Abstract
Transforming growth factor-β (TGF-β), a potent inducer of collagen synthesis, is implicated in fibrosis. Peroxisome proliferator-activated receptor-γ (PPAR-γ) is a nuclear hormone receptor that regulates adipogenesis and is recognized as a pleiotropic transcription factor. We demonstrated previously that activation of PPAR-γ by natural and pharmacologic ligands abrogated the stimulation of collagen gene expression induced by TGF-β in skin fibroblasts. The goal of this study was to characterize the physiologic role of endogenous PPAR-γ in regulation of TGF-β signaling and collagen gene expression. We found that basal collagen gene expression was markedly elevated in mouse embryonic fibroblasts (MEFs) lacking PPAR-γ and PPAR-γ ligand 15d-PGJ2 failed to reduce the elevated levels of collagen. Reconstitution of PPAR-γ null MEFs with ectopic PPAR-γ resulted in down-regulation of COL1A2 promoter activity. In contrast to control MEFs, PPAR-γ null MEFs displayed elevated expression of Type I TGF-β receptor TβRI, and produced more TGF-β1. Furthermore, PPAR-γ null MEFs showed Smad2 and Smad3 phosphorylation even in the absence of stimulation by exogenous TGF-β. Constitutive Smad2/3 phosphorylation in PPAR-γ null MEFs was associated with ligand-independent interaction of Smad3 with its cognate DNA recognition site and with p300, a coactivator previously implicated in mediating TGF-β responses. These results indicate that absence of PPAR-γ in MEFs is associated with constitutive up-regulation of collagen gene expression and Smad activation, at least in part, due to autocrine TGF-β stimulation. Importantly, in scleroderma skin fibroblasts, the levels of PPAR-γ were significantly less compared to healthy controls. Therefore, endogenous PPAR-γ may have a physiologic role in controlling Smad-dependent Type I collagen gene expression in fibroblasts and in tissue homeostasis.
INTRODUCTION
The synthesis of Type I collagen is stimulated by transforming growth factor-beta (TGF-β), and abnormal TGF-β signaling is implicated in the pathogenesis of fibrosis [1]. Transforming growth factor-β signals from cell surface receptors to the nucleus via both Smad and non-Smad pathways. Upon TGF-β receptor activation, cytoplasmic Smad2 and Smad3 heterodimerize with Smad4, translocate into the nucleus, and activate transcription through direct interaction with Smad-binding elements in appropriate target genes [2]. We have previously demonstrated that TGF-β stimulation of Type I collagen (COL1A2) transcription in fibroblasts requires cellular Smad3, and is abrogated by ectopically expressed Smad7 [3,4]. Furthermore, the interaction of activated Smad3 with the acetyltransferase p300 coactivator is essential for optimal TGF-β responses [5-8].
Peroxisome proliferator-activated receptor-gamma (PPAR-γ) is a nuclear hormone receptor and transcription factor that is essential for normal adipogenesis [9]. PPAR-γ is implicated in diabetes, glomerulosclerosis, and atherosclerosis [10-13]. Endogenous lipids and eicosanoids such as 15-deoxy-Δ12, 4 prostaglandin J2 (15d-PGJ2) acts as natural ligand for PPAR-γ. Activation of PPAR-γ can also be induced by thiazolidinedione class of anti-diabetic drugs such as troglitazone and activated PPAR-γ can enhance or repress transcription, depending upon the target gene and cell type [14].
We previously reported that in normal skin fibroblasts, PPAR-γ ligands abrogated the stimulation of collagen synthesis and α-smooth muscle actin expression induced by TGF-β [15]. Ectopic expression of PPAR-γ in these cells elicited a similar response. To determine whether ligand-induced suppression of collagen synthesis was PPAR-γ dependent or independent, we examined the regulation of collagen gene expression in mouse embryonic fibroblasts (MEFs) lacking PPAR-γ. Surprisingly, we observed that the level of Type I collagen was ~6-fold higher compared to heterozygous control and PPAR-γ ligands failed to suppress that increased collagen synthesis in the absence of PPAR-γ. Based on these observations, we further studied the molecular basis of upregulation of basal Type I collagen gene expression in this PPAR-γ null MEFs. Here, we report that MEFs lacking PPAR-γ constitutively displayed elevated collagen gene expression and PPAR-γ ligand 15d-PGJ2 failed to inhibit the elevated collagen synthesis in these cells. Forced reconstitution of ectopic PPAR-γ in these cells caused down-regulation of collagen synthesis. PPAR-γ null MEFs showed autocrine TGF-β stimulation with enhanced TGF-β production and TGF-β receptor expression. Furthermore, PPAR-γ null MEFs showed constitutive activation of endogenous Smad2/3 and its interaction with p300 in the absence of exogenous ligand. Activation of the Smad signal transduction pathway is associated with consequent up-regulation of collagen gene expression in PPAR-γ null MEFs in the absence of exogenous ligand. The parallel features of PPAR-γ null MEFs were seen in scleroderma skin fibroblasts where increased autocrine TGF-β signaling is associated with elevated collagen synthesis. Interestingly, the levels of PPAR-γ mRNA were significantly less in scleroderma patients compared to healthy controls. Together, these results suggest the existence of autocrine TGF-β signaling in PPAR-γ null MEFs and that endogenous PPAR-γ may have an important physiological role in modulating the intensity of intracellular TGF-β signaling and target gene expression.
MATERIALS AND METHODS
Reagents
Transforming growth factor-β2 (Genzyme, Framingham, MA), 15-deoxy-Δ(12,14) prostaglandin J2 (15d-PGJ2) (both from Biomol, Plymouth Meeting, PA), and SB431542 (GlaxoSmithKline, King of Prusia, PA) were used.
Cell culture
Mouse embryonic fibroblasts (MEFs) (gift of E. D. Rosen, Beth Israel Deacones Medical Center, Boston, MA) were established from PPAR-γ-floxed mouse embryos as described [9]. These MEFs were maintained in Dulbecco's Modified Eagle's Medium (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum, 1% vitamins, 1% penicillin/ streptomycin and 2 mM L-glutamine. The skin fibroblasts derived from scleroderma patients and healthy volunteers were established from biopsy specimens. All skin biopsies were performed following the rules of Institutional Review Board for Human studies. The skin fibroblasts were maintained in Eagle's Minimal Essential Medium (EMEM) supplemented with10% fetal bovine serum, 1% vitamins, 1% penicillin/ streptomycin and 2 mM L-glutamine.
Plasmids
The 772COL1A2-CAT reporter construct contains the -772 to +58 bp fragment of the human COL1A2 gene in front of chloramphenicol acetyltransferase (CAT) gene [16]. The expression vector pCMX-mPPAR-γ (from C.K. Glass, University of California, San Diego, CA) contains the full-length mouse PPAR-γ1 cDNA under the CMV promoter [10].
Transient transfection assay
At early confluence, MEFs were transiently transfected with 772COL1A2-CAT (1.25 μg/well), mPPAR-γ expression vector and empty vector along with pRLTK-luc as internal standard using Superfect Reagent (Qiagen, Valencia, CA). Transfected MEFs were pretreated with 15d-PGJ2 (10 μM) or DMSO for 60 min before the addition of TGF-β (12.5 ng/ml). Following further 48 h incubation, cultures were harvested, and whole cell lysates were assayed for their CAT activities by phase extraction procedure as described [3]. Results were normalized with protein concentration, and transfection efficiency was monitored by measuring renilla luciferase activity in each sample.
Western analysis
Whole cell lysates were prepared from MEFs using M-Per Mammalian Protein Extraction Buffer (Pierce, Rockford, IL). Equal amounts of proteins or aliquots of conditioned media were resolved by electrophoresis in 4-15% Tris-Glycine gradient gels (BIORAD, Hercules, CA), and membranes were immunoblotted with antibodies to human Type I collagen (Southern Biotechnology, Birmingham, AL), PPAR-γ (E-8), Smad7 (N-19), Smad4 (B8), Smad1/2/3 (H2), p300 (C-20) or actin (C-2) (all from Santa Cruz Biotech, Santa Cruz, CA ), TβRI, phospho-Smad2 or phospho-Smad 3 (Cell Signaling, Beverly, MA).
Quantitative PCR
Total RNA was extracted from PPAR-γ heterozygous and PPAR-γ null MEFs, SSc and healthy skin fibroblasts with TRIZOL reagent (Invitrogen Corp. Carlsbad, CA), and total RNA (1.0 mg) was reverse transcribed for RT-PCR analysis using Superscript III first Strand Synthesis System (Invitrogen, Carlsbad, CA). Real time PCR was performed with SYBR®GREEN Master Mix using ABI Prism 7500 sequence detector and SDS analysis software (PE Applied Biosystems, Forster City, CA). The following primers were used for COL1A1: F: 5’- GACGTCCTGGTGAAGTTGGT-3’; R: 5’ CAGGGAAGCCTCTTTCTCCT-3’. TGF-β1: F: 5’TACAGCAAGGTCCTTGCCCT-3’; R: 5’- GCAGCACGGTGACGCC-3’. PPAR-γ: F: 5'-GTCAAACGAGAGTCAGCC TTTAACG-3'; R: 5'-CCACGGAGCTGATCCCAA-3'. mGAPDH was amplified in parallel as an internal standard. Expression values were calculated using ΔCt method, normalized with endogenous GAPDH mRNA levels.
ELISA
At confluence, media from PPAR-γ null and PPAR-γ heterozygous MEFs was changed and after 8 h the levels of activated TGF-β1 in supernatants were determined by ELISA (R&D, Minneapolis, MN) according to the manufacturer's instruction.
DNA Affinity Precipitation Assays
The accumulation of Smad2/3 and p300 on the consensus Smad-binding element (SBE) was evaluated by DNA affinity precipitation assay (DAPA) as described [7]. Briefly, MEFs were incubated with TGF-β for 60 min, nuclear extracts were prepared, and equal amount of proteins (~200 μg) were incubated with biotin-labeled double-stranded deoxyoligonucleotides (3 μg) harboring the SBE sequences. After 30 min, 40 μl of streptavidin-agarose beads (4%) with 50% slurry (Sigma, St Louis, MO) was added, and mixtures were incubated at 4°C for 45 min on a rotator. The streptavidin-agarose beads were then precipitated by centrifugation, pellets were washed with cold PBS, bead-bound proteins were boiled in SDS-loading buffer, separated in 4-20% denatured gels and processed for immunoblot analysis using antibodies against phospho-Smad3, Smad4 or p300.
Immunofluorescence
PPAR-γ heterozygous and PPAR-γ null MEFs were seeded on coverslips were incubated with DMEM containing 10% FBS and TGF-β for 60 min. Cultures were washed in PBS and fixed in 4% paraformaldehyde for 30 min, followed by incubation with 0.25% Triton X-100 for 10 min and washing in PBS, and then stained with antibodies specific for Smad1/2/3 (Santa Cruz) or isotype control antibody. FITC-labeled anti-mouse IgG antibody was used as secondary antibody and nuclei were identified with 4'-6-Diamidino-2-phenylindole (DAPI). Images were obtained by digital capture in a Nikon Eclipse TE200 microscope (Fryer Company, Huntley, IL) and viewed under 200 X magnification.
Statistical analysis
The data were presented as means ± SD. The significance of differences between experimental or treated groups and control groups were determined by analysis of variance (ANOVA) and the value of p<0.05 by student t-test was considered statistically significant. Student t-tests were performed using GraphPad t-test calculator.
RESULTS
Elevated Type I collagen gene expression in PPAR-γ null MEFs
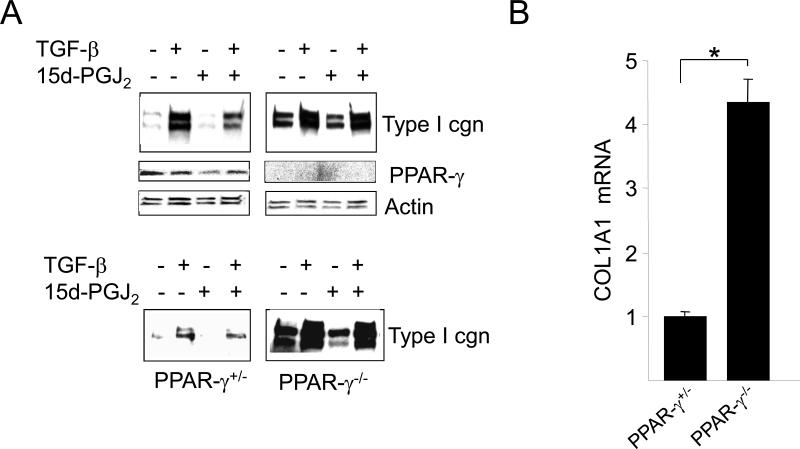
In order to study the contribution of cellular PPAR-γ in physiologic regulation of collagen gene expression, we pursued a loss-of-function approach using cells lacking PPAR-γ. Confluent cultures of PPAR-γ null MEFs and heterozygous control MEFs in parallel were pretreated with 15d-PGJ2 or DMSO for 60 min followed by TGF-β. At the end of 48 h incubation, cultures were harvested and whole cell lysates and supernatants were subjected to Western analysis. The results revealed that basal collagen production was substantially higher in PPAR-γ-null MEFs (Fig. 1A). Consistent with these findings, qPCR analysis indicated that levels of COL1A1 mRNA were >3-fold higher in PPAR-γ null MEFs compared to heterozygous MEFs (Fig. 1B). TGF-β caused a significant increase in the levels of intracellular, as well as secreted, Type I collagen in both heterozygous and PPAR-γ null MEFs. 15d-PGJ2 suppressed TGF-β-induced Type I collagen synthesis in heterozygous PPAR-γ control MEFs as expected. However, 15d-PGJ2 failed to inhibit TGF-β-induced Type I collagen synthesis in PPAR-γ null MEFs (Fig. 1A). Absence of PPAR-γ expression in PPAR-γ null MEFs was confirmed by Western blot analysis (Fig. 1A) as well as by RT-PCR (data not shown).
Figure 1. Elevated Collagen gene expression in PPAR-γ null MEFs.
Confluent PPAR-γ null and PPAR-γ heterozygous murine embryonic fibroblasts (MEF) were pretreated with 15d-PGJ2 (10 μM) for 60 min following incubation with TGF-β (12.5 ng/ml) for 48 h. A. Whole cell lysates (upper panels) and culture supernatants (lower panels) were examined by Western analysis. Representative immunoblots are shown. B. Total RNA was subjected to real-time qPCR analysis. The levels of COL1A1 mRNA are normalized to the GAPDH mRNA in each sample. The results represent the means ± SD of triplicate determinations. * denotes p<0.05
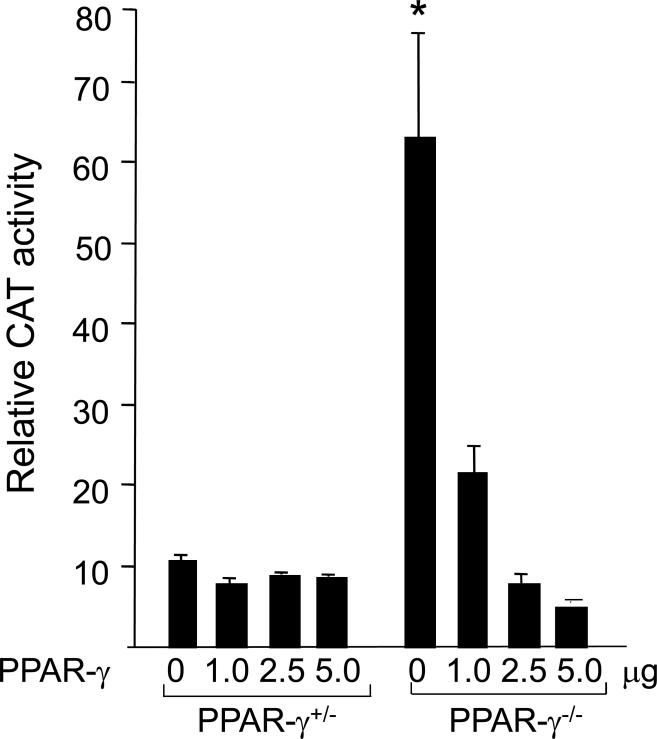
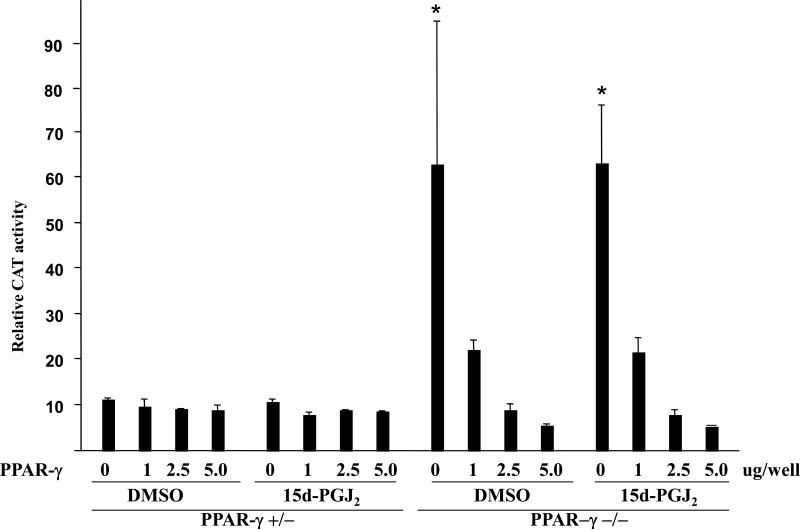
The regulation of collagen gene expression by PPAR-γ ligands was examined in transient transfection assays. Heterozygous and PPAR-γ null MEFs in parallel were cotransfected with 772COL1A2-CAT reporter construct and PPAR-γ expression vector. Following 24 h incubation with or without 15d-PGJ2, MEFs were harvested and cell lysates were assayed for their CAT activities. The results from multiple transfection assays indicated that the activity of the COL1A2 promoter was markedly elevated (~6-fold) in PPAR-γ null MEFs as was found for endogenous gene expression (Fig. 2). These results suggest that endogenous PPAR-γ is a repressor of collagen synthesis and regulation is at the level of transcription.
Figure 2. Ectopic PPAR-γ down-regulates basal COL1A2 transcription in PPAR-γ null MEFs.
PPAR-γ heterozygous and PPAR-γ null MEFs transiently transfected with 772COL1A2-CAT and PPAR-γ expression vector were treated with 15d-PGJ2 or DMSO for 60 min. Following 24 h incubation, cell lysates were prepared and assayed for their CAT activities. The results shown represent the means ± S.D. of triplicate determinations. * denotes p< 0.05.
Based upon these observations, we would predict that ectopic PPAR-γ might attenuate constitutively elevated COL1A2 promoter activity observed in PPAR-γ null MEFs, as well as restore their ability to respond to PPAR-γ ligands. The results from multiple assays showed that ectopic PPAR-γ had no effect on the basal COL1A2 promoter activity in heterozygous PPAR-γ MEFs. In contrast, ectopic PPAR-γ suppressed elevated collagen gene expression in PPAR-γ null MEFs in presence and absence of PPAR-γ ligand, 15d-PGJ2. The inhibitory effect was dose-dependent, with CAT activities reduced to the levels comparable to those noted in heterozygous control MEFs (Fig. 2). Therefore, lack of endogenous PPAR-γ largely accounted for constitutively elevated basal collagen synthesis in PPAR-γ null MEFs.
Autocrine TGF-β stimulation and exogenous ligand-independent Smad activation in PPAR-γ null MEFs
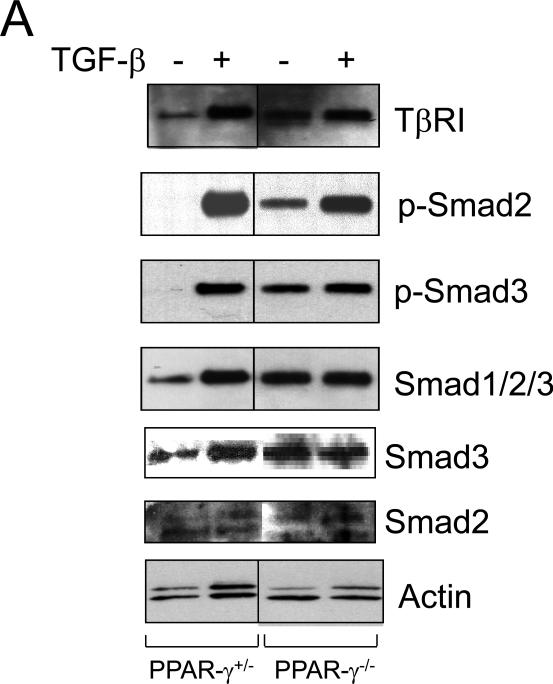
As recent reports demonstrated that activation of PPAR-γ caused suppression of TGF-β receptor and or TGF-β [17,18], we wondered whether elevated levels of Type I collagen was also due to increased expression of the Type I TGF-β receptor (TβR1), or increased endogenous production of TGF-β in PPAR-γ null MEFs. In order to address this issue, the level of TβRI was measured in PPAR-γ null MEFs and control MEFs. Western analysis showed that levels of TβRI were constitutively increased >2-fold in PPAR-γ null MEFs compared to control MEFs (Fig. 3A, upper panel). As expected, TβRI expression was stimulated by TGF-β in both PPAR-γ heterozygous and PPAR-γ null MEFs.
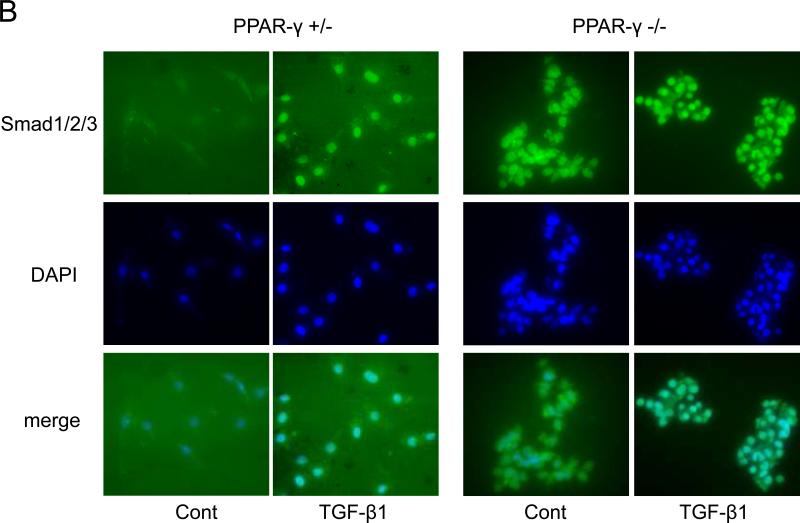
Figure 3. Elevated TβR1 levels and constitutive activation of endogenous Smad2/3 in PPAR-γ null MEFs.
A. Confluent PPAR-γ heterozygous and null PPAR-γ MEFs were incubated with TGF-β (12.5 ng/ml). After 23 h, cultures were harvested and whole cell lysates were examined by Western analysis. Representative immunoblots are shown. B. PPAR-γ heterozygous MEFs and PPAR-γ null MEFs were incubated with TGF-β and immunostained with Smad1/2/3 antibodies, and examined by fluorescent microscopy. Immunostained with Smad1/2/3, DAPI and merge images were shown.
We and others have shown previously that TGF-β stimulation of collagen gene expression required ALK5-dependent Smad3 phosphorylation and downstream Smad signal transduction [3,5,19-21]. To seek the mechanistic basis for constitutive collagen gene upregulation in the absence of endogenous PPAR-γ, we examined individual steps in the TGF-β/Smad signaling cascade upstream of collagen gene regulation. To evaluate alterations in Smad activity, PPAR-γ heterozygous and PPAR-γ null MEFs were incubated with TGF-β for 24 h, and Smad2/3 phosphorylation was examined in whole cell lysates. The results of Western analysis showed that in comparison to control MEFs, in MEFs lacking cellular PPAR-γ, endogenous Smad2 and Smad3 showed constitutive phosphorylation even in the absence of exogenous TGF-β, whereas levels of total Smad2 and Smad3 were comparable to control MEFs (Fig. 3A, lower panel). As expected, TGF-β induced phosphorylation of Smad2/3 in both PPAR-γ control MEFs and PPAR-γ null MEFs. To determine if constitutive Smad2/3 phosphorylation in PPAR-γ null MEFs was associated with nuclear accumulation, the subcellular distribution of Smad2/3 was examined by immunofluorescence. As shown in Fig. 3B, endogenous Smad2/3 were largely cytoplasmic in PPAR-γ heterozygous MEFs, and TGF-β induced their nuclear accumulation, as expected (Fig. 3B). In contrast, Smad2/3 showed constitutive nuclear accumulations in PPAR-γ null MEFs even in the absence of exogenous TGF-β.
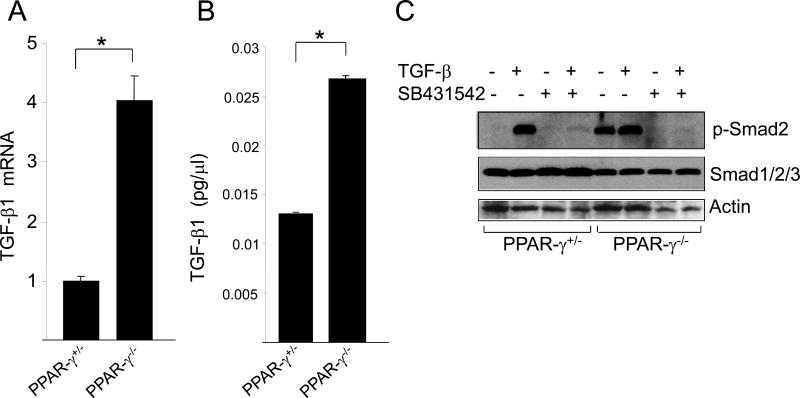
Constitutive Smad2/3 activation seen in PPAR-γ null MEFs may be due to autocrine stimulation of these cells by endogenous TGF-β. To address this possibility, TGF-β1 production and expression of TGF-β1 mRNA was examined. Results of qPCR analysis indicated that the levels of TGF-β1 mRNA were 4-fold higher in PPAR-γ null MEFs compared to PPAR-γ heterozygous control MEFs (Fig. 4A). The levels of secreted TGF-β1 determined by ELISA showed a 2-fold increase in supernatants from PPAR-γ null MEF cultures (Fig. 4B). Therefore, constitutive Smad2/3 activation in PPAR-γ null MEFs may reflect autocrine stimulation due to increased endogenous production of TGF-β, and or increased expression of the Type I TGF-β receptor (TβR1), in these cells.
Figure 4. Elevated TGF-β1 expression in PPAR-γ null MEFs.
A. Total RNA from confluent cultures of PPAR-γ heterozygous MEFs and null PPAR-γ MEFs was subjected to qPCR analysis TGF-β and normalized with the levels of GAPDH mRNA. The results represent the Mean ± SD of triplicate. * denotes p< 0.05. B. PPAR-γ heterozygous MEFs and PPAR-γ null MEFs were grown in culture and supernatants were harvested and the levels of TGF-β secreted into the media were determined by ELISA. C. Confluent cultures PPAR-γ heterozygous and PPAR-γ null MEFs were pretreated with SB431542 (10 μM) for 60 min followed by TGF-β, and after a further 23 h incubation, cell lysates were subjected to Western analysis. Representative immunoblots are shown.
To confirm the role of autocrine TGF-β stimulation in constitutive phosphorylation of Smad2/3 observed in PPAR-γ null MEFs, we examined the modulation of Smad2 activation in the presence of TβR1 kinase blockade. For this purpose, PPAR-γ heterozygous and PPAR-γ null MEFs were pretreated for 60 min with SB431542, a specific inhibitor of TβRI kinase activity and downstream Smad2/3 dependent signaling [22,23]. Cultures were harvested following incubation with TGF-β for 23 h, and whole cell lysates were subjected to Western analysis. The results showed that as expected, SB431542 blocked TGF-β-induced phosphorylation of Smad2 in PPAR-γ heterozygous MEFs (Fig. 4C). Importantly, SB431542 completely abrogated constitutive Smad2 phosphorylation in PPAR-γ null MEFs, suggesting that TβRI kinase activity was responsible for Smad2 activation observed in PPAR-γ null MEFs in the absence of exogenous ligand (Fig. 4C).
Constitutive Smad2/3 interaction with SBE and p300 accumulation in PPAR-γ null MEF in the absence of exogenous ligand
The transcriptional coactivator and histone acetyltransferase p300 has important roles in regulation of genes involved in cell proliferation, apoptosis and fibrosis, and is known to be intimately involved in TGF-β responses [24, 25]. We have shown previously that ligand-induced Smad2/3 interaction with p300 and consequent p300 recruitment to the Smad-binding element of the COL1A2 promoter was required for TGF-β stimulation of collagen synthesis [6,7]. The observation that PPAR-γ null MEFs showed constitutive Smad2/3 phosphorylation led us to consider whether elevated expression of collagen, a target gene for Smad2/3, in these cells could be due to constitutive interaction of Smad2/3 with the SBE and accumulation of p300 in that Smad complex. To address this question, the accumulation of Smad2/3 on the probe, and recruitment of endogenous p300 to the DNA-bound Smad2/3 transcriptional complex was examined by DNA affinity precipitation assays (DAPA). For this purpose, confluent MEFs were incubated with TGF-β for 60 min, and equal amounts of nuclear extracts were subjected to DAPA using oligonucleotide probes harboring the consensus Smad binding element SBE. The results showed that in PPAR-γ control MEFs, TGF-β enhanced the accumulation of phospho-Smad3 on its cognate binding site (Fig. 5) as shown previously in normal fibroblasts [6,7]. In marked contrast to control MEFs, phospho-Smad3 was found to be bound to SBE in a ligand-independent manner in PPAR-γ null MEFs (Fig. 5). Furthermore, TGF-β enhanced the accumulation of p300 in the Smad3-SBE complex in PPAR-γ heterozygous MEFs, whereas constitutive p300 accumulation on Smad3-SBE complex was seen in PPAR-γ null MEFs in the absence of exogenous ligand.
Figure 5. Constitutive phospho-Smad3 and p300 accumulation on SBE in PPAR-γ null MEFs.
PPAR-γ heterozygous and PPAR-γ null MEFs in parallel were incubated with or without TGF-β for 60 min. Nuclear extracts were subjected to DNA affinity precipitation assays using biotin-labeled oligodeoxynucleotides harboring Smad binding element (SBE) as probes.
Decreased levels of PPAR-γ expression in SSc skin fibroblasts
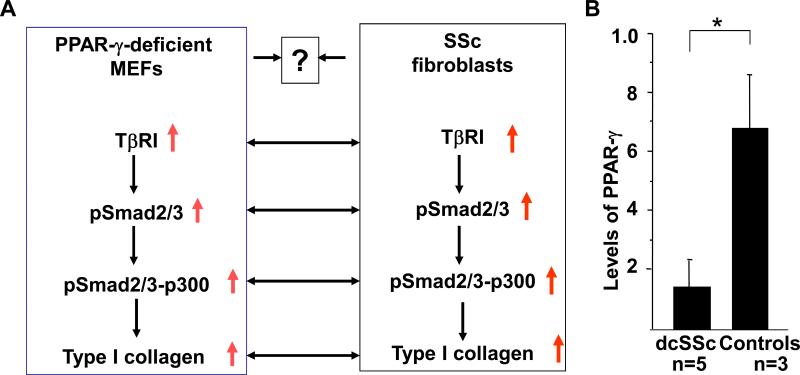
As PPAR-γ null MEFs possess many characteristics of SSc skin fibroblasts in the light of elevated TGF-β signaling, constitutive Smad activation, p300 recruitment in Smad containing transcriptional complex and elevated collagen synthesis, we asked whether SSc phenotype is due lack of PPAR-γ in fibroblasts (Fig. 6A). To address this issue, skin fibroblasts derived from SSc lesional skin and healthy controls were grown in culture and total RNA was extracted. The amount of PPAR-g mRNA was determined in SSc and healthy controls by quantitative PCR. The results showed that the levels of PPAR-γ mRNA was significantly decreased in SSc fibroblasts compared to healthy controls (Fig. 6B) indicating lack of PPAR-γ in SSc skin fibroblasts may be associated increased profibrotic responses.
Figure 6. PPAR-γ expression in SSc fibroblasts and parallelism between PPAR-γ null MEFs and SSc fibroblasts.
A. Lack of PPAR-γ may be associated with SSc phenotype. B. The total RNA was extracted from lesional SSc fibroblasts (five) and healthy control fibroblasts (three). The amount of PPAR-γ were determined in SSc and healthy skin fibroblasts by qPCR using PPAR-γ specific primers. The levels of β-actin mRNA were measured and used as control. Data represents means ± SD of triplicate determinations. * denotes p<0.05.
DISCUSSIONS
The nuclear hormone receptor PPAR-γ is intimately involved in adipogenesis and glucose homeostasis [26]. Like other nuclear receptors, PPAR-γ also functions as a ligand-dependent transcription factor that specifically recognizes conserved sequences [13]. Activation of PPAR-γ is involved in regulating the expression of multiple target genes [27]. Recent studies have identified a novel role of PPAR-γ in the regulation of collagen gene expression and in connective tissue homeostasis [28]. We and others have shown that PPAR-γ activation by troglitazone or 15d-PGJ2 in mesangial cells and fibroblasts results in inhibition of collagen synthesis, or abrogation of stimulation of collagen gene expression induced by TGF-β [15, 29-31].
In order to test whether ligand-induced suppression of TGF-β-induced collagen gene expression was PPAR-γ dependent, we undertook a loss-of function approach using PPAR-γ null MEFs and results showed that PPAR-γ ligand failed to inhibit the elevated levels of collagen gene expression in the absence of PPAR-γ. Interestingly, the basal level of collagen gene expression was very high in the PPAR-γ null MEFs compared to controls. The aim of the present report was to establish the molecular mechanism involved in the upregulation of basal collagen gene expression in PPAR-γ null MEFs.
Present study demonstrates that genetic deletion of PPAR-γ in MEFs causes an increased synthesis of Type I collagen and upregulation is due to increased transcription. This activation is associated with the lack of PPAR-γ because ectopic PPAR-γ dose-dependently suppresses collagen gene expression suggesting cellular PPAR-γ is a natural repressor of collagen synthesis. As increased TGF-β1 and TβRI are involved in upregulation of collagen synthesis and recent studies indicate that PPAR-γ is a suppressor of TGF-β1/TβRI [17,18], we wondered whether several fold elevation of collagen gene expression in PPAR-γ null MEFs were only due to lack of PPAR-γ or also due to elevation of autocrine TGF-β signaling. To address this issue we determined the levels of TβRI and TGF-β1 levels in PPAR-γ null MEFs and in heterozygous control. Indeed, the levels of TβRI and TGF-β1 are significantly elevated in the absence of PPAR-γ. These findings are in agreement with recent observations where treatment of mouse fibroblasts with 15d-PGJ2, a ligand of PPAR-γ, causes suppression of TGF-β1 gene expression in a PPAR-γ dependent manner [17]. Similarly, the activation of PPAR-γ by ligands also leads to suppression of TβRI and TβRII expression [18] which indicates that PPAR-γ negatively regulates TGF-β signaling and thus affects the downstream target genes like Type I collagen.
Type I collagen is the major extracellular matrix protein in skin and other tissues. While a physiological level of collagen in the matrix is necessary to maintain the tissue homeostasis, deregulated excessive synthesis and accumulation of collagen causes fibrosis in systemic sclerosis (SSc) [1]. The pleiotropic cytokine TGF-β is known to play a major role in the activation of fibroblasts and induction of collagen synthesis during wound healing [32]. Deregulation in this pathway leads to excessive collagen synthesis and tissue fibrosis. TGF-β transduces its signals via Smad-dependent and non-Smad pathways [33]. As TβRI and TGF-β1 are elevated in PPAR-γ null MEFs [present study] and TGF-β-induced collagen synthesis in fibroblasts is Smad dependent [3], we reasoned to study the levels and activity of TGF-β signaling molecules Smads in this cell line. Remarkably, the Smad2/3 are constitutively phosphorylated and located predominantly in the nucleus of cells lacking PPAR-γ. This activation of Smads is due to increased levels of TβRI kinase and activated TGF-β in PPAR-γ null MEFs because the phosphorylation can be blocked by a known ALK5 inhibitor SB431542 [22,23]. Taken together, present findings suggest that lack of PPAR-γ is associated with elevated levels of autocrine TGF-β signaling and activation of downstream Smad signal transduction pathway.
In TGF-β-induced Smad-signaling pathway, acetyltransferase p300 plays a pivotal role. Previously, we demonstrated that induced interaction of Smad with p300 is essential for induced collagen synthesis by activated fibroblasts [25]. The present study demonstrates the constitutive accumulation of acetyltransferase p300 on DNA bound p-Smads in PPAR-γ null MEFs. These results together further suggest that significant upregulation of collagen gene expression in PPAR-γ null MEFs is not only due to lack of PPAR-γ, a repressor of collagen gene, but also due to autocrine TGF-β signaling and constitutive Smad signaling and involvement of acetyltransferase p300.
Increased levels of TβRI/II and enhanced autocrine TGF-β signaling are implicated in the development of fibrosis in SSc [34,35]. Increased autocrine TGF-β signaling is also associated with constitutive activation of Smad2/3 in SSc [36]. Furthermore, the levels of acetyltransferase p300 and its interaction with Smad3 are significantly elevated in SSc fibroblasts [8,37]. The results of the present study clearly indicate the existence of elevated levels of TβRI/TGF-βl and downstream activated signaling molecules in PPAR-γ null MEFs. Therefore, the present model of PPAR-γ null MEF demonstrates parallel characteristics of SSc fibroblasts which ignite us to ask whether SSc phenotype is associated with impaired PPAR-γ signaling (Fig. 6A). Indeed, the levels of PPAR-γ mRNA and proteins in SSc fibroblasts are significantly lower compared to healthy controls (Fig. 6B) indicating that PPAR-γ may contribute to the SSc phenotype and may be a potential therapeutic target for SSc.
In summary, present study demonstrates that lack of PPAR-γ is associated with constitutive activation of autocrine TGF-β signaling via downstream Smad-p300 containing signaling cascade, and elevated collagen gene expression. As parallel characteristics are present in SSc fibroblasts, it is apparent that low levels of PPAR-γ in SSc may be associated with increased TGF-β-induced profibrotic signal and disruption of connective tissue homeostasis. Therefore, PPAR-γ null cells may be an excellent in vitro experimental model to pursue study on molecular basis of fibrosis.
Acknowledgements
We are grateful to Drs. Christopher K. Glass (University of California, San Diego, CA) for providing the mPPAR-γ expression vectors and Evan D. Rosen (Beth Israel Deaconess Medical Center, Boston, MA) for PPAR-γ null and heterozygous MEFs. This work was supported by grants from Scleroderma Foundation to AKG and the National Institutes of Health (AR-42309) to JV.
REFERENCES
- 1.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J. Cli. Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 3.Chen SJ, Yuan W, Mori Y, Levenson A, Trojanowska M, Varga J. Stimulation of type I collagen transcription in human skin fibroblasts by TGF-β: involvement of Smad 3. J. Invest. Dermatol. 1999;112:49–57. doi: 10.1046/j.1523-1747.1999.00477.x. [DOI] [PubMed] [Google Scholar]
- 4.Mori Y, Chen SJ, Varga J. Modulation of endogenous Smad expression in normal skin fibroblasts by transforming growth factor-β. Exp. Cell. Res. 2000;258:374–383. doi: 10.1006/excr.2000.4930. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh AK, Yuan W, Mori Y, Varga J. Smad-dependent stimulation of type I collagen gene expression in human skin fibroblasts by TGF-β involves functional cooperation withp300/CBP transcriptional coactivators. Oncogene. 2000;19:3546–3555. doi: 10.1038/sj.onc.1203693. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh AK, Yuan W, Mori Y, Chen SJ, Varga J. Antagonistic regulation of type I collagen gene expression by interferon-γ and transforming growth factor-β. Integration at the level of p300/CBP transcriptional coactivators. J. Biol. Chem. 2001;276:11041–11048. doi: 10.1074/jbc.M004709200. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh AK, Bhattacharyya S, Varga J. The tumor suppressor p53 abrogates Smad-dependent collagen gene induction in mesenchymal cells. J. Biol. Chem. 2004a;279:47455–47463. doi: 10.1074/jbc.M403477200. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya S, Ghosh AK, Pannu J, Mori Y, Takagawa S, Chen G, Trojanowska M, Gilliam AC, Varga J. Fibroblast expression of the coactivator p300 governs the intensity of profibrotic response to TGF- β. Arthritis Rheum. 2005;52:1248–1258. doi: 10.1002/art.20996. [DOI] [PubMed] [Google Scholar]
- 9.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferators-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy KJ, Routh RE, Shaw W, Walsh K, Welbourne TC, Johnson JH. Troglitazone halts diabetic glomerulosclerosis by blockade of mesangial expansion. Kidney Int. 2000;58:2341–2350. doi: 10.1046/j.1523-1755.2000.00418.x. [DOI] [PubMed] [Google Scholar]
- 12.Kon K, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Kitamura T, Takei Y, Sato N. Pioglitazone prevents early-phase hepatic fibrogenesis caused by carbon tetrachloride. Biochem. Biophys. Res. Commun. 2002;291:55–61. doi: 10.1006/bbrc.2002.6385. [DOI] [PubMed] [Google Scholar]
- 13.Lehrke M, Lazar MA. The many faces of PPARγ. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–439. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh AK, Bhattacharyya S, Lakos G, Mori Y, Chen S-J, Varga J. Peroxisome proliferator-activated receptor-γ signaling and profibrotic responses in normal skindisrupts TGF-β fibroblasts. Arthritis Rheum. 2004b;50:1305–1318. doi: 10.1002/art.20104. [DOI] [PubMed] [Google Scholar]
- 16.Ihn H, Ohnishi K, Tamaki T, LeRoy EC, Trojanowska M. Transcriptional regulation of the human α2(I) collagen gene. Combined action of upstream stimulatory and inhibitory cis-acting elements. J.Biol. Chem. 1996;271:26717–26723. doi: 10.1074/jbc.271.43.26717. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Yang EK, Kim SG. Peroxisome proliferator-activated receptor-gamma and retinoic acid X receptor alpha represses the TGFβ1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: role for Zf9 dephosphorylation. Mol. Pharmacol. 2006;70:415–425. doi: 10.1124/mol.106.022954. [DOI] [PubMed] [Google Scholar]
- 18.Zheng S, Chen A. Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G883–G893. doi: 10.1152/ajpgi.00450.2005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Ou J, Inagaki Y, Greenwel P, Ramirez F. Synergistic cooperation between Sp1 and Smad3/Smad4 mediates transforming growth factor beta1 stimulation of α2(I)-collagen (COL1A2) transcription. J. Biol Chem. 2000;275:39237–39245. doi: 10.1074/jbc.M003339200. [DOI] [PubMed] [Google Scholar]
- 20.Hayashida T, DeCaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad-signaling pathways enhances TGF-β dependent responses in human mesangial cells. FASEB J. 2003;17:1576–1588. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 21.Ishida W, Mori Y, Lakos G, Sun L, Shan F, Bowes S, Josiah S, Lee WC, Singh J, Ling LE, Varga J. Intracellular TGF-β receptor blockade abrogates Smad-dependent fibroblast activation in vitro and in vivo. J. Invest. Dermatol. 2006;126:1733–1744. doi: 10.1038/sj.jid.5700303. [DOI] [PubMed] [Google Scholar]
- 22.Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, et al. Inhibition of transforming growth factor (TGF)-β 1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol. Pharmacol. 2002;62:58–64. doi: 10.1124/mol.62.1.58. [DOI] [PubMed] [Google Scholar]
- 23.Mori Y, Ishida W, Bhattacharyya S, Li Y, Platanias LC, Varga J. Selective inhibition of activin receptor-like kinase 5 signaling blocks profibrotic transforming growth factor-β responses in skin fibroblasts. Arthritis Rheum. 2004;50:4008–4021. doi: 10.1002/art.20658. [DOI] [PubMed] [Google Scholar]
- 24.Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14:1553–1577. [PubMed] [Google Scholar]
- 25.Ghosh AK, Varga J. The transcriptional coactivator and acetyltransferase p300 in fibroblast biology and fibrosis. J. Cell. Physiol. 2007;213:663–671. doi: 10.1002/jcp.21162. [DOI] [PubMed] [Google Scholar]
- 26.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keen HL, Ryan MJ, Beyer A, Mathur S, Scheetz TE, Gackle BD, Faraci FM, Casavant TL, Sigmund CD. Gene expression profiling of potential PPAR-γ target genes in mouse aorta. Physiol. Genomics. 2004;18:33–42. doi: 10.1152/physiolgenomics.00027.2004. [DOI] [PubMed] [Google Scholar]
- 28.Lakatos HF, Thatcher TH, Kottmann RM, Garcia TM, Phipps RP, Sime PJ. The Role of PPARs in Lung Fibrosis. PPAR Res. 2007:p1–10. doi: 10.1155/2007/71323. Article ID: 71323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng F, Fornoni A, Elliot SJ, Guan Y, Breyer MD, Striker LJ, Striker GE. Upregulation of type I collagen by TGF-β in mesangial cells is blocked by PPARγ activation. Am. J. Physiol. Renal Physiol. 2002;282:F639–F648. doi: 10.1152/ajprenal.00189.2001. [DOI] [PubMed] [Google Scholar]
- 30.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPAR-γ agonists inhibit TGF-β induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;288:L1146. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 31.Yavrom S, Chen L, Xiong S, Wang J, Rippe RA, Tsukamoto H. Peroxisome proliferator-activated receptor gamma suppresses proximal α1(I) collagen promoter via inhibition of p300-facilitated NF-I binding to DNA in hepatic stellate cells. J. Biol. Chem. 2005;280:40650–40659. doi: 10.1074/jbc.M510094200. [DOI] [PubMed] [Google Scholar]
- 32.Warner S, Groose R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2002;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 33.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 34.Pannu J, Trojanowska M. Recent advances in fibroblast signaling and biology in scleroderma. Curr. Opin. Rheumatol. 2004;16:739–745. doi: 10.1097/01.bor.0000137894.63091.1a. [DOI] [PubMed] [Google Scholar]
- 35.Ihn H. Autocrine TGF-β signaling in the pathogenesis of systemic sclerosis. J. Dermatol Sci. 2007;XX:XX. doi: 10.1016/j.jdermsci.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Mori Y, Chen SJ, Varga J. Expression and regulation of intracellular SMAD signaling in scleroderma skin fibroblasts. Arthritis Rheum. 2003;48:1964. doi: 10.1002/art.11157. [DOI] [PubMed] [Google Scholar]
- 37.Ihn H, Yamane K, Asano Y, Jinnin M, Tamaki K. Constitutively phosphorylated Smad3 interacts with Sp1 and p300 in scleroderma fibroblasts. Rheumatol. (Oxf) 2006;45:157–165. doi: 10.1093/rheumatology/kei124. [DOI] [PubMed] [Google Scholar]