Abstract
Alcohol-related chronic myopathy is characterized by severe biochemical and structural changes to skeletal muscle. Our goals were to: (1) identify early regulatory elements that precede the overt manifestation of plantaris atrophy; and (2) circumvent these derangements by supplementing alcohol-fed rats with the glutathione precursor, procysteine. After 6 weeks of daily ingestion, before the development of overt atrophy of the plantaris muscle, alcohol increased several markers of oxidative stress and increased gene expressions of atrogin-1 and transforming growth factor-β1 (TGF-β1) by ~60- and ~65-fold, respectively, which were attenuated by procysteine supplementation. Interestingly, after 28 weeks of alcohol ingestion, when overt plantaris atrophy had developed, atrogin-1 and TGF-β1 gene expression had returned to baseline levels. Together, these findings suggest that alcohol-induced, redox-sensitive alterations drive pro-atrophy signaling pathways that precede muscle atrophy. Therefore, targeted anti-oxidant treatments such as procysteine supplementation may benefit individuals with chronic alcohol abuse, particularly if given prior to the development of clinically significant myopathy.
Keywords: alcoholic myopathy, atrogin-1, glutathione, oxidative stress, transforming growth factor-β1
Skeletal muscle myopathy due to excessive alcohol ingestion, termed alcoholic myopathy, is characterized by biochemical, physiological, and structural changes to skeletal muscle that lead to deficits in muscle function.31 In general, the degree and severity of alcoholic myopathy is proportional to the quantity and duration of alcohol consumption. Alcohol-related chronic myopathy is most evident in fast-twitch muscles16,29 (e.g., plantaris) and is characterized by progressive proximal weakness, muscle soreness and muscle atrophy, altered gait, and impaired mobility.17 Although the fundamental cause of alcohol-related myopathy is unknown, the diverse toxicology of chronic alcohol consumption suggests that the etiology of the disease is likely multifactorial. For instance, the toxic effects of alcohol on skeletal muscle may be due, in part, to increased apoptosis13; altered mitochondrial function5,6; increased acetaldehyde protein adduct formation38; decreased levels of the anabolic hormone, insulin-like growth factor-1 (IGF-1)21; reduced expression of the protein translation factor, p70 S6 kinase20; or increased expression of myostatin, a negative regulator of muscle growth.21
Alcohol-associated myopathy may occur independently of nutritional status or vitamin deficiencies.10 However, most studies suggest that increased skeletal muscle pro-oxidant levels and reductions to anti-oxidant capacity likely exacerbate symptoms of alcohol-related myopathy.1,12,19,26,30 Nevertheless, the effects of chronic alcohol abuse on skeletal muscle redox state and redox-sensitive signaling pathways that occur before the clinical presentation of alcohol-associated myopathy (e.g., atrophy) remain poorly defined.
Transforming growth factor-β (TGF-β) is a superfamily of cytokines that can be induced by reactive oxygen species and oxidative stress and plays important roles in growth and development, inflammation and repair, and immune responses.8 Our laboratory has previously shown that alcohol-induced oxidative stress increases expression of TGF-β1 and results in severe lung dysfunction.3 Further, TGF-β1 has been implicated in skeletal muscle catabolic conditions25 and has recently been shown to be regulated by atrogin-1, a novel E3 ubiquitin ligase associated with skeletal muscle atrophy.4,14 However, the influence of alcohol-induced oxidative stress on atrogin-1 and TGF-β1 expressions in skeletal muscle is unknown.
In this investigation, we analyzed the early alterations in redox state and the resultant effects on atrogin-1 and TGF-β1 expressions in skeletal muscles from alcohol-fed rats. Our objectives were to study these alcohol-induced derangements that precede the clinical manifestation of alcohol-related myopathy and to determine the effectiveness of anti-oxidant therapy in reducing these derangements. Based on strong evidence that chronic alcohol ingestion disrupts normal glutathione (GSH) metabolism,12 we supplemented the diets of alcohol-fed rats with the GSH precursor, procysteine, and found that GSH replacement therapy may be a clinically effective treatment option to circumvent alcohol-associated myopathy.
METHODS
Animals and Diet
Male Sprague–Dawley rats (200–250 g, n = 6 rats/group) were purchased from Charles River (Wilmington, Massachusetts) and housed in pairs under a 12:12-hour light–dark cycle. All procedures were approved by our institutional review board.
Rats were fed the Lieber–DeCarli liquid diet (Research Diets, New Brunswick, New Jersey) containing either alcohol or an isocaloric substitution with malt-in–dextrin (control diet) for 6 or 28 weeks as previously described.15,36 Alcohol was added gradually to acclimatize the rats to the diet. It was added as 18% of total calories for 1 week, then 27% of total calories for 1 week, and finally as 36% of total calories for 4 or 26 weeks, respectively. In a subgroup of rats fed alcohol for 6 weeks, a GSH precursor, procysteine (Sigma Co., St. Louis, Missouri), was added to the diets at a concentration of 0.35% (w/v).36 After 6 or 28 weeks, the rats were anesthetized with sodium pentobarbital and their plantaris muscles were removed, blotted dry, weighed, and prepared for further analyses.
Cross-Sectional Area Measurements
Plantaris muscles were embedded in OCT and immediately frozen in isopentane cooled in liquid nitrogen. Serial sections from the mid-belly of the plantaris muscle were cut at 14 μm and adhered to superfrost slides. Plantaris sections were processed for hematoxylin–eosin staining, dehydrated, mounted, and visualized with a Leica microscope. Approximately 125 fibers per muscle were analyzed and cross-sectional areas determined using ImageJ software (NIH, Bethesda, Maryland).
Total Protein Oxidation
Total protein oxidation was analyzed with the OxyBlot Protein Oxidation Detection Kit (Chemicon, Temecula, California) according to the manufacturer’s directions. Briefly, plantaris muscles were homogenized in buffer containing 250 mM sucrose, 5 mM ethylene-diamine tetraacetic acid (EDTA), 100 mM KCl, 20 mM Hepes, 2% β-mercaptoalcohol, and complete mini-protease inhibitor cocktail (Sigma). Carbonyl groups attached to the side chains of these protein lysates were derivatized to 2,4-dinitrophenylhydrazone and detected by dot-blot analysis. Densitometry was performed using a Chemidoc XRS system and analyzed using Quantity One software (Bio-Rad, Hercules, California). Samples were run in duplicate and total protein oxidation was expressed as fold change relative to controls.
High-Performance Liquid Chromatography
For determining the levels of GSH, glutathione disulfide (GSSG), cysteine (Cys), and cystine (Cyss) in plantaris muscle tissue, we used a variation of the high-performance liquid chromatography (HPLC) method described elsewhere.28 Each sample was extracted in 5% perchloric acid with 0.2 M boric acid and 10 μM γ-glutamylglutamate as an internal standard. Iodoacetic acid was added and the pH was adjusted to 9.0 ± 0.2. After incubation for 20 min to obtain S-carboxymethyl derivatives of thiols, dansyl chloride was added and the samples were incubated for 24 h in the dark. Samples were then separated on an amine column with solvents as previously described.15 Fluorescence detection was used for separation and quantification of the dansyl derivatives. The redox pairs (i.e., GSH and GSSG, Cys, and Cyss) were measured in parallel and expressed as picomoles/milligram.
Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
Plantaris muscles were immediately frozen in liquid nitrogen and stored at −80°C until processing for RT-PCR analyses. Trizol was added (1 ml/100 mg tissue) and the tissues homogenized using an electric tissue homogenizer. Total RNA (2.5 μg) was reverse transcribed in a 25–50-μl final reaction volume using random primers and M-MLV reverse transcriptase (Invitrogen, Carlsbad, California). The reverse transcription reaction was incubated at 65°C for 10 min, 80°C for 3 min, and 42°C for 60 min. RT-PCR products were analyzed using the iCycler iQ system (Bio-Rad). cDNA (5 μl of a 1:10 dilution) was amplified in a 12.5-μl reaction containing a 400-nm gene-specific primer pair and iQ Sybr Green Super-mix (Bio-Rad). Primers were as follows: atrogin-1, 5′-TCCAGACCCTCTACACATCCTT-3′ and 5′-CCTCTGCATGATGTTCAGTTGT-3′; TGF-β1, 5′-CTACTACGCCAAAGAAGTCACC-3′ and 5′-CTG-TATTCCGTCTCCTTGGTT-3′; and skeletal muscle actin, 5′-GCCTGCTATGTATGTGGCTATT-3′ and 5′-GGTGAGGATTTTCATCAGGTAG-3′. Samples were incubated at 95°C for 15 min, followed by 40 cycles of denaturation, annealing, and extension at 95°C, 58°C, and 72°C, respectively. As a control, RT-PCR was also performed on 2 μl of each RNA sample to confirm absence of contaminating genomic DNA. Fluorescence was recorded at the end of each annealing and extension step. All reactions were performed in triplicate and the starting quantity of the gene of interest was normalized to 18S rRNA for each sample. The delta–delta CT method24 was used to analyze alterations in gene expression and values were expressed as fold changes relative to control.
Statistics
One-way analyses of variance were performed followed by Student–Newman–Keuls post hoc tests using SigmaStat v2.0 software (Jandel, San Rafael, California). Significance was accepted at P ≤ 0.05.
RESULTS
Markers of Reduction and Oxidation (Redox) States
We first identified the effect of 6 weeks of alcohol ingestion on various markers of redox state in rat plantaris muscles, which did not show any atrophy, including measurable total protein oxidation and metabolites of the GSH system. The muscles displayed a nearly twofold increase in total protein oxidation relative to controls (Fig. 1). Supplementing the alcohol diets with the GSH precursor, procysteine, effectively decreased total protein oxidation in the plantaris to levels comparable to isocaloric-fed controls.
FIGURE 1.
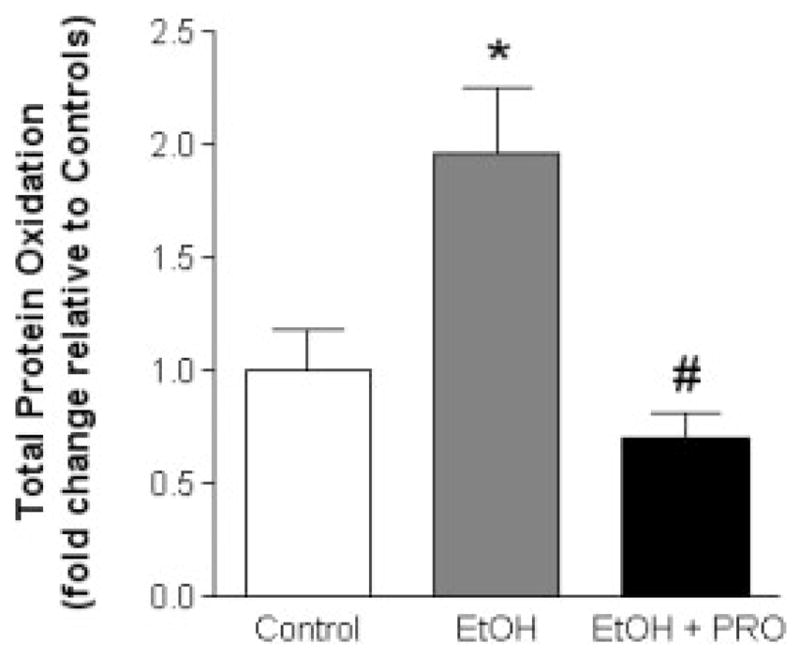
Total protein oxidation in rat plantaris muscles. The increase in total protein oxidation in rat plantaris muscles due to 6 weeks of chronic alcohol ingestion is attenuated by procysteine supplementation. Values are normalized to controls and expressed as mean + SEM. EtOH, alcohol-fed rats; PRO, procysteine-supplemented rats. Significant difference (P < 0.05) is shown compared with control group (*) or EtOH group (#).
We next used HPLC analysis to determine the levels of GSH and GSSG (Fig. 2), and the levels of Cys and Cyss (Fig. 3). Six weeks of alcohol ingestion significantly decreased GSH levels (Fig. 2A) and Cys levels (Fig. 3A), and increased the ratio of Cyss/Cys, a marker of the oxidative state of the Cys pool (Fig. 3C). Procysteine supplementation increased Cys levels and attenuated the alcohol-induced increase in Cyss/Cys (Fig. 3A and C, respectively).
FIGURE 2.
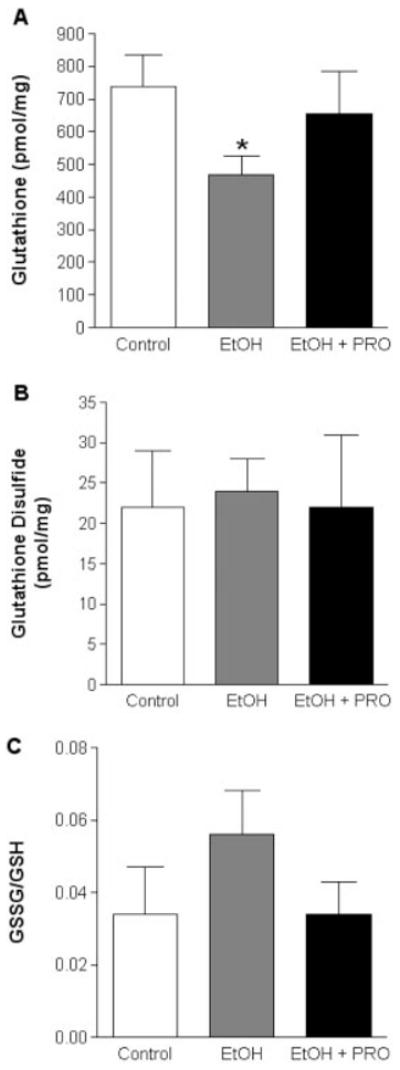
GSH and GSSG levels in plantaris muscles. Chronic alcohol ingestion for 6 weeks decreased the available pool of GSH (A), but had no effect on GSSG levels (B). The GSSG/GSH ratio, a marker of the oxidative state of the GSH pool, was unchanged between the groups (C). Values are expressed as mean + SEM. GSH, glutathione; GSSG, glutathione disulfide; EtOH, alcohol-fed rats; PRO, procysteine-supplemented rats. Significant difference (P < 0.05) is shown compared with control group (*).
FIGURE 3.
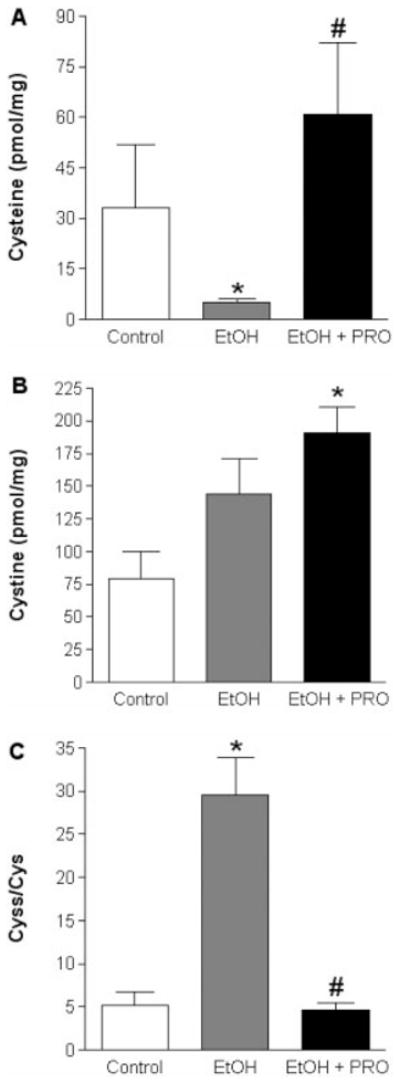
Cys and Cyss levels in plantaris muscles. Chronic alcohol ingestion for 6 weeks decreased Cys levels, which were restored to control levels following procysteine supplementation (A). Cyss levels were increased in plantaris muscles from EtOH + PRO animals (B). The Cyss/Cys ratio, a marker of the oxidative state of the Cys pool, was increased in alcohol-fed animals (C). Procysteine supplementation normalized this ratio to control levels. Values are expressed as mean + SEM. Cys, cysteine; Cyss, cystine; EtOH, alcohol-fed rats; PRO, procysteine-supplemented rats. Significant difference (P < 0.05) is shown compared with control group (*) or EtOH group (#).
Atrogin-1 and TGF-β1 Expressions
We then analyzed atrogin-1 and TGF-β1 mRNA levels in plantaris muscles from alcohol-fed rats supplemented with or without procysteine. Six weeks of alcohol ingestion increased atrogin-1 and TGF-β1 mRNA levels ~60-and ~65-fold in plantaris muscles from alcohol-fed rats (Fig. 4A and B, respectively). Procysteine supplementation in the diets of alcohol-fed rats significantly reduced both atrogin-1 and TGF-β1 mRNA levels; however, these levels were still higher than those from isocaloric-fed controls.
FIGURE 4.
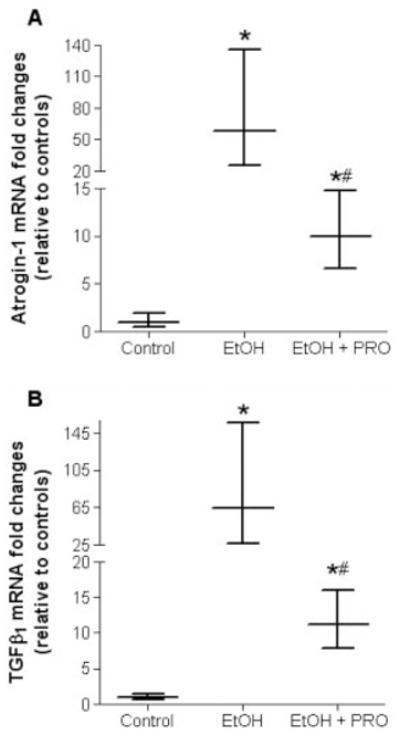
Atrogin-1 and TGF-β1 gene expressions in plantaris muscles. Real-time polymerase chain reaction analyses showed increased atrogin-1 (A) and TGF-β1 (B) mRNA levels in plantaris muscles from rats chronically fed alcohol for 6 weeks. These expression levels were sensitive to procysteine supplementation. As a control, mRNA levels of skeletal muscle actin were quantified and showed no difference between groups. Data are presented as mean ± range of potential values based on the 2−ΔΔCT method24 and expressed as fold changes relative to controls. EtOH, alcohol-fed rats; PRO, procysteine-supplemented rats. Significant difference (P < 0.05) is shown compared with control group (*) or EtOH group (#).
Plantaris Morphology and Gene Expression after 28 Weeks of Alcohol Ingestion
Because alcohol increased atrogin-1 and TGF-β1 gene levels in the absence of plantaris atrophy after 6 weeks, we next determined their expression levels when alcohol-induced atrophy was apparent. Plantaris muscle-fiber cross-sectional area (CSA) from alcohol-fed rats decreased ~32% compared with controls (Fig. 5A). Interestingly, gene expression levels of both atrogin-1 and TGF-β1 were unchanged (Fig. 5B and C, respectively).
FIGURE 5.
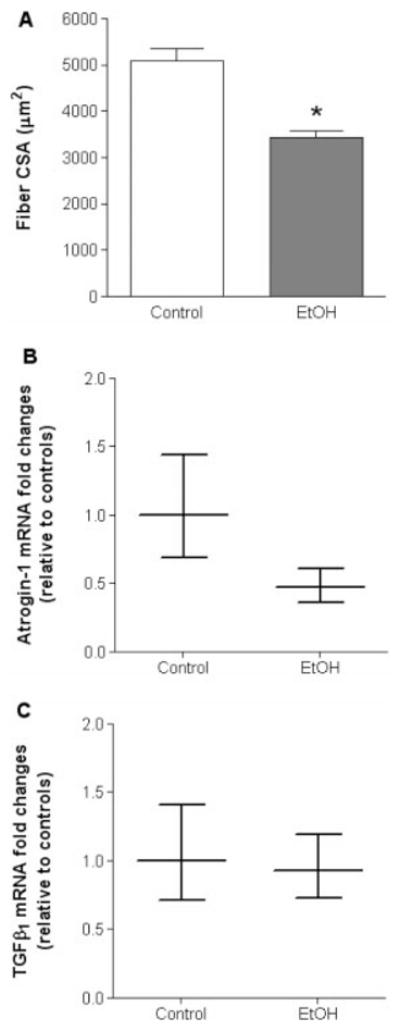
Plantaris fiber CSA, atrogin-1, and TGF-β1 gene expressions from rats chronically fed alcohol for 28 weeks. After 28 weeks of chronic alcohol ingestion, plantaris fiber CSA was significantly reduced compared with controls (A). However, no changes were observed in mRNA levels of atrogin-1 (B) or TGF-β1 (C). As a control, mRNA levels of skeletal muscle actin were quantified and showed no difference between groups. Data are presented as mean ± range of potential values based on the 2−ΔΔCT method24 and expressed as fold changes relative to controls. EtOH, alcohol-fed rats; PRO, procysteine-supplemented rats. Significant difference (P < 0.05) is shown compared with control group (*).
DISCUSSION
In this study, 6 weeks of daily alcohol ingestion caused significant oxidative stress in plantaris muscles. In parallel, we showed that chronic alcohol ingestion strongly induced atrogin-1 and TGF-β1 gene expressions before the onset of atrophy; once alcohol-induced atrophy was apparent, their expressions normalized. Together, these data reveal an important temporal relationship between these early pro-atrophy factors and the later clinical manifestation of alcohol-related myopathy. Procysteine supplementation reduced some markers of oxidative stress, and reduced atrogin-1 and TGF-β1 gene expressions, which implies that the attenuation of these early catabolic factors may prevent future skeletal muscle atrophy should alcohol abuse persist. These findings further suggest that anti-oxidant therapy, using GSH precursors such as procysteine or S-adenosyl-L-methionine (SAMe), may provide a feasible and clinically effective treatment option for alcohol-associated myopathy.
Our data support the notion that chronic alcohol ingestion alters redox balance in skeletal muscle.12,30 Type II fiber–rich muscles (i.e., fast muscles) are particularly sensitive to alcohol-induced alterations in redox state, in part because of lower GSH levels compared with slower muscles.23 GSH is a highly potent, non-vitamin anti-oxidant that detoxifies reactive oxygen species and plays an important role in maintaining redox balance in skeletal muscle.7,33 GSH levels may be restored by supplementing diets with precursor molecules such as N-acetylcysteine (NAC), SAMe, or procysteine. NAC, the only clinically available GSH precursor, was not effective in preventing alcohol-induced liver or lung dysfunction.15 In contrast, SAMe, an over-the-counter GSH precursor commonly used to treat alcoholic liver disease, has proven effective in experimental models.22 Our laboratory has shown that procysteine supplementation provides an effective means of replenishing the usable pool of GSH in lung tissue from alcohol-fed rats15,36 and abrogates alcohol-induced tissue dysfunction.3,36 Similarly, we have now shown that prolonged alcohol ingestion decreases the available pool of GSH and Cys in the plantaris, suggesting that those benefits of procysteine supplementation apparent in lung tissue3,36 may extend into skeletal muscle tissue. Although other investigators have tried anti-oxidant or anti-oxidant cofactor supplementation in the diets of alcoholics to improve derangements to skeletal muscle, the effectiveness of these interventions has been limited.9,18,32 One possible explanation for this lack of effectiveness in studies using vitamin anti-oxidant compounds is that levels of common vitamins, such as α-tocopherol, ascorbic acid, and retinol, are normal in serum and muscles from patients with alcohol-associated myopathy.11 Therefore, increasing the concentrations of these vitamins may provide only limited benefits.
Although debatable,29 a few studies have shown that daily alcohol ingestion for 6 weeks is insufficient to produce atrophy in predominantly fast muscles from older male rats.29,37 As we and others34 have shown, continued alcohol abuse in older animals will result in complications from alcohol-related myopathy. Nevertheless, despite the fact that 6 weeks of alcohol abuse failed to produce plantaris atrophy in our study, several known factors implicated in skeletal muscle catabolism are induced, including oxidative stress, atrogin-1, and TGF-β1.
Atrogin-1, also known as muscle atrophy F-box (MAFbx), is an E3 ubiquitin ligase that initiates adenosine triphosphate–dependent, ubiquitin-mediated proteolysis and is abundant in skeletal muscles undergoing atrophy.4,14 Atrogin-1 is thought to recognize and bind to specific phosphorylated proteins, promote their ubiquitination, and initiate degradation during skeletal muscle atrophy.4 Atrogin-1 mRNA expression is markedly increased in muscles that are wasting due to cancer, renal failure, and diabetes.14 Similar to alcoholic myopathy, these diseases are also associated with increased free-radical production and significant oxidative stress.27,35 Herein, we have provided evidence that skeletal muscles from alcohol-fed rats have increased atrogin-1 mRNA expression, which appears to be sensitive to oxidative stress. We assert that alcohol-induced, redox-sensitive atrogin-1 expression may prime skeletal muscle for future derangements should alcohol abuse persist. Six weeks of daily alcohol ingestion also increases the expression of TGF-β1, a cytokine implicated in the regulation of skeletal muscle mass.25 Atrogin-1 may regulate TGF-β signaling by degrading specific substrates associated with this pathway.2 Although procysteine supplementation alone did not completely normalize atrogin-1 and TGF-β1, the present findings suggest that these alcohol-induced expressions are, at least in part, amenable to GSH replacement therapy. Another possibility for the significant, albeit incomplete, reduction in atrogin-1 and TGF-β1 due to procysteine supplementation is that other redox-independent mechanisms may coregulate their expression levels. Nevertheless, procysteine supplementation reduced atrogin-1 and TGF-β1 expression levels by ~83%, which may be sufficient enough to abrogate the alcohol-induced, redox-sensitive mechanisms that lead to muscle derangements and dysfunctions.
Although 28 weeks of daily alcohol ingestion produced significant plantaris atrophy, gene expressions of atrogin-1 and TGF-β1 returned to control levels. Although it is possible that alcohol-related myopathy is independent of atrogin-1 and TGF-β1, the dramatic induction of these genes after 6 weeks of alcohol ingestion would appear to make this interpretation unlikely. Instead, these findings reveal an intriguing temporal association in which early, oxidant-mediated induction of atrogin-1 and TGF-β1 gene expression precedes the later development of overt muscle atrophy. Further, in light of the evolving evidence implicating atrogin-1 and TGF-β1 in the pathophysiology of muscle atrophy, these findings suggest a mechanistic as well as a temporal relationship. Taken together, our data support the hypothesis that these early, redox-sensitive inductions in pro-atrophy factors by daily alcohol ingestion represent significant pre-clinical alterations in the evolution of alcohol-related myopathy that are responsible, at least in part, for the establishment of a pro-atrophy signaling milieu. If correct, this pathophysiological scheme would then support the use of procysteine or other thiol supplementation to reduce atrogin-1 and TGF-β1 expression. Although the effectiveness of prescribing anti-oxidants or anti-oxidant cofactors as potential treatments for patients suffering from alcohol-associated myopathy has been debated, we have provided evidence that procysteine supplementation may represent a clinically effective treatment option that could attenuate this myopathy.
Acknowledgments
This work was supported by Grant AR052255-02 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (to J.S.O.) and by Grant P-50 AA013757 from the National Institute on Alcohol Abuse and Alcoholism (to D.M.G.).
Abbreviations
- CSA
cross-sectional area
- Cys
cysteine
- Cyss
cystine
- EtOH
alcohol
- GSH
glutathione
- GSSG
oxidized glutathione or glutathione disulfide
- IGF-1
insulin-like growth factor-1
- MAFbx
muscle atrophy F-box (atrogin-1)
- PRO
procysteine
- TGF-β1
transforming growth factor-β1
References
- 1.Adachi J, Asano M, Ueno Y, Reilly M, Mantle P, Peters TJ, et al. 7-α and 7 β-hydrocycholesterol-5-en 3β-ol in muscle as indices of oxidative stress: response to ethanol dosage in rats. Alcohol Clin Exp Res. 2000;24:675–681. [PubMed] [Google Scholar]
- 2.Aoyama Y, Urushiyama S, Yamada M, Kato C, Ide H, Higuchi S, et al. MFB-1, an F-box-type ubiquitin ligase, regulates TGF-beta signaling. Genes Cells. 2004;9:1093–1101. doi: 10.1111/j.1365-2443.2004.00792.x. [DOI] [PubMed] [Google Scholar]
- 3.Bechara RI, Pelaez A, Palacio A, Joshi PC, Hart CM, Brown LA, et al. Angiotensin II mediates glutathione depletion, transforming growth factor-beta1 expression, and epithelial barrier dysfunction in the alcoholic rat lung. Am J Physiol. 2005;289:L363–L370. doi: 10.1152/ajplung.00141.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum AI, Rubin E. Molecular injury to mitochondria produced by ethanol and acetaldehyde. Fed Proc. 1975;34:2045–2051. [PubMed] [Google Scholar]
- 6.Cederbaum AI. Introduction—serial review: alcohol, oxidative stress and cell injury. Free Radic Biol Med. 2001;313:1524–1526. doi: 10.1016/s0891-5849(01)00741-9. [DOI] [PubMed] [Google Scholar]
- 7.Chan KM, Decker EA. Endogenous skeletal muscle anti-oxidants. Crit Rev Food Sci Nutr. 1994;34:403–426. doi: 10.1080/10408399409527669. [DOI] [PubMed] [Google Scholar]
- 8.Clark DA, Coker R. Transforming growth factor-beta (TGF-β) Int J Biochem Cell Biol. 1998;30:293–298. doi: 10.1016/s1357-2725(97)00128-3. [DOI] [PubMed] [Google Scholar]
- 9.Durán Castellón MC, González-Reimers E, López-Lirola A, MartÍn Olivera R, Santolaria-Fernández F, Galindo-Martín L, et al. Alcoholic myopathy: lack of effect of zinc supplementation. Food Chem Toxicol. 2005;43:1333–1343. doi: 10.1016/j.fct.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Sola J, Estruch R, Grau JM, Pare JC, Urbano-Marquez A, Rubin E. The relation of alcoholic myopathy to cardiomyopathy. Curr Opin Neurol. 1994;9:400–405. doi: 10.7326/0003-4819-120-7-199404010-00001. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Sola J, Villegas E, Nicolas JM, Deulofeu R, Antunez E, Sacanella E, et al. Serum and muscle levels of alpha-tocopherol, ascorbic acid, and retinol are normal in chronic alcoholic myopathy. Alcohol Clin Exp Res. 1998;22:422–427. [PubMed] [Google Scholar]
- 12.Fernandez-Sola J, Garcia G, Elena M, Tobias E, Sacanella E, Estruch R, et al. Muscle anti-oxidant status in chronic alcoholism. Alcohol Clin Exp Res. 2002;26:1858–1862. [PubMed] [Google Scholar]
- 13.Fernandez-Sola J, Nicolas JM, Fatjo F, Garcia G, Sacanella E, Estruch R, et al. Evidence of apoptosis in chronic alcoholic skeletal myopathy. Hum Pathol. 2003;34:1247–1252. doi: 10.1016/j.humpath.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidot DM, Brown LA. Mitochondrial glutathione replacement restores surfactant synthesis and secretion in alveolar epithelial cells of ethanol-fed rats. Alcohol Clin Exp Res. 2000;24:1070–1076. [PubMed] [Google Scholar]
- 16.Hanid A, Slavin G, Mair W, Sowter C, Ward P, Webb J, et al. Fibre type changes in striated muscle of alcoholics. J Clin Pathol. 1981;34:991–995. doi: 10.1136/jcp.34.9.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiessling KH, Pilstrom L, Bylund AC, Piehl K, Saltin B. Effects of chronic ethanol abuse on structure and enzyme activities of skeletal muscle in man. Scand J Clin Lab Invest. 1975;35:601–607. doi: 10.1080/00365517509095786. [DOI] [PubMed] [Google Scholar]
- 18.Koll M, Beeso JA, Kelly FJ, Simanowski UA, Seitz HK, Peters TJ, et al. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Colch) 2003;104:287–294. doi: 10.1042/CS20020312. [DOI] [PubMed] [Google Scholar]
- 19.Koo-Ng R, Falkous G, Reilly M, Peters TJ, Mantle D, Preedy VR. Carbonyl levels in type I and II fiber-rich muscles and their response to chronic ethanol feeding in vivo and hydroxyl and superoxide radicals in vitro. Alcohol Clin Exp Res. 2000;24:1862–1868. [PubMed] [Google Scholar]
- 20.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-1 stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol. 2002;283:E917–E928. doi: 10.1152/ajpendo.00181.2002. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-1/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol. 2004;286:E916–E926. doi: 10.1152/ajpendo.00554.2003. [DOI] [PubMed] [Google Scholar]
- 22.Lieber CS, Casini A, Decarli LM, Kim C, Lowe N, Sasaki R, et al. S-Adenosyl-L-methionine attenuates alcohol-induced liver injury in the baboon. Hepatology. 1990;11:165–172. doi: 10.1002/hep.1840110203. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Yeo HC, Övervik-Douki E, Hagen T, Doniger SJ, Chu DW, et al. Chronically and acutely exercised rats: biomarkers of oxidative stress and endogenous anti-oxidants. J Appl Physiol. 2000;89:21–28. doi: 10.1152/jappl.2000.89.1.21. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg IE. The role of cytokines, chemokines, and adhesion molecules in the pathogenesis of idiopathic inflammatory myopathies. Curr Rheumatol Rep. 2000;2:216–224. doi: 10.1007/s11926-000-0082-y. [DOI] [PubMed] [Google Scholar]
- 26.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial DNA in mouse liver, brain, heart and skeletal muscles: protective effects of anti-oxidants. J Pharmacol Exp Ther. 2001;298:737–743. [PubMed] [Google Scholar]
- 27.Martin CJ, Goeddeke-Merickel CM. Oxidative stress in chronic kidney disease. Nephrol Nurs J. 2005;32:683–685. [PubMed] [Google Scholar]
- 28.Martin J, White JM. Fluorometric determination of oxidized and reduced glutathione in cells and tissues by high-performance liquid chromatography following derivatization with dansyl chloride. J Chromatogr. 1991;568:221–225. doi: 10.1016/0378-4347(91)80356-h. [DOI] [PubMed] [Google Scholar]
- 29.Preedy VR, Peters TJ. The effect of chronic ethanol ingestion on protein metabolism in type-I- and type-II-fibre-rich skeletal muscles of the rat. Biochem J. 1988;254:631–639. doi: 10.1042/bj2540631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preedy VR, Adachi J, Asano M, Koll M, Mantle D, Niemela O, et al. Free radicals in alcoholic myopathy: indices and preventive study. Free Rad Biol Med. 2001;32:683–687. doi: 10.1016/s0891-5849(01)00794-8. [DOI] [PubMed] [Google Scholar]
- 31.Preedy VR, Ohlendieck K, Adachi J, Koll M, Sneddon A, Hunter R, et al. The importance of alcohol-induced muscle disease. J Muscle Res Cell Motil. 2003;24:55–63. doi: 10.1023/a:1024842817060. [DOI] [PubMed] [Google Scholar]
- 32.Reilly ME, Patel VB, Peters TJ, Preedy VR. In vivo rates of skeletal muscle protein synthesis in rats are decreased by acute ethanol treatment but are not ameliorated by supplemental alpha-tocopherol. J Nutr. 2000;130:3045–3049. doi: 10.1093/jn/130.12.3045. [DOI] [PubMed] [Google Scholar]
- 33.Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72(suppl):653S–669S. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 34.Trounce I, Byrne E, Dennett X. Biochemical and morphological studies of skeletal muscle in experimental chronic alcoholic myopathy. Acta Neurol Scand. 1990;82:386–391. doi: 10.1111/j.1600-0404.1990.tb03322.x. [DOI] [PubMed] [Google Scholar]
- 35.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and anti-oxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Velasquez A, Bechara RI, Lewis JF, Malloy J, McCaig L, Brown LA, et al. Glutathione replacement preserves the functional surfactant phospholipid pool size and decreases sepsis-mediated lung dysfunction in ethanol-fed rats. Alcohol Clin Exp Res. 2002;26:1245–1251. doi: 10.1097/01.ALC.0000024269.05402.97. [DOI] [PubMed] [Google Scholar]
- 37.Vingren JL, Koziris LP, Gordon SE, Kraemer WJ, Turner RT, Westerlind KC. Chronic alcohol intake, resistance training, and muscle androgen receptor content. Med Sci Sports Exerc. 2005;37:1842–1848. doi: 10.1249/01.mss.0000176679.80555.cd. [DOI] [PubMed] [Google Scholar]
- 38.Worrall S, Niemela O, Parkkila S, Peters TJ, Preedy VR. Protein adducts in type I and type II fibre predominant muscles of the ethanol-fed rat: preferential localization in the sarcolemmal and subsarcolemmal region. Eur J Clin Invest. 2001;31:723–730. doi: 10.1046/j.1365-2362.2001.00848.x. [DOI] [PubMed] [Google Scholar]


