement of locomotor activity and stereotypic behavior for 1 h as described below. The doses of the drugs (as base equivalent) were 8.0 mg/kg for 10 mg/kg METH, and 0.91, 4.5, and 9.1 mg/kg for 1, 5, and 10 mg/kg BMY 14802, respectively. Locomotor data were collected simultaneously in this experiment by the method as described below.
Effects of selective sigma receptor agonists on BMY 14802 actions
Mice were weighed and divided randomly into five groups (n = 8 per group, except the group treated with 10 mg/kg PB 28 and 10 mg/kg BMY 14802, which was n = 4). Subjects were treated with 10 mg/kg METH 30 min after saline, BMY 14802, or combined injection of BMY 14802 and a selective sigma receptor agonist (SKF 10,047 or PB 28, the selective sigma1 and sigma2 receptor agonists, respectively). Doses of METH and BMY 14802 were 10 mg/kg. SKF 10,047 (4 mg/kg) was administered i.p., whereas 1 or 10 mg/kg PB 28 was injected into the tail vein (i.v.) based on the previous descriptions in the literature (Kamei et al., 1994, 1996; Kassiou et al., 2005). After the challenge injection, all mice were placed in the testing apparatus for measurement of locomotor activity and rating of stereotypic behavior for 1 h as described below. The doses of the drugs (as base equivalent) were 3.5 and 0.84 mg/kg for SKF 10,047 (4 mg/kg) and PB 28 (1 mg/kg), respectively.
To confirm the dose-response for inhibition of BMY 14802 action by SKF 10,047, additional mice (n = 6 per group) were treated with METH 30 min after BMY 14802 (10 mg/kg), or combined injection of BMY 14802 and various doses of SKF 10,047 (1, 4, and 10 mg/kg). The doses of the drugs (as base equivalent) were 0.88, 3.5, and 8.8 mg/kg for 1, 4, and 10 mg/kg SKF 10,047, respectively.
Effects of selective sigma receptor antagonists on METH-induced stereotypy
To confirm the involvement of sigma receptor subtypes which affect METH-induced stereotypy, additional experiments (n = 6 per group) similar to that of BMY 14802 (described above) were performed using BD 1047 (10 mg/kg, i.p.), a sigma1 receptor antagonist and SM-21 (1 mg/kg, i.p.), a sigma2 receptor antagonist. Mice were weighed, divided randomly into five groups, and treated with 10 mg/kg of METH 30 min after saline, BD 1047, SM-21, BD1047 + SKF 10,047, or BD 1047 + PB 28. The dose of METH was 10 mg/kg. Doses of BD 1047 and SM-21 were selected based on the literature (McCracken et al., 1999; Matsumoto and Mack, 2001). The doses of the drugs (as base equivalent) were 6.3 and 0.74 mg/kg for BD 1047 and SM-21, respectively.
Effect of pretreatment with histamine H1 receptor antagonists on BMY 14082 actions
To address whether histamine H1 receptor signaling is involved in BMY 14802 effects on METH-induced stereotypy, mice (n = 6 per group) were pretreated with 10 mg/kg BMY 14802 in combination with pyrilamine (10 mg/kg, i.p.), ketotifen (10 mg/kg, i.p.), or vehicle (saline) 30 min prior to METH and then tested for 1 h. Doses of pyrilamine and ketotifen were selected based on the literature (Kitanaka et al., 2007). The doses of the drugs (as base equivalent) were 7.1 and 7.3 mg/kg for pyrilamine and ketotifen, respectively.
Measurement of locomotor activity
Locomotor activity was measured in a transparent acrylic test box (30 × 30 × 35 cm) with approximately 25 g of fresh wood chips spread on the floor of the chamber using an Animex Auto apparatus (System MK-110; Muromachi Kikai Co., Ltd., Tokyo, Japan) in a quiet room as described previously (Kitanaka et al., 2003, 2005, 2007). The apparatus detects changes in electrical capacitance (oscillation frequency) in an LC oscillator circuit system under the floor of the apparatus as an animal moves horizontally in an electric field. In this set of experiments, sensitivity parameter was set at 580 (Kitanaka et al., 2003). Under this criterion, the count of oscillation frequency parallels the degree of horizontal locomotion. The acrylic test boxes were cleaned and wiped dry between sessions for each animal. All experiments were conducted between 9:00 and 16:00.
Rating of stereotypical behavior
Animals in the transparent acrylic test box undergoing locomotor testing were simultaneously observed for stereotypy for 1 h after drug administration by observers unaware of the treatments. The behavior was broken down into 30-s bins, and the predominant behavior was recorded for each bin. Since the animal's behavior was unchanged for long periods (>30 s) after drug treatment, it was possible to record the observations by hand. Behaviors scored were inactive (awake and inactive, or sleeping), ambulating, rearing, persistently locomoting, head bobbing (HB; up-and-down movements of the head), continuously sniffing with apparent exploratory behavior (SN), circling (CR), and continuous nail and/or wood chip biting or licking (BT), according to the method described previously with a slight modification (Kitanaka et al., 2005, 2007). In this study, the behavioral pattern “vigorous grooming with excess saliva” (Tatsuta et al., 2005) was not measured because very little grooming was observed. Ambulating and rearing were considered locomotor and exploratory behaviors and the last four categories were considered stereotypies. The cumulative number of bins within every 5 min period in which stereotypies were rated was shown as a time-course (maximal value = 10).
Statistical analysis
Values are presented as the mean ± the standard error of the means (SEM). Statistical analysis was performed using mixed factor analysis of variance (ANOVA) with or without repeated measures followed by the Bonferroni/Dunn test (Statview 5.0 for Apple Macintosh, SAS Institute, Inc., Cary, NC, USA). Statistical significance was set at P<0.05.
Results
The effect of BMY 14802 on METH-induced stereotypy and locomotion
Fig. 1 shows the time-course of the frequency of all stereotypical behavior after METH (or saline vehicle) treatment in mice. There was an increase in the overall frequency of stereotypy in mice after METH challenge, as compared with saline challenge, beginning at 5 minutes post-injection, reaching a maximum at 15 minutes post-injection, and continuing unabated for the duration of the test session. The frequency of stereotypy was unchanged after pretreatment with various doses of BMY 14802 or saline overall, and there was no change in the frequency of METH-induced stereotypy over time a result of BMY 14802 treatment. A repeated-measures ANOVA (BMY 14802 Dose × METH Treatment × Time) applied to the data represented in Fig. 1 yielded significant main effects of METH Treatment (F(1,56)=39520, P<0.0001) and Time (F(12,728)=711, P<0.0001), but no significant main effect of BMY 14802 Dose (F(3,56)=0.587, P=0.6258). This analysis also yielded a significant METH Treatment × Time interaction (F(12,728)=734, P<0.0001), but no significant BMY 14802 Dose × METH Treatment, BMY 14802 Dose × Time, or BMY 14802 Dose × METH Treatment × Time interactions (F(3,56)=0.514, P=0.6740, F(36,728)=0.338, P>0.9999, and F(36,728)=0.742, P=0.8656, respectively). Post-hoc pair-wise comparisons showed significant differences of time course between the time 5 and the time 10-60 and between the time 10 and the time 15-60 (Bonferroni/Dunn test, P<0.05).
Fig. 1.
Frequencies of stereotypy observed after a single administration of METH (10 mg/kg, i.p.). Values are shown as the means ± S.E.M. (n = 8 per group). BMY, BMY 14802 (1, 5, or 10 mg/kg); METH, methamphetamine (10 mg/kg); Sal, saline.
Four categories of stereotyped behaviors were observed and the frequency of each behavior was measured for 1 h (Figs. 2A-D). The total count of all observed stereotypical behaviors (HB+CR+BT+SN) is also shown in Fig. 2E. METH challenge increased the frequency of each category of stereotyped behavior, compared with saline challenge (essentially none of these behaviors were observed after saline injection). Two-way ANOVA (BMY 14802 Dose × METH Treatment) was applied separately for each behavior shown in Figs. 2A-D. The ANOVA showed significant main effects of METH Treatment (F(1,56)=51.882, P<0.0001, F(1,56)=9.003, P<0.001, F(1,56)=1606.6, P<0.0001, and F(1,56)=200.36, P<0.0001 for HB, CR, BT, and SN, respectively). Furthermore, pretreatment with doses of BMY 14802 affected significantly the expression of HB, BT, and SN, but not CR. Thus, there was a significant effect of BMY 14802 Dose (F(3,56)=5.028, P<0.001, F(3,56)=23.323, P<0.001, and F(3,56)=27.183, P<0.0001 for HB, BT, and SN, respectively, but no significant main effect of BMY 14802 Dose for CR, F(3,56)=0.405, P=0.7498). The frequency of sniffing was increased by BMY 14802, while the frequency of biting was reduced. The frequency of head bobbing appeared to be affected in a somewhat biphasic manner; reduction of head bobbing at a moderate dose, but increases at a higher dose. Thus, the ANOVA also indicated significant BMY 14802 Dose × METH Treatment interactions (F(3,56)=4.606, P<0.01, F(3,56)=22.626, P<0.0001, and F(3,56)=25.303, P<0.001 for HB, BT, and SN, respectively, but not for CR, F(3,56)=0.405, P=0.7498). Regarding the METH Treatment, post-hoc comparisons indicated significant differences in the frequencies of the four stereotypical behavior components between the METH-treated and the saline-treated mice (Bonferroni/Dunn test, P<0.05). Post-hoc comparisons indicated significant differences in the frequencies of stereotyped behavior components between the doses indicated in Figs. 2A-D (Bonferroni/Dunn test, P<0.05). Despite these differences in the frequency of individual stereotypical behaviors, the total incidence of stereotypy was unaffected. A separate two-way ANOVA applied to the total frequency of stereotypic behavior represented in Fig. 2E indicated a significant main effect of METH Treatment (F(3,56)=39519, P<0.0001), but no significant main effect of BMY 14802 Dose (F(3,56)=0.587, P=0.6258), nor any METH Treatment × BMY 14802 Dose interaction (F(3,56)=0.514, P=0.6740). Regarding the METH Treatment, post-hoc comparisons indicated a significant difference of frequencies of total stereotyped behavior between the METH-treated and the saline-treated mice (Fig. 2E; Bonferroni/Dunn test, P<0.05). Post hoc comparisons confirmed that pretreatment with BMY 14802 significantly increased METH-induced sniffing but decreased biting in a dose-dependent manner, without affecting the total frequency of stereotypy (Figs. 2B-C), while the biphasic effect on head-bobbing was confirmed by a significant difference between 5 and 10 mg/kg of BMY 14802 (Fig. 2A).
Fig. 2.
Stereotyped behavior in response to saline (open column) or 10 mg/kg of METH (solid column) in mice pretreated with 0, 1, 5, and 10 mg/kg of BMY 14802. Behavior was scored in 30-s bins, and total numbers for 1 h are shown. (A) head-bobbing; (B) circling; (C) nail and/or wood chip biting; (D) sniffing; (E) Total number of stereotypic scores (“Total”) includes the numbers of head-bobbing, circling, nail and/or wood chip biting, and sniffing. Values are shown as the means ± S.E.M. (n = 8 per group). *P<0.05, compared with two columns indicated with a horizontal bar (Bonferroni/Dunn test). †P<0.05, compared with saline-challenged group (Bonferroni/Dunn test).
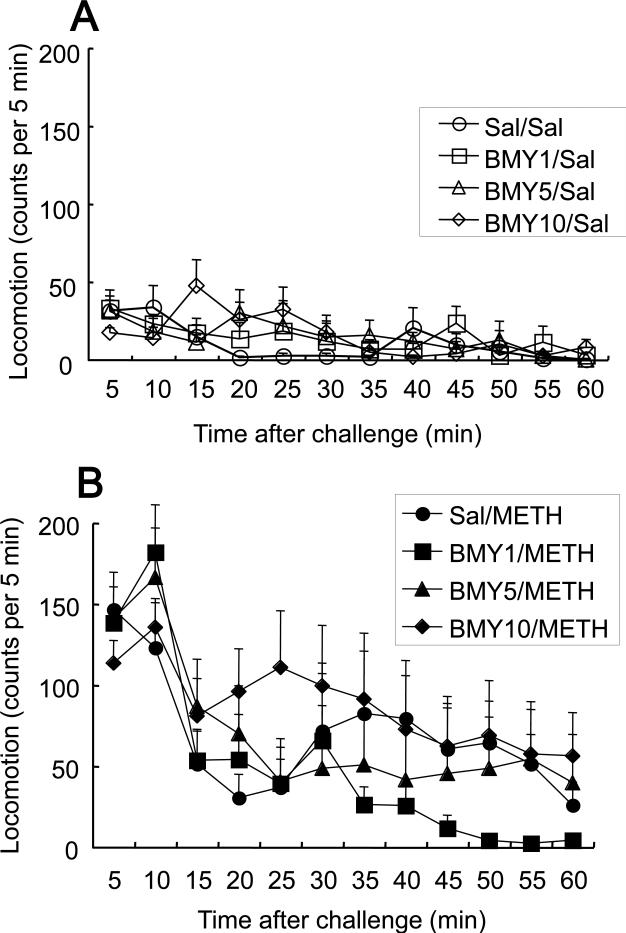
METH treatment increased locomotion compared to saline treatment, but BMY 14802 did not substantially affect locomotion in either case. Locomotor activity was analyzed by separate repeated-measures ANOVA (BMY 14802 Dose × Time) applied to the saline-treated (Fig. 3A) and METH-treated (Fig. 3B) subjects. There was a significant main effect of Time for saline-treated (F(11,336)=3.905, P<0.0001) and METH-treated (F(11,336)=12.168, P<0.0001) subjects, but no significant main effect of BMY 14802 Dose for either treatment (F(3,28)=0.320, P=0.8110 and F(3,28)=0.602, P=0.6190 for saline- and METH-treated subjects, respectively). The BMY 14802 Dose × Time interaction was not significant either (F(33,336)=1.277, P=0.1483 and F(33,336)=1.073, P=0.3651 for saline- and METH-treated subjects, respectively). Overall, BMY 14802 did not affect locomotor activity alone nor did it affect METH-induced hyperactivity.
Fig. 3.
Locomotor activity after a single administration of saline (A) or METH (10 mg/kg, i.p.) (B) in mice pretreated with 0, 1, 5, and 10 mg/kg of BMY 14802 or saline (as vehicle). Values are shown as the means ± S.E.M. (n = 8 per group). BMY, BMY 14802 (1, 5, or 10 mg/kg); METH, methamphetamine (10 mg/kg); Sal, saline.
Effects of selective sigma receptor agonists on BMY 14802 actions
In preliminary experiments, pretreatment of mice with 4 mg/kg SKF 10,047 (a putative sigma1 agonist) or with 1 mg/kg PB 28 (a putative sigma2 agonist) had no effect on METH-induced stereotypical behavior in terms of either overall frequency of total stereotypy, the frequency of individual behaviors or the time-course of stereotypy induced by METH (data not shown, n = 3 per group).
Next, we investigated whether selective sigma receptor agonists (SKF 10,047 for sigma1 and PB 28 for sigma2) could affect BMY 14802 actions on METH-induced stereotypies (Fig. 4). Modulation of sigma receptor subtypes by selective agonists affected the frequency of METH-induced biting and sniffing, but not other stereotypical behaviors, nor did they affect the total stereotypy. ANOVA yielded a significant main effect of pretreatment (i.e. saline versus BMY 14802, BMY 14802 + SKF 10,047, and BMY 14802 + PB 28) for SN and BT (F(3,28)=82.618, P<0.0001 and F(3,28)=49.150, P<0.0001, respectively), but not HB, CR, or Total stereotypy (F(3,28)=0.655, P=0.5865, F(3,28)=0.489, P=0.6928, and F(3,28)=0.329, P=0.8044, respectively). As in the previous experiment, BMY 14802 increased METH-induced sniffing and reduced METH-induced biting (Bonferroni/Dunn test, P<0.05 versus saline). Pretreatment with SKF 10,047 or PB 28 reduced the increase in METH-induced sniffing produced by BMY 14802 (Bonferroni/Dunn test, P<0.05 versus BMY 14802 pretreatment alone), although this effect was much more pronounced in SKF 10,047-treated subjects (Bonferroni/Dunn test, P<0.05 versus PB 28 pretreatment). Nonetheless, neither treatment alone completely eliminated the effects of BMY 14802: all BMY 14802-treated subjects were significantly different from subjects treated with METH alone. Pretreatment with SKF 10,047 or PB 28 also reduced the decreases in METH-induced biting produced by BMY 14802 (Bonferroni/Dunn test, P<0.05 versus saline). Again, the effects of SKF 10,047 were much greater (Bonferroni/Dunn test, P<0.05 versus PB 28 pretreatment), but neither agonist alone completely reversed the effects of BMY 14802 and all BMY 14802-treated subjects were significantly different from subjects treated with METH alone.
Fig. 4.
Effects of putative agonists for sigma receptor subtypes on METH-induced stereotypy with BMY 14802 pretreatment. Values are shown as the means ± S.E.M. (n = 8 per group). BMY, BMY 14802 (10 mg/kg); BT, nail and/or wood chip biting; CR, circling; HB, head-bobbing; METH, methamphetamine (10 mg/kg); Sal, saline; SKF, SKF 10,047 (4 mg/kg); SN, sniffing; PB, PB 28 (1 mg/kg). *P<0.05, compared with Sal/METH group (Bonferroni/Dunn test). †P<0.05, compared with BMY/METH group (Bonferroni/Dunn test). ‡P<0.05, compared with SKF-BMY/METH group (Bonferroni/Dunn test).
A higher dose of PB 28 (10 mg/kg) did not produce greater reversal of the effects of BMY 14802 on METH-induced stereotypy. A similar behavioral pattern of METH-induced stereotypy was observed compared to that produced by 1 mg/kg of PB 28 combined with BMY 14802 (HB, 1.5 ± 0.3; CR, 1.0 ± 0.4; SN, 31.5 ± 2.4; BT, 70.3 ± 3.5; Total, 104.3 ± 3.4; n = 4).
In a further study, the effects of SKF 10,047 were shown to dose-dependently inhibit the BMY 14802-induced transition from biting to sniffing behavior in METH-challenged mice (Fig. 5), producing increased biting and decreased sniffing behavior compared to mice treated only with METH and BMY 14802. Repeated-measures analysis of the dose effect of SKF 10,047 on METH-induced stereotypic behavior in mice pretreated with BMY 14802 yielded a significant main effect of SKF 10,047 dose for sniffing (F(3,20)=72.541, P<0.0001) and biting (F(3,20)=55.976, P<0.0001), respectively. There was no effect of SKF 10,047 on other types of stereotypic behavior induced by METH, including head-bobbing (F(3,20)=0.380, P=0.7686), circling (F(3,20)=0.328, P=0.8052), and total stereotypy (F(3,20)=2.322, P=0.1060). Post-hoc comparisons showed that the frequency of sniffing was greater in the 0 mg/kg SKF 10,047 group compared to all other groups (Bonferroni/Dunn test, P<0.05), and also in the 1 mg/kg SKF 10,047 group compared to higher dose groups (Bonferroni/Dunn test, P<0.05). For biting, post-hoc comparisons showed that biting in the 0 mg/kg SKF 10,047 group was less than all other groups (Bonferroni/Dunn test, P<0.05), and biting in the 1 mg/kg SKF 10,047 group was less than in higher dose groups (Bonferroni/Dunn test, P<0.05).
Fig. 5.
Dose-response for inhibition of BMY 14802 action by SKF 10,047. Values are shown as the means ± S.E.M. (n = 6 per group). Mice were treated with METH (10 mg/kg) 30 min after combined pretreatment with BMY 14802 (10 mg/kg) and SKF 10,047 (0, 1, 4, and 10 mg/kg). BT, nail and/or wood chip biting; CR, circling; HB, head-bobbing; SN, sniffing. *P<0.05, compared with 0 mg/kg SKF 10,047 (Bonferroni/Dunn test). †P<0.05, compared with 1 mg/kg SKF 10,047 (Bonferroni/Dunn test).
Effects of selective sigma receptor antagonists on METH-induced stereotypy
The effects of putative selective antagonists for sigma receptor subtypes (BD 1047 for sigma1 and SM-21 for sigma2, respectively) on METH-induced stereotypies were investigated in a further experiment (Fig. 6). Two groups were also pretreated with the selective agonists (SKF 10,047 for sigma1 and PB 28 for sigma2) in addition to the selective sigma1 antagonist (BD 1047). Pretreatment with the selective sigma1 antagonist BD 1047 had an effect similar to that observed previously for the non-selective sigma antagonist BMY 14802; BD 1047 increased the effects of METH on sniffing and decreased the effects of METH on biting (Bonferroni/Dunn test, P<0.05 versus saline pretreatment). This effect was not observed after treatment with the selective sigma2 antagonist SM-21. Comparison of the five treatment groups compared by ANOVA yielded a significant main effect of pretreatment for SN and BT (F(4,25)=55.816, P<0.0001 and F(4,25)=31.822, P<0.0001, for each measure respectively), but not for HB, CR, or Total stereotypy (F(4,25)=0.196, P=0.9384, F(4,25)=0.159, P=0.9571, and F(4,25)=0.1.328, P=0.2871, for each measure respectively). This effect was not observed after treatment with the selective sigma2 antagonist SM-21. The effect of BD 1047 on METH-induced stereotypies was completely reversed by the selective sigma1 agonist SKF 10,047 (Bonferroni/Dunn test, P<0.05 versus BD 1047 pretreatment alone) and partially reversed by the selective sigma2 agonist PB 28 (Bonferroni/Dunn test, P<0.05 versus BD 1047 pretreatment alone).
Fig. 6.
Effect of pretreatment with putative sigma receptor antagonists on METH-induced stereotypy in mice with or without putative agonists. Values are shown as the means ± S.E.M. (n = 6 per group). BD, BD 1047 (10 mg/kg); BT, nail and/or wood chip biting; METH, methamphetamine (10 mg/kg); Sal, saline; SKF, SKF 10,047 (4 mg/kg); SM, SM-21 (1 mg/kg); SN, sniffing; PB, PB 28 (1 mg/kg). *P<0.05, compared with Sal/METH group (Bonferroni/Dunn test). †P<0.05, compared with BD/METH group (Bonferroni/Dunn test). ‡P<0.05, compared with SM/METH group (Bonferroni/Dunn test). #P<0.05, compared with BD-SKF/METH group (Bonferroni/Dunn test).
Effect of pretreatment with histamine H1 receptor antagonists on BMY 14082 actions
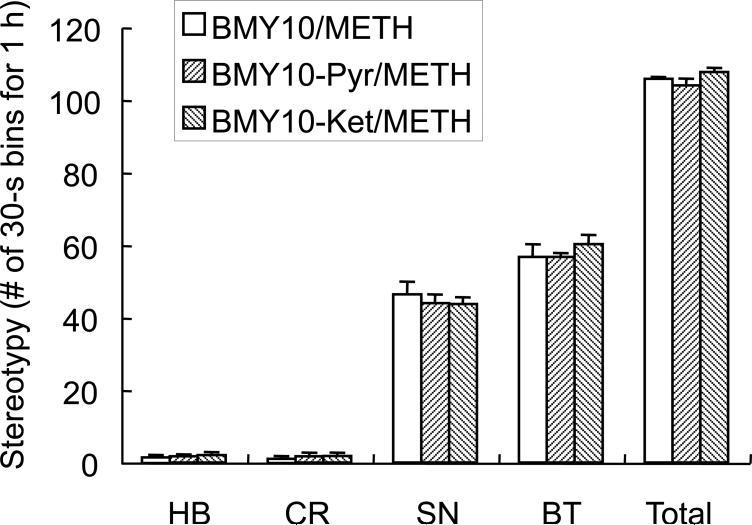
Pretreatment with the central histamine H1 receptor antagonists pyrilamine or ketotifen had no effect on BMY 14802 modulation of METH-induced stereotypies, as is shown in Fig. 7. ANOVA yielded no significant main effect of pretreatment (i.e. BMY 14802 versus BMY 14802 + pyrilamine, or BMY 14802 + ketotifen) on HB, CR, SN, BT, or Total stereotypy (F(2,15)=0.130, P=0.8790, F(2,15)=0.172, P=0.8437, F(2,15)=0.299, P=0.7456, F(2,15)=0.613, P=0.5547, and F(2,15)=2.097, P=0.1574, for each measure respectively).
Fig. 7.
Effect of pretreatment with histamine H1 receptor antagonists on BMY 14082 action on METH-induced stereotypy in mice. Values are shown as the means ± S.E.M. (n = 6 per group). BMY, BMY 14802 (10 mg/kg); Ket, ketotifen (10 mg/kg); METH, methamphetamine (10 mg/kg); Pyr, pyrilamine (10 mg/kg).
Discussion
Sigma receptor antagonists have been reported to inhibit psychostimulant (cocaine, amphetamine, and METH) actions including locomotor hyperactivity, behavioral sensitization, striatal dopamine release, and neurotoxicity in rodents (Ujike et al., 1992, 1994; Terleckyj and Sonsalla, 1994; Izenwasser et al., 1998; Takahashi et al., 2000; Nguyen et al., 2005; Matsumoto et al., 2007), although there have also been some negative findings (Skuza and Rogóz, 2006). These studies suggest that sigma receptor antagonists might have therapeutic potential for the treatment of psychostimulant abuse or psychoses. The intracellular signaling systems of the sigma receptors have been less well investigated with regard to potential involvement in regulation of psychostimulant-induced stereotypical behavior (Guitart et al., 2004), which may impact upon measures of locomotion in some of the studies mentioned above.
In the present study, we demonstrated that sigma receptor antagonists, such as BMY 14802 (a non-specific sigma receptor antagonist) and BD 1047 (a selective sigma1 receptor antagonist), altered the pattern of METH-induced stereotypy without affecting the total amount of stereotypical behavior observed. In particular, the incidence of stereotypical biting was reduced but the incidence of stereotypical sniffing was increased (Fig. 2), and this effect appeared to be mediated primarily by sigma1 receptors (Fig. 6). The two compounds BMY 14802 and BD 1407 are different from each other with regard to chemical structure and receptor subtype specificity, but the effects of the two compounds on METH-induced stereotypical behavior were identical (Figs. 4 and 6). These effects on METH-induced stereotypy are very similar to those produced by HMT inhibitors such as metoprine and SKF 91488, which increase brain histamine levels and activate histamine H1 receptors. These drugs also affect the pattern of METH-induced stereotypy rather than the total amount of stereotypy, producing an increase in METH-induced sniffing, but reducing METH-induced biting (Kitanaka et al., 2007). Although the mechanism underlying these effects is uncertain, they may involve a shift in activity between striatal subregions implicated in the control of each of these behaviors, the central-anterior caudate putamen and the nucleus accumbens (Costall et al., 1977).
However, with regard to locomotor behavior there are distinct differences between the effects of sigma compounds and the effects of histamine compounds. In the case of the HMT inhibitors, metoprine shows a biphasic effect on locomotion in mice: activation at 10 mg/kg and no effect at 20 mg/kg. The biphasic action of metoprine on locomotion may be associated with elevations in extracellular histamine levels produced by metoprine (Kalivas, 1982). In contrast to these findings, BMY 14802 did not stimulate locomotor activity alone nor did it inhibit METH-induced hyperactivity. The failure to observe effects of sigma1 receptor antagonists on METH-induced hyperlocomotion (Fig. 3B) may be due to ceiling effects since the high dose of METH used (10 mg/kg) induced persistent expression of stereotypy in all mice about 20 min after the drug challenge. By contrast, about only 30% of mice exhibited stereotypy when 5 mg/kg of METH was used (authors’ unpublished observation). Since the intention of the study was to examine individual components of METH-induced stereotypy the higher dose was chosen so that all subjects would exhibit stereotypy. It is possible that at lower doses of METH sigma effects on both hyperlocomotion, and perhaps overall levels of stereotypy, might be observed. In any case, at this higher METH dose sigma compounds and histamine compounds appear to have similar effects on METH-induced stereotypy, but quite distinct effects on METH-induced locomotion. Furthermore, even though their effects on METH-induced stereotypy are quite similar, the mechanisms appear to be independent, as indicated by the failure of histamine antagonists to alter BMY 14802 actions on METH-induced stereotypy.
Thus, the present study, as well as our previous report (Kitanaka et al., 2007), suggests that two receptor systems (histamine H1 receptor and sigma1 receptor signaling systems) independently alter the expression pattern of the METH-induced stereotypies, producing a shift from biting behavior to sniffing in a very similar manner, even though the effects appear to be independent of each other. These two receptor systems might share common unidentified intracellular signaling pathway(s) which influence stereotyped behavior induced by METH in distinct neural pathways involved in the production of stereotypical behavior (Costall et al., 1975, 1977; Iversen, 1977; Eichler et al., 1980; Schulz et al., 1981), but such speculation will require clarification in further studies.
The most likely mechanism underlying these effects is a sigma1 mediated mechanism since (1) two sigma receptor antagonists with different chemical structures (BMY 14802 and BD 1047) produced similar results (Figs. 4, 6), and (2) SKF 10,047, a putative sigma1 receptor agonist, blocked the effects of BMY 14802 on METH-induced stereotypy in a dose-dependent manner (Fig. 5). Regional distribution of brain sigma1 receptors suggests a role in motor behavior as sigma1 receptor binding sites are found in brain regions associated with the mesolimbic and nigrostriatal dopamine systems (Largent et al., 1986; McCann et al., 1994; Guitart et al., 2004). The molecular basis which may underlie the interaction of sigma1 receptor antagonists with METH actions is a matter of speculation, but sigma compounds have been shown to influence dopamine release (Weatherspoon and Werling, 1999) and dopamine transporter (DAT) function (Derbez et al., 2002). However, such explanations would not account for the shift in responses from one type of stereotypical behavior to another, since both are associated with elevations in dopaminergic function. Furthermore, BMY 14802 did not stimulate locomotor activity nor inhibit METH-induced hyperactivity, both of which are profoundly affected by other manipulations of DAT. Therefore, alteration of DAT activity alone, or generally, may not be the primary mechanism involved in these actions of sigma1 receptor antagonists. There is some suggestion that BMY 14802 can act directly on striatal dopamine D1 and D2 receptors (Terleckyj and Sonsalla, 1994), but since the effects of this compound were confirmed with a selective sigma1 antagonist and reversed by a selective sigma1 receptor agonist this possibility seems unlikely. Stereotyped licking, biting and other orofacial behaviors are known to involve nigrostriatal dopaminergic neurotransmission (Iversen, 1977) and distinct striatal subregions (Costall et al., 1977; Kelley et al., 1988; Delfs and Kelley, 1990). Importantly, it appears that control of sniffing and biting are mediated by different striatal subregions (Costall et al., 1977). There is no evidence that sigma ligands affect the compartmentalization of METH in distinct striatal subregions, which might be hypothesized to affect for the transition of stereotypical behavior from biting to sniffing.
Regarding the involvement of the sigma2 receptor subtype in the BMY 14802 actions, our results are less clear. PB 28 (1 mg/kg, i.v.), a putative sigma2 receptor agonist, partially inhibited the BMY 14802- and BD 1047- (a putative sigma1 receptor antagonist) induced transition of the stereotyped behavior from biting to sniffing (Figs. 4, 6). A higher dose of PB 28 did not have greater effects than the lower dose however. PB 28 is thought to be a sigma2 receptor-preferring agonist (Kassiou et al., 2005), although an in vitro study demonstrated that it acts as a sigma1 receptor antagonist as well (Azzariti et al., 2006), so perhaps the relatively weaker actions of PB 28 in the present study are due to non-specific effects on the sigma1 receptor.
In humans, the expression pattern, but not severity (or intensity), of stereotyped behavior has often been a focus of investigation and clinical interest (Groves and Rebec, 1976), since patterns of stereotyped behavior observed in amphetamine abusers are more varied and complex than those observed in animal models (Randrup and Munkvad, 1967). Based on this clinical perspective, sigma1 receptor antagonists might not necessarily be thought to improve conditions associated with aberrant repetitive behavior since the agents had no effect on the overall frequency of METH-induced stereotypy. However, by altering the pattern of the stereotyped behavior, producing a shift from biting to sniffing, might be considered to be effective under certain circumstances, such as in the treatment of self-injurious behavior associated with chronic drug use or other conditions such as autism (Matson and Lovullo, 2008). In animal models for psychostimulant abuse, biting behavior has often been considered to be a more severe pattern of stereotypy than other behaviors, such as sniffing (e.g. Costall et al., 1975; Braestrup, 1977). It may be better to describe these behaviors as distinct, separable, and sometimes competing behavioral entities, rather than on a continuum. The psychostimulant-induced vigorous biting behavior in rodents appears to be particularly relevant to self-injurious behavior in humans (Mueller et al., 1982; Kita et al., 2000; Mori et al., 2004), and continuous sniffing may be more related to anxiety (Blanchard et al., 2000; Markham et al., 2006). In this regard, sigma1 receptor antagonists might be useful for the treatment of self-injurious behavior observed in psychostimulant abusers as well as in several neuropsychiatric disorders, including autism spectrum disorders, Lesch-Nyhan syndrome, Tourette's syndrome, and de Lange syndrome (Aman, 1982; Winchel and Stanley, 1991; Moy et al., 2008).
References
- Aman MG. Stimulant drug effects in developmental disorders and hyperactivity – toward a resolution of disparate findings. J Autism Dev Disord. 1982;12:385–398. doi: 10.1007/BF01538326. [DOI] [PubMed] [Google Scholar]
- Azzariti A, Colabufo NA, Berardi F, Porcelli L, Niso M, Simone GM, Perrone R, Paradiso A. Cyclohexylpiperazine derivative PB28, a σ2 agonist and σ1 antagonist receptor, inhibits cell growth, modulates P-glycoprotein, and synergizes with anthracyclines in breast cancer. Mol Cancer Ther. 2006;5:1807–1816. doi: 10.1158/1535-7163.MCT-05-0402. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Neural substrates of psychostimulant-induced arousal. Neuropsychopharmacology. 2006;31:2332–2340. doi: 10.1038/sj.npp.1301159. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Hebert M, Dulloog L, Markham C, Figueira R, Nishimura O, Newsham K, Kaawaloa JN, Blanchard DC. Cocaine-induced sniffing stereotypy changes in response to threat. Pharmacol Biochem Behav. 2000;66:249–256. doi: 10.1016/s0091-3057(00)00183-0. [DOI] [PubMed] [Google Scholar]
- Braestrup C. Changes in drug-induced stereotyped behavior after 6-OHDA lesions in noradrenaline neurons. Psychopharmacology (Berl) 1977;51:199–204. doi: 10.1007/BF00431741. [DOI] [PubMed] [Google Scholar]
- Budygin EA. Dopamine uptake inhibition is positively correlated with cocaine-induced stereotyped behavior. Neurosci Lett. 2007;429:55–58. doi: 10.1016/j.neulet.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costall B, Marsden CD, Naylor RJ, Pycock CJ. Stereotyped behaviour patterns and hyperactivity induced by amphetamine and apomorphine after discrete 6-hydroxydopamine lesions of extrapyramidal and mesolimbic nuclei. Brain Res. 1977;123:89–111. doi: 10.1016/0006-8993(77)90645-x. [DOI] [PubMed] [Google Scholar]
- Costall B, Naylor RJ, Neumeyer JL. Differences in the nature of the stereotyped behaviour induced by aporphine derivatives in the rat and in their actions in extrapyramidal and mesolimbic brain areas. Eur J Pharmacol. 1975;31:1–16. doi: 10.1016/0014-2999(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Derbez AE, Mody RM, Werling LL. Sigma2-receptor regulation of dopamine transporter via activation of protein kinase C. J Pharmacol Exp Ther. 2002;301:306–314. doi: 10.1124/jpet.301.1.306. [DOI] [PubMed] [Google Scholar]
- Dickson PR, Lang CG, Hinton SC, Kelley AE. Oral stereotypy induced by amphetamine microinjection into striatum: an anatomical mapping study. Neuroscience. 1994;61:81–91. doi: 10.1016/0306-4522(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Eichler AJ, Antelman SM, Black CA. Amphetamine stereotypy is not a homogenous phenomenon: sniffing and licking show distinct profiles of sensitization and tolerance. Psychopharmacology (Berl) 1980;68:287–290. doi: 10.1007/BF00428117. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Matsumoto RR, Thompson TL, Patrick RL, Bowen WD, Walker JM. Motor effects of two σ ligands mediated by nigrostriatal neurons. Synapse. 1989;4:254–258. doi: 10.1002/syn.890040311. [DOI] [PubMed] [Google Scholar]
- Groves PM, Rebec GV. Biochemistry and behavior: some central actions of amphetamine and antipsychotic drugs. Annu Rev Psychol. 1976;27:91–127. doi: 10.1146/annurev.ps.27.020176.000515. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA. Biphasic effect of sigma receptor ligands on the extracellular concentration of dopamine in the striatum of the rat. J Neural Transm. 1999;106:849–856. doi: 10.1007/s007020050205. [DOI] [PubMed] [Google Scholar]
- Guitart X, Codony X, Monroy X. Sigma receptors: biology and therapeutic potential. Psychopharmacology (Berl) 2004;174:301–319. doi: 10.1007/s00213-004-1920-9. [DOI] [PubMed] [Google Scholar]
- Hamamura T, Akiyama K, Akimoto K, Kashihara K, Okumura K, Ujike H, Otsuki S. Co-administration of either a selective D1 or D2 dopamine antagonist with methamphetamine prevents methamphetamine-induced behavioral sensitization and neurochemical change, studied by in vivo intracerebral dialysis. Brain Res. 1991;546:40–46. doi: 10.1016/0006-8993(91)91156-u. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T-P. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18:269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- Iversen SD. Neural substrates mediating amphetamine response. In: Ellinwood EH, Kilbey MM, editors. Cocaine and other stimulants. Plenum Press; New York: 1977. pp. 31–45. [Google Scholar]
- Izenwasser S, Thompson-Montgomery D, Deben SE, Chowdhury IN, Werling LL. Modulation of amphetamine-stimulated (transporter mediated) dopamine release in vitro by σ2 receptor agonists and antagonists. Eur J Pharmacol. 1998;346:189–196. doi: 10.1016/s0014-2999(98)00063-6. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Histamine-induced arousal in the conscious and pentobarbital-pretreated rat. J Pharmacol Exp Ther. 1982;222:37–42. [PubMed] [Google Scholar]
- Kamei H, Kameyama T, Nabeshima T. SKF-10,047 reverses stress-induced motor suppression: interaction with dopaminergic system. Eur J Pharmacol. 1994;260:39–46. doi: 10.1016/0014-2999(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Kamei H, Kameyama T, Nabeshima T. (+)-SKF-10,047 and dextromethorphan ameliorate conditioned fear stress via dopaminergic systems linked to phenytoin-regulated sigma1 sites. Eur J Pharmacol. 1996;309:149–158. doi: 10.1016/0014-2999(96)00346-9. [DOI] [PubMed] [Google Scholar]
- Kassiou M, Dannals RF, Liu X, Wong DF, Ravert HT, Scheffel UA. Synthesis and in vivo evaluation of a new PET radioligand for studying sigma-2 receptors. Bioorg Med Chem. 2005;13:3623–3626. doi: 10.1016/j.bmc.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology (Berl) 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6-OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Kita T, Matsunari Y, Saraya T, Shimada K, O'Hara K, Kubo K, Wagner GC, Nakashima T. Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice. Int J Dev Neurosci. 2000;18:521–530. doi: 10.1016/s0736-5748(00)00022-8. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Takemura M. 2-Phenylethylamine in combination with l-deprenyl lowers the striatal level of dopamine and prolongs the duration of the stereotypy in mice. Pharmacol Biochem Behav. 2005;82:488–494. doi: 10.1016/j.pbb.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Morita Y, Takemura M. Blockade of brain histamine metabolism alters methamphetamine-induced expression pattern of stereotypy in mice via histamine H1 receptors. Neuroscience. 2007;147:765–777. doi: 10.1016/j.neuroscience.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Morita Y, Takemura M. Toxicity of an alkaloid extracted from the plant Lobelia inflata: association with methamphetamine-induced behavioral abnormalities. Chudoku Kenkyu (Jpn J Toxicol) 2008;21:189–191. (in Japanese) [PubMed] [Google Scholar]
- Kitanaka N, Kitanaka J, Takemura M. Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur J Pharmacol. 2003;474:63–70. doi: 10.1016/s0014-2999(03)02015-6. [DOI] [PubMed] [Google Scholar]
- Kramer JC, Fischman VS, Littlefield DC. Amphetamine abuse: pattern and effects of high doses taken intravenously. JAMA. 1967;201:305–309. doi: 10.1001/jama.201.5.305. [DOI] [PubMed] [Google Scholar]
- Largent BL, Gundlach AL, Snyder SH. Pharmacological and autoradiographic discrimination of sigma and phencyclidine receptor binding sites in brain with (+)-[3H]SKF 10,047, (+)-[3H]-3-[3-hydroxyphenyl]-N-(1-propyl)piperidine and [3H]-1-[1-(2-thienyl)cyclohexyl]piperidine. J Pharmacol Exp Ther. 1986;238:739–748. [PubMed] [Google Scholar]
- Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophrenia Bull. 1990;16:97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- Markham CM, Yang M, Blanchard RJ, Blanchard DC. Effects of D-amphetamine on defensive behaviors related to fear and anxiety. Pharmacol Biochem Behav. 2006;83:490–499. doi: 10.1016/j.pbb.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Matson JL, Lovullo SV. A review of behavioral treatments for self-injurious behaviors of persons with autism spectrum disorders. Behav Modif. 2008;32:61–76. doi: 10.1177/0145445507304581. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Mack AL. (±)-SM-21 attenuates the convulsive and locomotor stimulatory effects of cocaine in mice. Eur J Pharmacol. 2001;417:R1–R2. doi: 10.1016/s0014-2999(01)00891-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. Effects of UMB24 and (±)-SM 21, putative σ2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol Biochem Behav. 2007;86:86–91. doi: 10.1016/j.pbb.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann DJ, Weissman AD, Su TP. Sigma-1 and sigma-2 sites in rat brain: comparison of regional, ontogenetic, and subcellular patterns. Synapse. 1994;17:182–189. doi: 10.1002/syn.890170307. [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. Two novel sigma receptor ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol. 1999;370:225–232. doi: 10.1016/s0014-2999(99)00113-2. [DOI] [PubMed] [Google Scholar]
- Moison D, De Deurwaerdère P, Cagnotto A, Marrazzo A, Prezzavento O, Ronsisvalle G, Mennini T, Spampinato U. Intrastriatal administration of sigma ligands inhibits basal dopamine release in vivo. Neuropharmacology. 2003;45:945–953. doi: 10.1016/s0028-3908(03)00253-3. [DOI] [PubMed] [Google Scholar]
- Mori T, Ito S, Kita T, Sawaguchi T. Effects of dopamine- and serotonin-related compounds on methamphetamine-induced self-injurious behavior in mice. J Pharmacol Sci. 2004;96:459–464. doi: 10.1254/jphs.fpj04040x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, Crawley JN, Duncan GE, Bodfish JM. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Saboda S, Palmour R, Nyhan WL. Self-injurious behavior produced in rats by daily caffeine and continuous amphetamine. Pharmacol Biochem Behav. 1982;17:613–617. doi: 10.1016/0091-3057(82)90332-x. [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. Involvement of sigma (σ) receptors in the acute actions of methamphetamine: Receptor binding and behavioral studies. Neuropharmacology. 2005;49:638–645. doi: 10.1016/j.neuropharm.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Okuyama S, Kawashima N, Chaki S, Yoshikawa R, Funakoshi T, Ogawa SI, Suzuki Y, Ikeda Y, Kumagai T, Nakazato A, Nagamine M, Tomisawa K. A selective dopamine D4 receptor antagonist, NRA0160: a preclinical neuropharmacological profile. Life Sci. 1999;65:2109–2125. doi: 10.1016/s0024-3205(99)00476-2. [DOI] [PubMed] [Google Scholar]
- Randrup A, Munkvad I. Stereotyped activities produced by amphetamine in several animal species and man. Psychopharmacologia (Berl) 1967;11:300–310. doi: 10.1007/BF00404607. [DOI] [PubMed] [Google Scholar]
- Rebec GV, Bashore TR. Critical issues in assessing the behavioral effects of amphetamine. Neurosci Biobehav Rev. 1984;8:153–159. doi: 10.1016/0149-7634(84)90030-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Schulz EM, Wright JW, Harding JW. Distinctions between stereotyped sniffing and licking in rats with methamphetamine and apomorphine. Pharmacol Biochem Behav. 1981;15:521–523. doi: 10.1016/0091-3057(81)90289-6. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. An escalating dose “binge” model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551–2566. doi: 10.1523/JNEUROSCI.17-07-02551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuza G, Rogóz Z. Effect of BD 1047, a sigma1 receptor antagonist, in the animal models predictive of antipsychotic activity. Pharmacol Rep. 2006;58:626–635. [PubMed] [Google Scholar]
- Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol. 2005;75:406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Miwa T, Horikomi K. Involvement of σ1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett. 2000;289:21–24. doi: 10.1016/s0304-3940(00)01258-1. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Effects of monoamine oxidase inhibitors on methamphetamine-induced stereotypy in mice and rats. Neurochem Res. 2005;30:1377–1385. doi: 10.1007/s11064-005-8390-2. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lobeline attenuates methamphetamine-induced atereotypy in adolescent mice. Neurochem Res. 2006;31:1359–1369. doi: 10.1007/s11064-006-9180-1. [DOI] [PubMed] [Google Scholar]
- Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M. Lack of effect of anticonvulsant topiramate on methamphetamine-induced stereotypy and rewarding property in mice. Pharmacol Biochem Behav. 2007;87:48–55. doi: 10.1016/j.pbb.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Terleckyj I, Sonsalla PK. The sigma receptor ligand (±)-BMY 14802 prevents methamphetamine-induced dopaminergic neurotoxicity via interactions at dopamine receptors. J Pharmacol Exp Ther. 1994;269:44–50. [PubMed] [Google Scholar]
- Ujike H, Kanzaki A, Okumura K, Akiyama K, Otsuki S. Sigma (σ) antagonist BMY 14802 prevents methamphetamine-induced sensitization. Life Sci. 1992;50:PL29–34. doi: 10.1016/0024-3205(92)90466-3. [DOI] [PubMed] [Google Scholar]
- Ujike H, Kuroda S, Otsuki S. σ Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42:355–402. [PubMed] [Google Scholar]
- Weatherspoon JK, Werling LL. Modulation of amphetamine-stimulated [3H]dopamine release from rat pheochromocytoma (PC12) cells by sigma type 2 receptors. J Pharmacol Exp Ther. 1999;289:278–284. [PubMed] [Google Scholar]
- Winchel RM, Stanley M. Self-injurious behavior: a review of the behavior and biology of self-mutilation. Am J Psychiatry. 1991;148:306–317. doi: 10.1176/ajp.148.3.306. [DOI] [PubMed] [Google Scholar]
- Woods-Kettelberger A, Kongsamut S, Smith CP, Winslow JT, Corbett R. Animal models with potential applications for screening compounds for the treatment of obsessive-compulsive disorder. Expert Opin Invest Drugs. 1997;6:1369–1381. doi: 10.1517/13543784.6.10.1369. [DOI] [PubMed] [Google Scholar]