Abstract
An increasing body of evidence shows that structural modifications of chromatin, the DNA–protein complex that packages genomic DNA, do not only participate in maintaining cellular memory (e.g., cell fate), but they may also underlie the strengthening and maintenance of synaptic connections required for long-term changes in behavior. Accordingly, epigenetics has become a central topic in several neurobiology fields such as memory, drug addiction, and several psychiatric and mental disorders. This interest is justified as dynamic chromatin modifications may provide not only transient but also stable (or even potentially permanent) epigenetic marks to facilitate, maintain, or block transcriptional processes, which in turn may participate in the molecular neural adaptations underlying behavioral changes. Through epigenetic mechanisms the genome may be indexed in response to environmental signals, resulting in specific neural modifications that largely determine the future behavior of an organism. In this review we discuss recent advances in our understanding of how epigenetic mechanisms contribute to the formation of long-term memory and drug-seeking behavior and potentially how to apply that knowledge to the extinction of memory and drug-seeking behavior.
An introduction to epigenetics and chromatin modification
Beginning approximately 6–7 years ago, there was a major movement in basic and clinical research to understand the role of epigenetics in neurobiology, especially the neurobiology of learning and memory, drug addiction, and cognitive disorders. That is not to say that epigenetic mechanisms have not been on scientists’ minds for the better part of a half-century. The term epigenetics was originally coined by Waddington in 1942 to describe the examination of “causal mechanisms” whereby “the genes of the genotype bring about phenotypic effects” (Haig 2004). The term has now taken several new definitions, especially in the neurosciences. In non-neuroscience fields, the term epigenetics refers to a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence (Berger et al. 2009). In a recent book on epigenetics by Allis et al. (2007), one can find two different definitions. In one section epigenetics is defined as “change in phenotype that is heritable but does not involve DNA mutation.” It should be noted that in most cases the term heritable is being applied to somatic cellular memory. In another section epigenetics is defined as “changes in gene transcription through modulation of chromatin, which is not brought about by changes in the DNA sequence.” Notably, the term heritable is not part and parcel of the latter definition.
As neuroscientists are by definition interested in the function of neurons, which are postmitotic differentiated cells, the definition of epigenetics normally used by neuroscientists has also dropped the heritable component (Abel and Zukin 2008; Barrett and Wood 2008; Graff and Mansuy 2008; Levenson and Sweatt 2005). As the number of publications relating to epigenetics have gone from 50 in 1989 to nearly 6000 in 2008, it is obvious that regardless of how researchers define epigenetics, it has taken a central position in research. Realizing this emerging theme, the National Institutes of Health (NIH) held a workshop in 2007 to examine the possibility of supporting research aimed at furthering our understanding of epigenetics through the NIH Roadmap program. NIH describes epigenetics as “refer[ing] to both heritable changes in gene activity and expression (in the progeny of cells or of individuals) and also stable, long-term alterations in the transcriptional potential of a cell that are not necessarily heritable” (http://nihroadmap.nih.gov/epigenomics/index.asp).
The purpose of this review is to examine the contribution of current literature to our understanding of the role of epigenetic mechanisms involved in learning and memory as well as drug addiction processes as it relates to the development of therapeutic strategies. Epigenetic mechanisms have been found to be central to the initial formation and consolidation of memory (Barrett and Wood 2008) and central to the cellular and behavioral responses to drugs of abuse, including the potential transition in response from acute exposure (drug experimentation) to chronic exposure (compulsive drug use; Renthal and Nestler 2008). The important hypothesis to emerge from examining addiction from a learning and memory perspective is that addiction may be established by abnormal powerful and persistent memory mechanisms leading to severe changes in behavior.
With regard to learning and memory, there is a process called experimental extinction in which a conditioned response is weakened and thus the strength of the original memory is greatly diminished. Recently, we and others have demonstrated that extinction of fear-related memories can be facilitated by compounds called histone deacetylase (HDAC) inhibitors (Bredy and Barad 2008; Bredy et al. 2007; Lattal et al. 2007). Even more recently we have shown that HDAC inhibition can also facilitate the extinction of drug-seeking behavior in a manner that significantly attenuates reinstatement (the reappearance of drug-seeking behavior following exposure to drug-associated cues; Malvaez et al. 2009). Thus, it may be possible to modulate memory processes such as extinction via epigenetic mechanisms resulting in stable extinction of memories associated with fear/anxiety/phobias as well as extinction of drug-seeking behavior. In this review we discuss recent advances in our understanding of how epigenetic mechanisms contribute to the formation of long-term memory and drug-seeking behavior and potentially how to apply that knowledge to the extinction of memory and drug-seeking behavior.
The main epigenetic mechanism we discuss in this review is that of chromatin modification because it is the best studied in learning and memory and thus the most relevant to a discussion about the extinction of memory and drug-seeking behavior. Chromatin (the material of which chromosomes are made) is defined as the complex of genomic DNA, histones, and nonhistone proteins found in the nucleus. The repeating subunit of chromatin is called the nucleosome, which consists of 146 bp of DNA wrapped around a histone octamer. This histone octamer consists of pairs of the core histone proteins H2A, H2B, H3, and H4. The amino terminal tails of these core histone proteins are the sites of numerous post-translational modifications (e.g., acetylation, methylation, phosphorylation) carried out by an equally large number of histone-modifying enzymes (acetyltransferases, deacetylases, methyltransferases, demethylases, kinases; see Kouzarides 2007 for review). The manipulation of chromatin via the addition of functional groups to histone tails is referred to as chromatin modification, which serves two main purposes: The first is to provide recruitment signals for nonhistone proteins involved in transcriptional activation and silencing (Kouzarides 2007; Taverna et al. 2007). The second is to relax chromatin by disrupting contacts between nucleosomes and also interactions between histone tails and genomic DNA (Kouzarides 2007). One main functional consequence of these modifications is to modulate transcriptional regulation.
Of course, histone modification is only one of many mechanisms by which chromatin structure can be regulated, with the ultimate aim of modulating transcription. Chromatin structure can also be regulated via chromatin (or nucleosome) remodeling, which refers to ATP-dependent enzymatic complexes (e.g., SWI/SNF, ISWI, INO80, NURD) that restructure, mobilize, and remove nucleosomes to regulate access to genomic DNA for transcriptional activation (Saha et al. 2006a, b). Chromatin structure may also be manipulated and regulated via histone variant incorporation (e.g., H3.3, macroH2A, H2AZ, H2AX) (Ausio 2006). Another level of regulation comes from the dynamic crosstalk between histone modifications and DNA methylation (Vaissiere et al. 2008). In this review we focus mainly on chromatin modification via histone acetylation because this area has been examined more thoroughly with respect to learning and memory at this time.
Learning and memory as it relates to drug addiction
In this section we set the stage for why we believe that gaining an understanding of the role of chromatin modification in learning and memory processes will not only elucidate fundamental aspects of memory formation and storage, but also give tremendous insight into facilitating extinction of memory and how it may relate to extinction of drug-seeking behavior.
Drug addiction is defined as a behavioral syndrome characterized by compulsive drug seeking and loss of control over drug intake regardless of medical illness, engaging in criminal activity, and other adverse consequences (Hyman et al. 2006; Kalivas et al. 2005; McLellan et al. 2000). Despite attempts to control drug intake and periods of prolonged abstinence, addicted individuals remain at a high risk of relapse to drug use (Hyman et al. 2006; Kalivas et al. 2005; McLellan et al. 2000; Robinson and Berridge 2003). These long-lasting effects may be accounted for by stable changes in cellular function leading to stable changes in neuronal plasticity. Understanding the molecular and neural mechanisms underlying the consolidation, persistence, and re-emergence of drug-seeking behavior is crucial for the improvement of treatments for drug addiction (Hyman 2005; Jones and Bonci 2005; Robinson and Berridge 2003).
Previous research has identified the initial pharmacological actions of addictive drugs and the homeostatic cellular adaptations triggered by drug exposure that lead to tolerance, dependence, and withdrawal (Anagnostaras and Robinson 1996; Kalivas and Duffy 1993; Nestler and Aghajanian 1997). Although the avoidance of withdrawal may perpetuate drug use, it does not explain compulsive drug use. Relapse episodes occur after extended periods of abstinence, long after withdrawal symptoms have abated (Berke and Hyman 2000; McLellan et al. 2000; Robinson and Berridge 2000).
Relapse to compulsive drug use is more readily triggered by exposure to cues such as paraphernalia, places, or people previously associated with drug use (O’Brien et al. 1998). Imaging studies have found that drug-associated cues elicit drug cravings that correlate with activation of brain regions implicated in stimulus-reward associations (Childress et al. 1999; Ehrman et al. 1992; Kilts et al. 2001). Animal models of drug-seeking behavior have shown that drugs of abuse have the ability to strengthen the association between drug cues and drug-related responses (Everitt and Robbins 2005; Hyman et al. 2006), providing insight on how drugs of abuse are able to sustain drug-seeking behavior. In the addicted state, the acute psychoactive effects alone do not maintain drug-related behavior; rather, drug-related stimuli trigger drug-seeking behavior through associative learning. From these observations, the dominant hypothesis is that the underlying molecular mechanisms of compulsive drug use are similar to those that underlie long-term associative memory (Everitt et al. 2008; Hyman 2005; Hyman et al. 2006; Robbins et al. 2008).
In the next sections of this review we examine the literature describing a role for chromatin modification in learning and memory as well as drug addiction. These two topics have been reviewed by Barrett and Wood (2008) with regard to learning and memory and by Renthal and Nestler (2008) with regard to drug addiction. Thus, we highlight recent advances and what we perceive to be the most important key questions at this time with regard to how epigenetic mechanisms contribute to the formation of long-term memory and drug-seeking behavior and potentially how to apply that knowledge to the extinction of memory and drug-seeking behavior.
Role of chromatin modification in learning and memory
One of the main reasons the regulation of histone modifications came to the forefront in examining the molecular mechanisms underlying learning and memory is that the regulation of histone modification is strongly correlated with transcriptional regulation. Why is transcriptional regulation so important to learning and memory? It is generally accepted that long-term forms of synaptic plasticity, the strengthening in communication between neurons, and long-term memory processes are dependent on transcription (reviewed in Alberini 2009). Although transcription factors such as cAMP response element binding protein (CREB) have been studied for quite a long time in the field of learning and memory, the coactivators such as CREB-binding protein (CBP) were only recently examined (reviewed in Barrett and Wood 2008). CBP is a potent histone acetyltransferase (HAT) shown to be necessary for long-lasting forms of transcription-dependent synaptic plasticity and long-term memory (Alarcon et al. 2004; Bourtchouladze et al. 2003; Guan et al. 2002; Korzus et al. 2004; Oike et al. 1999; Oliveira et al. 2007; Stefanko et al. 2009; Vecsey et al. 2007; Wood et al. 2005, 2006). At the same time that CBP and histone acetylation were identified as critical to the molecular mechanism involved in memory processes, histone deacetylases (HDACs) were also proving to be critical for synaptic plasticity and memory (Guan et al. 2002; Levenson et al. 2004; Yeh et al. 2004). In general, impairments in HAT function and decreases in histone acetylation result in impaired synaptic plasticity and long-term memory, whereas increasing histone acetylation (via HDAC inhibition) results in enhanced synaptic plasticity and long-term memory. These initial studies demonstrated that histone-modifying enzymes and histone modifications are pivotal to the molecular mechanisms underlying long-term memory processes. We have reviewed this literature in Barrett and Wood (2008), thus we continue here with the most recent advances.
Although the best studied epigenetic mechanism involved in memory formation may be histone acetylation, recent advances in understanding DNA methylation and how it controls transcription required for memory formation have radically challenged previously held notions. One of the most controversial issues is whether DNA methylation is reversible (Szyf 2009). One of the first studies to address this issue with regard to synaptic plasticity demonstrated that DNA within promoter regions of reelin and Bdnf (genes involved in mechanisms of hippocampal synaptic plasticity) exhibited rapid and reversible changes in methylation upon inhibition of DNA (cytosine-5) methyltransferase (DNMT) (Levenson et al. 2006). In the same study the authors show that blocking DNMT inhibits hippocampal long-term potentiation, a form of synaptic plasticity (Levenson et al. 2006). In a different study, contextual fear conditioning was shown to increase expression of DNMT in the hippocampus and infusion of a DNMT inhibitor into the hippocampus-blocked long-term memory for contextual fear (Miller and Sweatt 2007). Miller and Sweatt (2007) also demonstrated that fear conditioning is associated with rapid changes in methylation of DNA in the promoter regions of PP1 (which is associated with preventing memory formation) and reelin (which as mentioned above is associated with mechanisms of promoting synaptic plasticity). Similar rapid changes in methylation have been demonstrated in regulating exonspecific Bdnf transcripts during contextual fear learning (Lubin et al. 2008). In summary, these landmark studies demonstrate that DNA methylation is dynamically regulated and that these rapid changes in methylation are crucial for engaging long-term synaptic plasticity and long-term memory formation.
One exciting possibility that interests many researchers is the potential for chromatin modifications and DNA methylation being involved in establishing more stable transcription profiles leading to stable changes in cellular function and ultimately persistent changes in behavior. One approach used to demonstrate this idea as feasible is represented by work done on the effects of maternal behavior on the adult behavior of offspring (Weaver et al. 2004). This research, performed in the labs of Michael Meaney and Moshe Szyf, essentially demonstrates that maternal behavior causes changes in the DNA methylation patterns of the exon 17 glucocorticoid receptor promoter of offspring and that this correlates with long-term behavioral changes in those offspring (Meaney and Szyf 2005). This remarkable finding suggests that epigenetic modifications can be stable and result in persistent changes in behavior.
Currently, we have little understanding of the molecular mechanisms underlying long-term memory lasting beyond 24 h. Considering the role of chromatin modification and DNA methylation in cell-fate decisions and other stable epigenetic phenomena, it seems reasonable that perhaps these mechanisms are also involved in establishing and maintaining specific neuronal functions serving persistent memory storage. This possibility is only beginning to be explored and presents seriously difficult problems in correlating epigenetic modifications at single genes with their changed expression and ultimately changes in behavior of the organism (as demonstrated with regard to the effects of maternal behavior discussed above). However, there are some more simple approaches we can take in the meantime.
For example, we recently took advantage of a behavioral task called novel object recognition (NOR) to demonstrate that histone acetylation is involved in mechanisms that can transform a learning event that does not normally result in long-term memory into an event that is now remembered long-term (Stefanko et al. 2009). In the same study we also show that histone acetylation is involved in generating a form of long-term memory that persists beyond the point at which normal memory for NOR fails. This behavioral task is well suited to address the role of histone acetylation in modulating long-term memory formation because it is so easily manipulated in mice. In this task, a mouse is exposed to two of the same objects during training. Either 90 min (short-term memory) or 24 h (long-term memory) later the mouse is given a retention test in the same context using one familiar object and one novel object. The innate behavior of the mouse is to explore the novel object, but that depends on the mouse having a memory for the familiar object. A short training period results in no short- or long-term memory. However, mice treated with an HDAC inhibitor following training still exhibit no short-term memory, but they do exhibit excellent long-term memory (Stefanko et al. 2009). Thus, a learning event that does not normally result in long-term memory is transformed into one that does. In a different experiment, a longer training period was used that results in both short-term and 24-h long-term memory. However, memory for the familiar object fails by 7 days post-training. Treating a mouse with an HDAC inhibitor following this longer training period does not affect performance in the 24-h retention test (most likely because animals have hit a ceiling), but animals examined at 7 days continue to have excellent memory for the familiar object (Stefanko et al. 2009). These data indicate that although the effects of HDAC inhibitors are transient (as far as anyone can observe using the antibodies commercially available), the effects on long-term memory and changes in behavior are much more persistent. One reason we examined how HDAC inhibition modulates long-term memory for NOR is because every single genetically modified Cbp mutant mouse studied to date exhibits significant long-term memory impairments for NOR (but exhibit normal short-term memory; discussed in Barrett and Wood 2008). This suggested that CBP and histone acetylation are pivotal for this type of memory (see also Fontan-Lozano et al. 2008). The other main reason is that one can study a time point at which normal memory fails for NOR allowing the persistence of memory to be examined.
In the next section we explore how we and others have applied the ideas of modulating memory formation and the persistence of memory to the extinction of memory. Extinction is an experience-dependent change in behavior in which an animal learns that the relationship between previously associated stimuli is severed. As we discuss below, chromatin modification has been shown to be pivotal to extinction learning and the persistence of extinction. With regard to learning and memory, we and others have shown that extinction of fear memories can be facilitated by HDAC inhibition. With regard to drug-seeking behavior, we have shown that extinction of cocaine-context-associated memories can be facilitated by HDAC inhibition in a persistent manner that prevents reinstatement of the drug-seeking behavior. These new findings provide novel insight into how HDAC inhibition (and numerous FDA-approved HDAC inhibitors) may be used in combination with behavioral extinction therapy to overcome fear and anxiety disorders as well as drug-seeking behavior.
Chromatin modification and the extinction of fear-related memories
Anxiety disorders have been suggested to be the manifestation of maladaptive fear learning in which a stimulus becomes a cue for fear and anxiety (Garakani et al. 2006). It has been suggested that the inability to inhibit or extinguish these memories contributes to the persistence of anxiety disorders. Much progress has been made in understanding the neural basis of fear learning by studying the classical animal model of fear conditioning (Garakani et al. 2006; Maren and Quirk 2004) in which fear memories are formed by pairing an initially neutral stimulus to an unconditioned stimulus (e.g., a shock). Although significant advances have been made, a major challenge in treating anxiety disorders is understanding how to permanently suppress these associative emotional memories.
Currently, the most effective treatment for anxiety disorders is exposure therapy, which is a form of extinction in which fear-evoking stimuli are presented in the absence of aversive consequences (Norberg et al. 2008a; Rothbaum and Davis 2003; Shearer 2007). Extinction, first described by Pavlov (1927) nearly 100 years ago, is an experience-dependent process by which the magnitude of a previously learned behavioral response is reduced. The molecular mechanisms of extinction have been the focus of much investigation. Studies have suggested that many of the molecular mechanisms involved in extinction are similar to those in initial associative learning (Mueller et al. 2008). It is generally thought that extinction involves new learning, which interferes with retrieval of the original memory but does not erase that original memory. This idea is supported by “uncovering phenomena” such as spontaneous recovery, renewal, and reinstatement that are able to reveal that the original memory is intact (Bouton 1993; Pearce 1987). However, defining extinction by the basis of whether the original memory can be retrieved is a specious argument because persistent extinction would result in the original memory remaining intractable. This issue is well described in reviews by Lattal et al. (2006) and Lattal and Stafford (2008).
Transcription, a process critical for long-term memory (Alberini 2009), has recently been implicated in extinction. Several studies have used transcription inhibitors during extinction training and observed completely blocked or partially impaired extinction (Mueller et al. 2008; Vianna et al. 2003; Yang and Lu 2005). These experiments support a role for transcription in extinction, but as these drugs may have other effects, such as apoptosis (Fraschini et al. 2005; Shim et al. 2004), they are not sufficient evidence to prove a role for gene expression in extinction processes. Other studies have examined the mRNA levels of specific genes following extinction. They have found that a number of genes are upregulated during this period in brain regions that are implicated in extinction (Chhatwal et al. 2005, 2006; Heldt and Ressler 2007; Herry and Mons 2004; Mickley et al. 2007). Furthermore, the promoter region of one of these genes, BDNF, has altered histone acetylation, a modification that regulates transcription, following extinction (Bredy et al. 2007). These results together suggest that gene expression plays a role in extinction learning, but more precise experimental approaches are necessary to establish this link and understand how gene expression is regulated.
The major goal of current studies is to enhance extinction of maladaptive fear memories. In the basic research setting, a number of drugs have been used to enhance extinction training (Ledgerwood et al. 2005; Walker et al. 2002; Woods and Bouton 2006). Based on the similarity of exposure therapy to extinction, this has also been tested in the clinical setting, although to a more limited degree. D-cycloserine (DCS), a partial NMDA agonist, has been administered in combination with exposure therapy (Ressler and Mayberg 2007). These studies have generally observed enhanced efficacy (Kushner et al. 2007; Ressler et al. 2004), although some have not (Guastella et al. 2007; Storch et al. 2007). A meta-analysis of studies of DCS treatment in combination with exposure therapy found that DCS is useful for augmenting exposure-based therapy (Norberg et al. 2008b). Although exposure-based therapies can reduce the expression of fear, extinguished fear often reappears. Thus, approaches to enhancing extinction need to focus on methods that not only enhance the rate of extinction but also generate a more persistent form of extinction.
One potential mechanism that may produce long-lasting behavioral effects is stable changes in cellular function leading to stable changes in neuronal plasticity. Extinction is a form of learning, and, as mentioned previously, learning involves modulation of gene expression by histone modification, which can be altered by drugs such as HDAC inhibitors. Several studies have examined the ability of HDAC inhibitors to enhance extinction. Lattal et al. (2007) found that intrahippocampal or systemic administration of an HDAC inhibitor enhanced extinction of contextual fear conditioning. Bredy et al. (2007; Bredy and Barad 2008) showed that administration of a variety of HDAC inhibitors enhanced extinction of cued fear and that histone acetylation at promoters of specific genes is altered during extinction. Due to the ability of HDAC inhibitors to enhance extinction, decreasing behavioral responses, and their ability to increase the persistence of memory (suggesting that they may prolong the effectiveness of extinction), HDAC inhibitors provide an excellent candidate as companions to extinction therapy in humans. The ability of HDAC inhibitors to not only enhance memory but also to create a more persistent form of memory may allow HDAC inhibitor-enhanced extinction to be resistant to the return of fear in the form of spontaneous recovery, reinstatement, or renewal. Although further studies are needed to show that this is the case for fear learning or anxiety, we have recently demonstrated this phenomenon in the context of drug-seeking behavior (Malvaez et al. 2009). We now turn to the role of chromatin modification in drug addiction.
Role of chromatin modification in drug addiction
Addictive drugs cause persistent changes in the structure and function of the brain’s circuitry, leading to long-lasting changes in behavior. Understanding the mechanisms responsible for the persistence of drug-associated behaviors is a major focus of addiction research. As implicated in long-lasting forms of learning and memory, altered gene expression is thought to also contribute to the development and persistence of drug addiction (Hyman et al. 2006; McClung and Nestler 2008). Fast growing evidence supports the notion that drugs of abuse may trigger chromatin modifications that modulate transcription profiles involved in drug-induced neural and behavioral changes (McClung and Nestler 2008). These studies have focused mainly on histone acetylation and/or phosphorylation. In this section we focus on acetylation, as the effects of drugs of abuse on histone phosphorylation have been recently reviewed (Brami-Cherrier et al. 2009).
Studies assessing the ability of addictive drugs to promote increases of global histone acetylation in the central nervous system have provided conflicting results (Brami-Cherrier et al. 2005; Cassel et al. 2006; Kim and Shukla 2006; Pandey et al. 2008; Shen et al. 2008). This disparity might be due, at least in part, to the diversity in the treatment conditions, brain regions analyzed, or even the subjects’ developmental stage. As illustrated by a recent study (Pascual et al. 2009), the same drug treatment can produce a different result depending on the subjects’ age (e.g., young, but not old, rats exhibit histone acetylation changes). Furthermore, opposite changes are observed in adjacent brain areas within the same subject (e.g., increased acetylation in the nucleus accumbens but decreased in striatum; Pascual et al. 2009). How to interpret the meaning of changes in global histone acetylation is another major problem, as these global changes may not have physiological relevance.
Technical limitations in measuring global histone acetylation may also contribute to the conflicting results that have been observed. The complex and narrow pattern of histone modifications triggered by drugs of abuse might not be captured if analyzing global changes. Indeed, if histone acetylation changes regulate gene expression, it is conceivable that these changes do not occur for the whole genome of heterogeneous neuronal populations of large anatomical brain structures (i.e., striatum) but rather at the promoters of a restricted group of genes of particular neuronal ensembles (Sanchis-Segura et al. 2009). A study by Kumar et al. (2005) showed that acute administration of cocaine transiently increases acetylation of histone H4 at the c-fos and fosB gene promoters but not at the promoters of other genes (i.e., β-tubulin, core histone H4 genes) whose expression is not altered by cocaine. These results support the notion that changes in histone modification in response to drug exposure are not global but are limited to specific genes.
Another perplexing issue is whether the most commonly used HDAC inhibitors (TSA, SAHA, NaBut, VPA), which have nonspecific activity with respect to blocking HDACs, may ultimately generate effects on behavior via specific molecular pathways. Although this idea is counterintuitive, recent findings suggest that indeed nonspecific HDAC inhibitors may affect behavior via defined pathways. For example, Vecsey et al. (2007) demonstrated that the nonspecific HDAC inhibitor TSA enhances long-term potentiation via a CREB:CBP-dependent mechanism. Similarly, Guan et al. (2009) recently demonstrated that the nonspecific HDAC inhibitor SAHA enhances memory via an HDAC2-dependent mechanism. Together, these two studies suggest that nonspecific inhibitors may ultimately cause cellular and behavioral changes via specific mechanisms. This idea is further supported by gene expression data in Vecsey et al. (2007) in which the authors examined a defined pool of 12 genes involved in learning and memory and showed that TSA affects only the expression of Nr4a1 and Nr4a2 (these are immediate early genes and transcription factors) in the hippocampus after fear conditioning. Thus, although nonspecific HDAC inhibitors may have general effects on histone acetylation, the molecular mechanisms involved in memory formation (such as CBP activity) become pivotal to the ability of HDAC inhibitors to modulate memory via specific mechanisms.
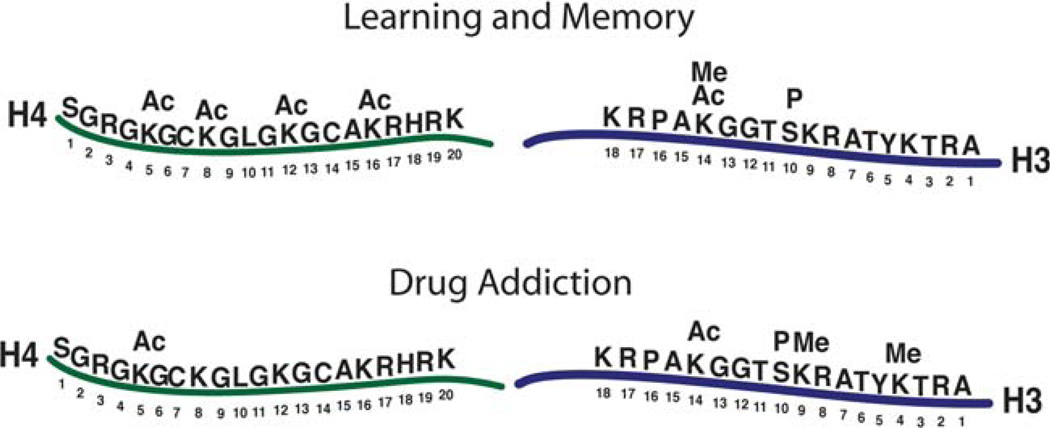
Although still specific, chronic drug exposure has been shown to result in a different pattern of histone modifications from that of acute drug exposure. In response to chronic cocaine exposure, there is no change in histone H4 acetylation at the c-fos promoter, which correlates with a return to basal levels of c-fos expression following chronic drug exposure (Kumar et al. 2005). Furthermore, while acute drug exposure does not change histone H3 acetylation at the fosB promoter, chronic exposure does increase histone H3 acetylation at this locus (Kumar et al. 2005). Interestingly, in response to the same chronic drug exposure, the core histone proteins can be differentially modified at different loci of genes. For example, histone H3 acetylation, but not histone H4 acetylation, increases at the promoters of genes (i.e., cdk5, bdnf, npy) whose expression is induced by chronic cocaine exposure (Freeman et al. 2008; Kumar et al. 2005). Histone H3 acetylation at the promoter of egr-1 has been shown to decrease with cocaine abstinence, which correlates with decreased egr-1 expression (Freeman et al. 2008). Taken together, these results highlight the complexity and specificity of histone modifications triggered by drugs of abuse. Figure 1 illustrates the different residues on the core histone proteins H3 and H4 that have been implicated in regulating gene expression during learning and memory processes as well as following drug exposure.
Fig. 1.
This schematic illustrates the amino terminal tails of two of the core histone proteins, histones H3 and H4, which are the sites of numerous post-translational modifications. Sites marked with a modification (Me methylation, P phosphorylation, Ac acetylation) are those that have been examined with regard to learning and memory processes and drug addiction, respectively
Studying specific changes in histone modifications has been useful in understanding how drugs of abuse can trigger changes in transcription profiles. However, this kind of correlational evidence might be insufficient when trying to assess the biological and behavioral significance of histone modifications (Kouzarides 2007). A more causal relationship between the ability of drugs to trigger changes in histone acetylation and the functional consequences of these changes may be discerned by focusing on the enzymes mediating those post-translational modifications (i.e., HATs and HDACs).
Recent studies have shown that CBP plays an important role in drug-related behaviors. A study by Levine et al. (2005) showed that in response to cocaine CBP is recruited to the promoter of FosB, a gene implicated in the persistence of drug-related behaviors, and positively correlates with histone H4 acetylation and FosB expression. Similarly, it has been reported that acute ethanol increases CBP immunoreactivity at the central and medial (but not basolateral) amygdala where it promotes acetylation of histones H3 and H4 and increases npy expression (Pandey et al. 2008). Furthermore, cbp mutant mice show decreased sensitivity to chronic cocaine, which correlates with decreased CBP occupancy at the FosB promoter and decreased histone H4 acetylation (Levine et al. 2005). Although the role of CBP has been extensively studied with regard to synaptic plasticity and memory, few studies have addressed its role in the mechanisms driving gene expression via histone acetylation required for neuronal and behavioral changes associated with drugs of abuse.
As described above, drugs of abuse can modulate histone acetylation at specific loci. One mechanism by which acetylation can be modulated is by repressing HDAC activity. In support of this view are studies that have shown decreased HDAC activity in response to drug exposure (Pandey et al. 2008; Romieu et al. 2008). Through an enzymatic cascade that involves different Ca2+/calmodulin-dependent kinases, repeated administration of cocaine is associated with increased HDAC5 phosphorylation in the nucleus accumbens (Chawla et al. 2003; Mattson et al. 2005), facilitating its nuclear export and blocking its inhibition of gene expression (Renthal et al. 2007). Conversely, addictive drugs can also recruit HDACs to specific loci and suppress expression of particular genes. HDAC1 seems to be recruited by DeltaFosB (which accumulates with repeated drug exposure) to promote the return of c-fos expression to a basal level, as observed in response to chronic drug treatment (Renthal et al. 2008). Together, these studies provide substantial evidence indicating that drugs of abuse affect the ability of HDACs to modulate gene transcription and, consequently, drug-induced neuroplasticity.
Pharmacological manipulations of HDAC activity have also provided insight on the role of histone acetylation in the development of addictive behavior. Studies using HDAC inhibitors, which produce a hyperacetylated state, have shown that several behavioral effects induced by drugs of abuse are enhanced. It has been demonstrated that administration of an HDAC inhibitor increases cocaine self-administration (Sun et al. 2008) and also enhances the ability of cocaine to produce conditioned place preference (CPP; Kumar et al. 2005; Renthal et al. 2007; Schroeder et al. 2008). These results suggest that similar to observations showing that HDAC inhibition enhances the acquisition of context-shock-associated memories (Vecsey et al. 2007), HDAC inhibition may facilitate acquisition of context-drug memories.
This conclusion has been further supported by elegant experiments involving genetic manipulation of specific HDACs. Overexpression of HDAC4 or HDAC5 (but not HDAC9) in the nucleus accumbens results in a dramatic reduction of cocaine-induced CPP (Renthal et al. 2007). This finding, coupled with the observation that chronic cocaine exposure induces HDAC5 phosphorylation and its nuclear export, provides a potential mechanism by which the expression of HDAC5 target genes are disinhibited (Renthal et al. 2007). Experimental evidence consistent with this potential mechanism has been provided from HDAC5 knockout mice, which show enhanced responses to chronic, but not acute, cocaine administration (Renthal et al. 2007). Similarly, a recent study showed that selective deletion of CaMKIV in the striatum resulted in increased HDAC4 phosphorylation in the striatum as well as enhanced CPP after cocaine administration (Bilbao et al. 2008). Although the exact underlying effect of CaMKIV deletion may be difficult to interpret, phosphorylation of HDAC4 may drive enhanced nuclear export of HDAC4, increasing gene expression contributing to enhanced CPP. Taken together, these results suggest that histone deacetylases modulate the formation and/or consolidation of drug-associated memories.
Interestingly, both genetic and pharmacological manipulations of HATs and HDACs have also revealed a role for histone acetylation in the development of drug-induced behavioral (e.g., locomotor) sensitization. Several HDAC inhibitors have been shown to increase drug-induced sensitization (Kalda et al. 2007; Sanchis-Segura et al. 2009; Shen et al. 2008; but see also Romieu et al. 2008), an enhancement that was also observed in CaMKIV-deficient mice exhibiting increased HDAC4 phosphorylation (Bilbao et al. 2008). These findings are consistent with the observation that the development of cocaine-induced locomotor sensitization is blunted in mice lacking one allele of the CBP gene (Levine et al. 2005). The involvement of HATs and HDACs on behavioral sensitization strengthens the notion that histone acetylation plays a modulatory role in drug-induced changes underlying the development and maintenance of addictive behavior.
In summary, growing evidence shows that drugs of abuse modify HAT and HDAC activity, leading to changes in histone acetylation that modulates expression of genes implicated in addictive behavior. Studies have demonstrated that chromatin modification triggered by drugs of abuse modulate the formation and/or consolidation of drugassociated memories and potentially contribute to the formation and persistence of drug-seeking behaviors. These studies parallel findings in the field of learning and memory in which chromatin modification via histone acetylation is pivotal for long-lasting forms of synaptic plasticity and long-term memory formation. As discussed above for learning and memory, similar mechanisms are involved in the acquisition and extinction of memory. These studies have shown that HDAC inhibitors can facilitate the consolidation of both the initial memory and extinction of the memory. In the next section we examine very recent findings that HDAC inhibitors can facilitate extinction of drug-seeking behavior and significantly attenuate reinstatement of drug-seeking behavior.
Chromatin modification and the extinction of drug-associated memories
As introduced in previous sections of this review, drugs of abuse modify brain motivation and reward pathways as well as learning and memory processes (Everitt and Robbins 2005; Hyman et al. 2006; Kalivas and O’Brien 2008). As drug exposure becomes chronic, more persistent changes in behavior develop. The molecular mechanisms sustaining these maladaptive memories are very persistent and cannot be readily reversed, influencing the individual’s behavior and maintaining a high risk of relapse even after protracted drug abstinence.
As with treatments for anxiety disorders, one approach to treating substance abuse is to use extinction techniques in which the patient learns that the environmental cues or behavioral responses no long produce the substance of abuse (Heather and Bradley 1990; O’Brien et al. 1990; Raw and Russell 1980). Although extinction therapy can reduce conditioned responses elicited by drug-associated cues, these behavioral changes are readily reversed (Conklin and Tiffany 2002). Therefore, it is important to focus on methods that enhance extinction and also prevent the reinstatement of drug-seeking behaviors.
Recent studies have demonstrated that extinction of drug-related behaviors can be enhanced pharmacologically (Taylor et al. 2009). Most of the research has been focused at the synaptic level, more specifically on the role of different glutamatergic receptors in extinction of drug-seeking behavior. The most studied pharmacological treatment has been the partial N-methyl-D-aspartate (NMDA) glutamate receptor agonist D-4-animo-3-isoxazolalidone (D-cycloserine, DCS). DCS facilitates extinction of cocaine- and alcohol-seeking behavior (cocaine: Botreau et al. 2006; Paolone et al. 2009; alcohol: Vengeliene et al. 2008). However, the ability of DCS to prevent the reversal of extinguished drug-seeking behavior is inconsistent (Groblewski et al. 2009; Kelley et al. 2007; Paolone et al. 2009; Thanos et al. 2009; Vengeliene et al. 2008). Although problems exist with using DCS, this line of research has demonstrated the enormous potential of using pharmacological agents to modify extinction learning, which can then be used in conjunction with behavioral therapy. In this regard, a recent study from our lab investigated the role of HDAC inhibitors in facilitating extinction of drug-induced behaviors.
In this review we have summarized the available information suggesting that chromatin modification is involved in different kinds of long-term memory, including drug-related memories. We have recently started to investigate the role of chromatin-modifying enzymes in the extinction of drug-seeking behavior. In a very recent study, we show for the first time that extinction of cocaine-seeking behavior (as measured in the CPP paradigm) was significantly facilitated in mice treated with the HDAC inhibitor sodium butyrate (Malvaez et al. 2009). We found that post-training HDAC inhibition administration resulted in a faster and greater loss of preference for the cocaine-paired context. Moreover, HDAC inhibition modulated extinction in such a way that priming-induced reinstatement of drug seeking was also significantly attenuated. These results demonstrate that both the rate of extinction and its persistence were enhanced by HDAC inhibition. Moreover, these results are consistent with the observation that HDAC inhibition facilitates the consolidation of extinction of fear memories.
The mechanisms underlying the ability of HDAC inhibition to enhance extinction are currently unknown. Extinction is thought to involve new learning while preserving the original memory intact (Bouton 2004; Bouton and Moody 2004). As described above, several studies have suggested that HDAC inhibitors facilitate transcriptional activation during memory consolidation. Therefore, one possibility is that facilitation of extinction via HDAC inhibition promotes the consolidation of new associations formed during extinction. Indeed, increased histone acetylation facilitates long-term memory formation of latent inhibition (Levenson et al. 2004), a phenomenon theoretically linked to extinction (Bouton 2004). In any case, our findings on the ability of HDAC inhibition to facilitate extinction of contextual fear (Bredy et al. 2007; Lattal et al. 2007) and facilitate extinction of drug-seeking behavior (Malvaez et al. 2009) add to a growing body of evidence that indicates that the fundamental mechanisms of gene expression regulation via chromatin modification are involved in long-term synaptic plasticity and long-term memory processes as well as persistent behavioral responses.
Considering the complexity of chromatin modifications, relating a specific chromatin modification to long-lasting changes in behavior is a daunting task. Here we have reviewed evidence indicating that epigenetic regulation is involved in long-term forms of memory processes as well as persistent drug-induced behavioral responses. Chromatin-modifying enzymes such as HATs and HDACs are clearly modulating both initial memory consolidation and memory extinction processes. We believe that these enzymes can serve as potential therapeutic targets for both anxiety and drug abuse disorders.
Acknowledgments
Preparation of this review was supported by grants from the National Institute of Drug Abuse (MAW) and the National Institute of Mental Health (MAW).
Contributor Information
Melissa Malvaez, Department of Neurobiology and Behavior, Center for the Neurobiology of Learning and Memory, University of California, Irvine, Irvine, CA 92697-3800, USA.
Ruth M. Barrett, Department of Neurobiology and Behavior, Center for the Neurobiology of Learning and Memory, University of California, Irvine, Irvine, CA 92697-3800, USA
Marcelo A. Wood, Email: mwood@uci.edu, Department of Neurobiology and Behavior, Center for the Neurobiology of Learning and Memory, University of California, Irvine, Irvine, CA 92697-3800, USA.
Carles Sanchis-Segura, Email: csanchis@psb.uji.es, Area de Psicobiologia, Universitat Jaume I, Castellon, Spain.
References
- Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon JM, Malleret G, Touzani K, Vronskaya S, Ishii S, et al. Chromatin acetylation, memory, and LTP are impaired in CBP +/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42:947–959. doi: 10.1016/j.neuron.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis CD, Jenuwein T, Reinberg D. Epigenetics. illustrated ed. Cold Spring Harbor: CSHL Press; 2007. [Google Scholar]
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Ausio J. Histone variants—the structure behind the function. Brief Funct Genomic Proteomic. 2006;5:228–243. doi: 10.1093/bfgp/ell020. [DOI] [PubMed] [Google Scholar]
- Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger SL, Kouzarides T, Shiekhattar R, Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke JD, Hyman SE. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- Bilbao A, Parkitna JR, Engblom D, Perreau-Lenz S, Sanchis-Segura C, et al. Loss of the Ca2+/calmodulin-dependent protein kinase type IV in dopaminoceptive neurons enhances behavioral effects of cocaine. Proc Natl Acad Sci USA. 2008;105:17549–17554. doi: 10.1073/pnas.0803959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. d-Cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, et al. A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci Biobehav Rev. 2004;28:663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Roze E, Girault JA, Betuing S, Caboche J. Role of the ERK/MSK1 signalling pathway in chromatin remodelling and brain responses to drugs of abuse. J Neurochem. 2009;108:1323–1335. doi: 10.1111/j.1471-4159.2009.05879.x. [DOI] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Hervé D, Darragh J, Corvol JC, Pages C, Arthur SJ, Girault JA, Caboche J. Parsing molecular and behavioral effects of cocaine in mitogen- and stress-activated protein kinase-1-deficient mice. J Neurosci. 2005;25(49):11444–11454. doi: 10.1523/JNEUROSCI.1711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Barad M. The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem. 2008;15:39–45. doi: 10.1101/lm.801108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredy TW, Wu H, Crego C, Zellhoefer J, Sun YE, et al. Histone modifications around individual BDNF gene promoters in prefrontal cortex are associated with extinction of conditioned fear. Learn Mem. 2007;14:268–276. doi: 10.1101/lm.500907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–492. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem. 2003;85:151–159. doi: 10.1046/j.1471-4159.2003.01648.x. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fontan-Lozano A, Romero-Granados R, Troncoso J, Munera A, Delgado-Garcia JM, et al. Histone deacetylase inhibitors improve learning consolidation in young and in KA-induced-neurodegeneration and SAMP-8-mutant mice. Mol Cell Neurosci. 2008;39:193–201. doi: 10.1016/j.mcn.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Fraschini A, Bottone MG, Scovassi AI, Denegri M, Risueno MC, et al. Changes in extranucleolar transcription during actinomycin D-induced apoptosis. Histol Histopathol. 2005;20:107–117. doi: 10.14670/HH-20.107. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Patel KM, Brucklacher RM, Lull ME, Erwin M, et al. Persistent alterations in mesolimbic gene expression with abstinence from cocaine self-administration. Neuropsychopharmacology. 2008;33:1807–1817. doi: 10.1038/sj.npp.1301577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garakani A, Mathew SJ, Charney DS. Neurobiology of anxiety disorders and implications for treatment. Mt Sinai J Med. 2006;73:941–949. [PubMed] [Google Scholar]
- Graff J, Mansuy IM. Epigenetic codes in cognition and behaviour. Behav Brain Res. 2008;192:70–87. doi: 10.1016/j.bbr.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of D-cycloserine on extinction and reconditioning of ethanol-seeking behavior in mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–493. doi: 10.1016/s0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Haig D. The (dual) origin of epigenetics. Cold Spring Harb Symp Quant Biol. 2004;69:67–70. doi: 10.1101/sqb.2004.69.67. [DOI] [PubMed] [Google Scholar]
- Heather N, Bradley BP. Cue exposure as a practical treatment for addictive disorders: why are we waiting? Addict Behav. 1990;15:335–337. doi: 10.1016/0306-4603(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Kalda A, Heidmets LT, Shen HY, Zharkovsky A, Chen JF. Histone deacetylase inhibitors modulate the induction and expression of amphetamine-induced behavioral sensitization partially through an associated learning of the environment in mice. Behav Brain Res. 2007;181:76–84. doi: 10.1016/j.bbr.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. J Neurosci. 1993;13:266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelley JB, Anderson KL, Itzhak Y. Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport. 2007;18:777–780. doi: 10.1097/WNR.0b013e3280c1e2e7. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, et al. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim JS, Shukla SD. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 2006;41:126–132. doi: 10.1093/alcalc/agh248. [DOI] [PubMed] [Google Scholar]
- Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Lattal KM, Stafford JM. What does it take to demonstrate memory erasure? Theoretical comment on Norrholm et al. (2008) Behav Neurosci. 2008;122:1186–1190. doi: 10.1037/a0012993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Radulovic J, Lukowiak K. Extinction: [corrected] does it or doesn’t it? The requirement of altered gene activity and new protein synthesis. Biol Psychiatry. 2006;60:344–351. doi: 10.1016/j.biopsych.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Barrett RM, Wood MA. Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci. 2007;121:1125–1131. doi: 10.1037/0735-7044.121.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Sweatt JD. Epigenetic mechanisms in memory formation. Nat Rev Neurosci. 2005;6:108–118. doi: 10.1038/nrn1604. [DOI] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, et al. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levenson JM, Roth TL, Lubin FD, Miller CA, Huang IC, et al. Evidence that DNA (cytosine-5) methyltransferase regulates synaptic plasticity in the hippocampus. J Biol Chem. 2006;281:15763–15773. doi: 10.1074/jbc.M511767200. [DOI] [PubMed] [Google Scholar]
- Levine AA, Guan Z, Barco A, Xu S, Kandel ER, et al. CREB-binding protein controls response to cocaine by acetylating histones at the fosB promoter in the mouse striatum. Proc Natl Acad Sci USA. 2005;102:19186–19191. doi: 10.1073/pnas.0509735102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of histone modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.07.032. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology. 2008;33:3–17. doi: 10.1038/sj.npp.1301544. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284:1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28:456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Mickley GA, Hoxha Z, Bacik S, Kenmuir CL, Wellman JA, et al. Spontaneous recovery of a conditioned taste aversion differentially alters extinction-induced changes in c-Fos protein expression in rat amygdala and neocortex. Brain Res. 2007;1152:139–157. doi: 10.1016/j.brainres.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–869. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Diefenbach GJ, Tolin DF. Quality of life and anxiety and depressive disorder comorbidity. J Anxiety Disord. 2008a;22:1516–1522. doi: 10.1016/j.janxdis.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008b;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, et al. Truncated CBP protein leads to classical Rubinstein-Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism. Hum Mol Genet. 1999;8:387–396. doi: 10.1093/hmg/8.3.387. [DOI] [PubMed] [Google Scholar]
- Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn Mem. 2007;14:564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. An investigation of the physiological activity of the cerebral cortex. London: Oxford University Press; 1927. Conditioned reflexes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM. A model for stimulus generalization in Pavlovian conditioning. Psychol Rev. 1987;94:61–73. [PubMed] [Google Scholar]
- Raw M, Russell MA. Rapid smoking, cue exposure and support in the modification of smoking. Behav Res Ther. 1980;18:363–372. doi: 10.1016/0005-7967(80)90001-7. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renthal W, Maze I, Krishnan V, Covington HE, III, Xiao G, et al. Histone deacetylase 5 epigenetically controls behavioral adaptations to chronic emotional stimuli. Neuron. 2007;56:517–529. doi: 10.1016/j.neuron.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Renthal W, Carle TL, Maze I, Covington HE, III, Truong HT, et al. Delta FosB mediates epigenetic desensitization of the c-fos gene after chronic amphetamine exposure. J Neurosci. 2008;28:7344–7349. doi: 10.1523/JNEUROSCI.1043-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction. 2000;95 Suppl 2:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- Romieu P, Host L, Gobaille S, Sandner G, Aunis D, et al. Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats. J Neurosci. 2008;28:9342–9348. doi: 10.1523/JNEUROSCI.0379-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Saha A, Wittmeyer J, Cairns BR. Chromatin remodelling: the industrial revolution of DNA around histones. Nat Rev Mol Cell Biol. 2006a;7:437–447. doi: 10.1038/nrm1945. [DOI] [PubMed] [Google Scholar]
- Saha A, Wittmeyer J, Cairns BR. Mechanisms for nucleosome movement by ATP-dependent chromatin remodeling complexes. Results Probl Cell Differ. 2006b;41:127–148. doi: 10.1007/400_005. [DOI] [PubMed] [Google Scholar]
- Sanchis-Segura C, Lopez-Atalaya JP, Barco A. Selective boosting of transcriptional and behavioral responses to drugs of abuse by histone deacetylase inhibition. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.125. [DOI] [PubMed] [Google Scholar]
- Schroeder FA, Penta KL, Matevossian A, Jones SR, Konradi C, et al. Drug-induced activation of dopamine D(1) receptor signaling and inhibition of class I/II histone deacetylase induce chromatin remodeling in reward circuitry and modulate cocaine-related behaviors. Neuropsychopharmacology. 2008;33:2981–2992. doi: 10.1038/npp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer SL. Recent advances in the understanding and treatment of anxiety disorders. Prim Care. 2007;34:475–504. doi: 10.1016/j.pop.2007.05.002. v–vi. [DOI] [PubMed] [Google Scholar]
- Shen HY, Kalda A, Yu L, Ferrara J, Zhu J, et al. Additive effects of histone deacetylase inhibitors and amphetamine on histone H4 acetylation, cAMP responsive element binding protein phosphorylation and DeltaFosB expression in the striatum and locomotor sensitization in mice. Neuroscience. 2008;157:644–655. doi: 10.1016/j.neuroscience.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Shim D, Kang HY, Jeon BW, Kang SS, Chang SI, et al. Protein kinase B inhibits apoptosis induced by actinomycin D in ECV304 cells through phosphorylation of caspase 8. Arch Biochem Biophys. 2004;425:214–220. doi: 10.1016/j.abb.2004.03.028. [DOI] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proc Natl Acad Sci USA. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, Murphy TK, Lewis MH, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang L, Jiang B, Hui B, Lv Z, et al. The effects of sodium butyrate, an inhibitor of histone deacetylase, on the cocaine- and sucrose-maintained self-administration in rats. Neurosci Lett. 2008;441:72–76. doi: 10.1016/j.neulet.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14:1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56 Suppl 1:186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Bermeo C, Wang GJ, Volkow ND. D-cycloserine accelerates the extinction of cocaine-induced conditioned place preference in C57bL/c mice. Behav Brain Res. 2009;199:345–349. doi: 10.1016/j.bbr.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, et al. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB: CBP-dependent transcriptional activation. J Neurosci. 2007;27:6128–6140. doi: 10.1523/JNEUROSCI.0296-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Vianna MR, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiol Learn Mem. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. [DOI] [PubMed] [Google Scholar]
- Waddington CH. The epigenotype. Endeavor. 1942;1:18–20. [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intraamygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, et al. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Attner MA, Oliveira AM, Brindle PK, Abel T. A transcription factor-binding domain of the coactivator CBP is essential for long-term memory and the expression of specific target genes. Learn Mem. 2006;13:609–617. doi: 10.1101/lm.213906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-cycloserine facilitates extinction but does not eliminate renewal of the conditioned emotional response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol. 2004;65:1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]