Abstract
Purpose
Secondary somatic BRCA1/2 mutations may restore BRCA1/2 protein in hereditary ovarian carcinomas. In cell lines, BRCA2 restoration mediates resistance to platinum chemotherapy and poly (ADP-ribose) polymerase (PARP) inhibitors. We assessed primary and recurrent BRCA1/2-mutated ovarian carcinomas to define the frequency of secondary mutations and correlate these changes with clinical outcomes.
Methods
Neoplastic cells were isolated with laser capture microdissection, and DNA was sequenced at the site of the known germline BRCA1/2 mutation. When secondary mutations were found that restored wild-type sequence, haplotyping was performed using single nucleotide polymorphisms in tumor and paired lymphocyte DNA to rule out retention of the wild-type allele.
Results
There were 64 primary and 46 recurrent ovarian carcinomas assessed. Thirteen (28.3%) of 46 (95% CI, 17.3% to 42.6%) recurrent carcinomas had a secondary mutation compared with two (3.1%) of 64 (95% CI, 1.0% to 10.7%) primary carcinomas (P = .0003, Fisher's exact test). Twelve (46.2%) of 26 (95% CI, 28.7% to 64.7%) platinum-resistant recurrences had secondary mutations restoring BRCA1/2, compared with one (5.3%) of 19 (95% CI, 1.2% to 24.8%) platinum-sensitive recurrences (P = .003, Fisher's exact test). Six (66.7%) of nine (95% CI, 34.8% to 87.8%) women with prior breast carcinoma had a recurrent carcinoma with a secondary mutation, compared with six (17.1%) of 35 (95% CI, 8.2% to 32.8%) with no history of breast carcinoma (P = .007, Fisher's exact test).
Conclusion
Secondary somatic mutations that restore BRCA1/2 in carcinomas from women with germline BRCA1/2 mutations predict resistance to platinum chemotherapy and may also predict resistance to PARP inhibitors. These mutations were detectable only in ovarian carcinomas of women whom have had previous chemotherapy, either for ovarian or breast carcinoma.
INTRODUCTION
Ten percent to 15% of ovarian carcinomas occur in women with heterozygous germline mutations in BRCA1 and BRCA2 (BRCA1/2).1,2 BRCA1/2 are tumor suppressor genes, and BRCA1/2 proteins are instrumental in repairing damaged DNA, through homologous recombination.3,4 The vast majority of primary carcinomas in patients with BRCA1/2 mutations have deletions of the wild-type BRCA1/2 allele,5–7 leading to a neoplasm deficient in BRCA1/2 protein. BRCA1/2-deficient carcinomas have decreased capacity to repair DNA and are sensitive to chemotherapy with DNA cross-linking agents such as cisplatin and carboplatin (platinum agents)8–10 and are susceptible to synthetic lethality from poly (ADP-ribose) polymerase (PARP) inhibitors.11–13 Women with BRCA1/2-associated ovarian carcinoma have improved survival compared with those with sporadic ovarian carcinomas when treated with platinum agents.14–16 Despite this, the majority of women with BRCA1/2-mutated ovarian carcinomas ultimately develop recurrent disease that is resistant to platinum agents.
Our group and another have recently outlined a novel mechanism for resistance to platinum chemotherapy using BRCA2-deficient cancer cell lines.17–19 Secondary mutations that restore functional BRCA2 protein can be induced by exposing BRCA2-deficient pancreatic or ovarian cancer cell lines to cisplatin or PARP inhibitors. The resulting clones with restored BRCA2 are resistant to both cisplatin and PARP inhibitors.17–19 Furthermore, secondary mutations restoring wild-type sequence or a functional reading frame have been seen in a small number of ovarian neoplasms from both BRCA1 and BRCA2 mutation carriers.17–20
This study aimed to define the frequency of secondary mutations that restore BRCA1/2 function within the ovarian carcinomas of BRCA1/2 mutation carriers and correlate these changes with therapeutic outcomes.
METHODS
Patients with primary and/or recurrent ovarian, peritoneal, or fallopian tube carcinomas and a confirmed BRCA1/2 mutation were identified using the University of Washington and Cedars-Sinai Women's Cancer Institute Gynecologic Oncology Tissue Banks, as approved by the Human Subjects Committee of the institutional review board at each institution, as well as one patient from Edinburgh Cancer Research Centre with institutional review board approval through Fred Hutchinson Cancer Research Center. Neoplastic samples were most often formalin-fixed paraffin embedded blocks, with some samples frozen in optimal cutting-temperature compound. One neoplastic sample was isolated from a malignant pleural effusion, using the CELLection Epithelial Enrich protocol (Invitrogen, Carlsbad, CA).
Neoplastic cells in blocks were isolated from surrounding normal cells by laser capture microdissection using a Veritas system (Moelcular Devices, Sunnyvale, CA). Neoplastic DNA was extracted using the PicoPure DNA extraction kit (Arcturus; Applied Biosystems, Foster City, CA), and neoplastic and normal DNA (from lymphocytes) was amplified by polymerase chain reaction (PCR), using primers flanking the known mutation in BRCA1 or BRCA2. PCR products were purified and sequenced with BigDye Terminator v3.1 (Applied Biosystems) using the ABI 3100 Genetic Analyzer (Applied Biosystems). Sequences at the mutation site were analyzed for relative ratios of wild-type and mutant sequences by comparing fluorescent peak heights. In cases with suspected secondary mutations, intragenic heterozygous single nucleotide polymorphisms (SNPs) were identified in lymphocyte DNA and compared with neoplastic DNA to rule out contamination with non-neoplastic cells or retention of the wild-type allele. If a heterozygous SNP was not identified, microsatellite testing was used.
Platinum sensitivity was defined by the response to platinum chemotherapy after the carcinoma was sampled (rather than the usual definition using the treatment-free interval before the recurrence). Carcinomas that responded to platinum chemotherapy and were in remission for at least 6 months after chemotherapy were defined as platinum sensitive; all others were labeled platinum resistant.
The number of patients with a secondary mutation was too small to adequately assess a survival difference between those with and without secondary mutations.
Statistical analysis was performed using Instat or Prism software (Graphpad, San Diego, CA). Contingency tables were made for comparison of categorical variables, and P values were derived using Fisher's exact test.
RESULTS
Description of Samples
We analyzed 64 primary (48 BRCA1, 16 BRCA2) and 46 recurrent (32 BRCA1, 14 BRCA2) ovarian, fallopian tube, and primary peritoneal carcinomas (subsequently collectively referred to as ovarian carcinomas). The 46 recurrent carcinomas were from 44 women, as two women had two separate recurrences analyzed. Seven primary and 15 recurrent carcinomas were previously published.17,18,20 All carcinomas had deletion of the wild-type BRCA1/2 allele, as determined by no wild-type sequence being detected or by confirmation with SNPs or microsatellite testing.
Secondary Mutations in Primary and Recurrent Ovarian Carcinomas
Secondary mutations restoring BRCA1/2 were more common in recurrent ovarian carcinomas (13 [28.3%] of 46; 95% CI, 17.3% to 42.6%) than in primary carcinomas (two [3.1%] of 64; 95% CI, 1.0% to 10.7%; P = .0003, Fisher's exact test). Secondary mutations were more common in recurrent ovarian carcinomas of BRCA2 mutation carriers (six [46.2%] 13; 95% CI, 23.0% to 71.1%,), compared with those of BRCA1 mutation carriers (six [19.4%] 31; 95% CI, 9.2% to 36.4%), but this did not achieve statistical significance (P = .13; Appendix Fig A1, online only).
Of the 46 recurrences, previous chemotherapy regimen information was complete on 42. Twenty-five recurrences were sampled after only one previous chemotherapy regimen, and four had secondary mutations (16%; 95% CI, 6.5% to 34.9%). In contrast, 17 recurrences were sampled after two or more chemotherapy regimens, and eight contained secondary mutations restoring BRCA1/2 (47%; 95% CI, 26.0% to 69.2%; P = .04, Fisher's exact test). Of the four recurrent cases with secondary mutations sampled after only one chemotherapy regimen for ovarian carcinoma, three had received chemotherapy previous to their ovarian cancer diagnosis for breast carcinoma.
Cases with secondary mutations are summarized in Table 1. Two secondary mutations were insertions or deletions that restored a functional reading frame, 11 were reversions to wild-type sequence, one was a synonymous mutation restoring the wild-type amino acid sequence, and one was loss of the mutant BRCA2 allele. An example of sequences from a patient with a secondary mutation is shown in Figure 1.
Table 1.
Characteristics of Women With Secondary Mutations
| Patient | Inherited Mutation | Carcinoma | Stage of Primary | Secondary Mutation | Response of Primary Carcinoma to Platinum | Response of Recurrent Carcinoma to Platinum | History of Previous Breast Cancer | Progression-Free Survival (months) | Interval Since Last Chemotherapy* (months) | Overall Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Primary carcinomas | ||||||||||
| UWF35 | B1.120A>G | Primary fallopian tube | IA | Reversion to wild-type | Nonevaluable | Yes, with chemotherapy | 27 (censored) | 27 (censored) | ||
| UW40 | B1.2594delC | Primary ovarian | IV | Reversion to wild-type | Resistant | Yes, with chemotherapy | 8 | 23 | ||
| Recurrent carcinomas | ||||||||||
| CS1 | B2.5193delC | Recurrent ovarian | IIIC | B2.5177insA | Sensitive | Resistant | No | 24 | 19 | 125 (censored) |
| UWF52 | B1.2800delAA | Recurrent ovarian | IIIC | Reversion to wild-type | Resistant | Resistant | Yes, chemotherapy unknown | 11 | 5 | 27 |
| UWF51 | B1.300T>G | Recurrent primary peritoneal | IIIC | Reversion to wild-type | Resistant | Resistant | No | 6 | 0 | 69 |
| UW400 | B2.4075delGT | Recurrent ovarian | IIIC | Reversion to wild-type | Sensitive | Resistant | Yes, with chemotherapy | 12 | 7 | 16 (censored) |
| UW91 | B1.185delAG | Recurrent ovarian | IIIC | Reversion to wild-type | Sensitive | Resistant | Yes, chemotherapy unknown | 52 | 47 | 89 |
| UW40 | B1.2594delC | Recurrent ovarian | IV | B1.2606_ 2628del23 | Resistant | Resistant | Yes, with chemotherapy | 8 | 2 | 23 |
| UW304 | B2.6174delT | Recurrent ovarian | IIIB | Reversion to wild-type | Sensitive | Resistant | Yes, tamoxifen | 24 | 7 | 98 |
| UW200 | B2.4689delAC | Recurrent ovarian | IIIB | Reversion to wild-type | Sensitive | Sensitive | No | 28 | 23 | 74 (censored) |
| UW200 | B2.4689delAC | 2nd recurrent ovarian† | IIIB | Reversion to wild-type | Sensitive | Resistant | No | 28 | 13 | 74 (censored) |
| UWF27 | B1.185delAG | Recurrent ovarian | IIC | Reversion to wild-type | Sensitive | Resistant | Yes, with chemotherapy | 17 | 12 | 53 |
| UW80 | B1.185delAG | Recurrent ovarian | IIIC | Reversion to wild-type | Sensitive | Resistant | No | 25 | 11 | 109 |
| CS2 | B1.1912T>A B2.8765delAG | Recurrent ovarian | IIC | Loss of mutant BRCA2 allele | Sensitive | Resistant | No | 38 | 33 | 90 |
| PEO1 | B2.5193C>G | Recurrent ovarian | IIIC | B2.5193C>T | Sensitive | Resistant | No | 22 | 16 | 34 |
Defined as the time off chemotherapy before the sampled recurrence.
This is the second recurrent carcinoma for patient UW200, which occurred 13 months after completing chemotherapy for the first recurrent carcinoma. It was ultimately platinum resistant because of disease progression within 2 months of completing another course of platinum chemotherapy.
Fig 1.
Samples from individual with germline BRCA2 5193delC mutation demonstrating a secondary somatic mutation restoring BRCA2 in her ovarian carcinoma. (A) Lymphocyte DNA sequence of the heterozygous germline BRCA2 5193delC mutation. (B) DNA sequence from her primary ovarian carcinoma. Only mutant 5193delC sequence is seen, consistent with loss of the wild-type BRCA2 allele in neoplastic cells. (C) DNA sequence from recurrent ovarian carcinoma obtained 10 years after initial diagnosis. This patient had multiple chemotherapeutic regimens for recurrent disease, including a poly (ADP-ribose) polymerase (PARP) inhibitor before surgical resection of this recurrence (CS1 in Table 1). An insertion of an A base is seen at nucleotide 5177, which restores the open reading frame disrupted by the 5193delC mutation and restoring full-length BRCA2 protein. Both the original BRCA2 5193delC sequence and the 5177insA/5193delC combination sequence are seen, suggesting that the secondary mutation occurs in just one of a duplicated mutant allele or in just a proportion of the neoplastic cells analyzed. Postoperatively, the recurrent carcinoma was resistant to platinum chemotherapy and subsequently to a trial of a PARP inhibitor. (D) Single nucleotide polymorphism (SNP) haplotyping differentiates the germline mutant and wild-type alleles. Lymphocyte DNA demonstrates an A/G SNP within BRCA2. The primary carcinoma has only G, indicating that the G is on the mutant allele. The recurrent carcinoma also has predominantly G, indicating that the secondary mutation occurred on the mutant allele. (E) Allele diagrams show the relative positions of the SNP, the original mutation, and the secondary mutation.
Secondary Mutations and Platinum Resistance
Of 64 primary ovarian carcinomas, four (6.3%) were primarily platinum resistant, 56 were platinum sensitive, three were of unknown platinum sensitivity, and one was nonevaluable for response. One of four platinum-resistant primary ovarian carcinomas had a secondary mutation restoring BRCA1 (25%; 95% CI, 5.3% to 71.4%), as compared with 0 of 56 platinum-sensitive primary carcinomas (95% CI, 0% to 6.3%; P = .07, Fisher's exact test; Fig 2A).
Fig 2.
Secondary mutations in primary and recurrent carcinomas by platinum sensitivity and a history of previous breast cancer. (A) and (C) refer to primary ovarian carcinomas. (B) and (D) refer to recurrent ovarian carcinomas. (A) One (25%) of four platinum-resistant primary ovarian carcinomas had secondary mutations restoring BRCA1/2, compared with 0 of 56 platinum-sensitive primary ovarian carcinomas (P = .07, Fisher's exact test). Three carcinomas had unknown platinum status and one individual was nonevaluable. (B) Twelve (46.2%) of 26 platinum-resistant recurrent ovarian carcinomas had secondary mutations restoring BRCA1/2, compared with one (5%) of 19 platinum-sensitive recurrent ovarian carcinomas (P = .003). One carcinoma had unknown platinum status. Two carcinomas with secondary mutations are from the same patient (one sensitive and one resistant), and two carcinomas without secondary mutations are from the same patient (one sensitive and one resistant). (C) Two (10.5%) of 19 primary ovarian carcinomas obtained from women with a history of breast carcinoma had secondary mutations restoring BRCA1, compared with 0 of 45 obtained from women with BRCA1/2 mutations and no history of breast carcinoma (P = .08, Fisher's exact test). (D) Six (66.7%) of nine recurrent ovarian carcinomas from women with a history of breast carcinoma before their diagnosis of ovarian carcinoma had secondary mutations restoring BRCA1/2, compared with six (17.1%) of 35 recurrent ovarian carcinomas from women with no prior history of breast carcinoma (P = .007, Fisher's exact test).
In contrast, of 46 recurrent ovarian carcinomas, 26 (56.5%) were platinum resistant (defined as recurrence within 6 months of stopping secondary chemotherapy after the recurrent carcinoma was sampled), 19 were platinum sensitive, and one was unknown. Twelve of 26 platinum-resistant recurrences had secondary mutations restoring BRCA1/2 (46.2%; 95% CI, 28.7% to 64.7%), as compared with one of 19 platinum-sensitive recurrences (5.3%; 95% CI, 1.2% to 24.8%; P = .003, Fisher's exact test, Fig 2B).
One primary carcinoma with a secondary mutation occurred in a 0.8-mm stage IA fallopian tube carcinoma diagnosed at the time of risk-reducing salpingo-oophorectomy, in a carrier of a germline BRCA1 120A>G mutation who was previously treated with chemotherapy for breast cancer (UWF35, Table 1). She received three cycles of docetaxel and carboplatin for the tubal carcinoma and has no evidence of recurrence 2 years later. She was considered nonevaluable for response given her lack of residual disease after initial surgery. The other patient with a secondary mutation in a primary carcinoma had a germline BRCA1 2594delC mutation and stage IV primary platinum-resistant disease (UW40, Table 1).20 She also was treated previously with chemotherapy for breast cancer (unknown agents). She subsequently experienced recurrence within 3 months of completing primary platinum-containing chemotherapy.
The patient with a secondary mutation in a platinum-sensitive recurrent carcinoma (UW200) initially presented with stage IIIB ovarian carcinoma, treated with primary surgery and six cycles of carboplatin and paclitaxel. She then experienced recurrence 3 years later, when the recurrent sample with a secondary mutation was obtained. This was a small-volume recurrence that was removed with no visible residual disease. She was treated with intraperitoneal cisplatin and intravenous paclitaxel for six cycles and did not experience recurrence again for 13 months after completing that regimen. Her recurrence at that time was also found to have a secondary mutation and was platinum resistant with disease progression within 2 months of completing platinum-based therapy. Interestingly, her recurrent carcinoma has subsequently had a partial response on a clinical trial combining carboplatin, gemcitabine, and a PARP inhibitor. However, the lack of a complete response to platinum is consistent with platinum resistance.
Secondary Mutations and Response to PARP Inhibition
Six patients with platinum-resistant recurrent ovarian carcinomas were treated with PARP inhibitors (Table 2). Three carcinomas had secondary mutations restoring BRCA1/2 (including the one just described), two did not respond, and one had a partial response. Two patients who did not have secondary mutations were noted to have a complete response to PARP inhibition, and one had a partial response.
Table 2.
Summary of Platinum-Resistant Recurrent Ovarian Carcinomas Treated With PARP Inhibition
| Patient | Inherited Mutation | Carcinoma | Stage of Primary Carcinoma | Secondary Genetic Change in Carcinoma | PARP-Inhibitor Containing Regimen | Best Response to PARP-Inhibitor Containing Regimen |
|---|---|---|---|---|---|---|
| CS1 | B2.5193delC | Recurrent ovarian | IIIC | 5177insA | AZD-2281 | Progressive disease |
| UW200 | B2.4689delAC | Recurrent ovarian | IIIB | Reversion to wild-type | ABT-888 with carboplatin and gemcitabine | Partial response |
| UW80 | B1.185delAG | Recurrent ovarian | IIIC | Reversion to wild-type | AZD-2281 | Progressive disease |
| CS25 | B1.185delAG | Recurrent ovarian | IIC | No secondary mutation | AZD-2281 and carboplatin | Complete response |
| CS15 | B1.5382insC | Recurrent ovarian | IIIC | No secondary mutation | AZD-2281 | Complete response |
| UW336 | B1.5083del19 | Recurrent ovarian | IIIC | No secondary mutation | AZD-2281 | Partial response |
Abbreviation: PARP, poly (ADP-ribose) polymerase.
Secondary Mutations and History of Previous Breast Carcinoma and Chemotherapy
A history of breast carcinoma occurring before the primary ovarian carcinoma was present in 19 (29.7%) of 64 patients who had primary carcinomas sequenced. Two (10.5%) of 19 (95% CI, 3.2% to 31.7%) had secondary mutations in their primary ovarian carcinoma after a previous breast carcinoma, compared with 0 of 45 with no history of breast carcinoma (95% CI, 0 to 7.7%; P = .08, Fisher's exact test; Fig 2C). Data regarding prior treatment with chemotherapy was incomplete; however, at least 11 of 19 had chemotherapy for breast carcinoma before diagnosis with ovarian carcinoma. Interestingly, the two primary carcinomas with secondary mutations that restored BRCA1 had received their chemotherapy for breast carcinoma 10 and 11 years before their diagnosis of fallopian tube or ovarian carcinoma, respectively.
Of 44 women with recurrent ovarian carcinomas (two women had two recurrences), nine (20.5%) had been previously treated for breast carcinoma. Six of nine recurrent ovarian carcinomas in women with a history of breast carcinoma had secondary mutations (66.7%; 95% CI, 34.8% to 87.8%), compared with six of 35 recurrent ovarian carcinomas in women with no history of breast carcinoma (17.1%; 95% CI, 8.2% to 32.8%; P = .007, Fisher's exact test; Fig 2D). Information on chemotherapy for breast carcinoma was incomplete; however, at least four of nine women had chemotherapy for breast carcinoma before their primary ovarian carcinoma, and three of four had secondary mutations restoring BRCA1/2. To date, no secondary mutation has been identified in a primary or recurrent ovarian carcinoma in an individual without any history of prior chemotherapy. Of women with breast carcinoma and a secondary mutation, the range of time from breast cancer diagnosis to ovarian cancer diagnosis was 60 to 156 months (median, 115.5 months).
Five women received neoadjuvant chemotherapy immediately before surgery for their primary ovarian carcinoma (range, three to four cycles), and none had detectable secondary mutations in their primary carcinomas.
Timing of Secondary Mutations
Patient UW40 had a 23 base-pair (bp) deletion downstream from her BRCA1 2594delC mutation that restores a functional reading frame (Fig 3A), as previously described.20 Primers were designed to PCR amplify only the sequence containing the secondary 23-bp deletion. Using Sanger sequencing, this 23-bp deletion was detectable only in the recurrent carcinoma and not in the paired primary carcinoma from the same individual. However, PCR amplification using the mutation-specific primers detected the secondary mutation in the patient's primary carcinoma (Fig 3B), indicating that the secondary mutation in the recurrent carcinoma was present in a minority of neoplastic cells at the time of diagnosis.
Fig 3.
A secondary mutation in the recurrent ovarian carcinoma identified at low levels in the primary carcinoma. (A) Sequences from UW40. Lymphocyte DNA demonstrates the BRCA1 heterozygous germline mutation 2594delC and two heterozygous single nucleotide polymorphisms (SNPs). The primary ovarian carcinoma sequence shows both the 2594delC mutation and wild-type sequence, consistent with a secondary mutation restoring wild-type sequence. SNPs within the primary carcinoma confirm that only one allele is present, indicating that the secondary mutation occurred on the mutant allele, and the wild-type sequence present was not from retained wild-type allele. The recurrent ovarian carcinoma sequence has a 23-bp deletion that, when combined with the 2594delC mutation, restores the open reading frame. The 23-bp deletion is not detectable in the primary carcinoma using conventional sequencing. Adapted with permission from Swisher et al.20 (B) Mutation-specific primers were designed to amplify sequences with the 23-bp deletion only using polymerase chain reaction. As expected, the 23-bp deletion amplifies well in the recurrent carcinoma (Rec Ca). This 23-bp deletion sequence can also be detected in two separate samples of the primary ovarian carcinoma, from the ovary (Prim Ca Ovary) and from a metastatic lesion in the omentum (Prim Ca Om). Control samples of an unrelated individual's carcinoma (Control DNA), the UW40's lymphocyte DNA (Lymph), and water (H20) do not have detectable sequence.
DISCUSSION
In this large series of BRCA1/2-mutated ovarian, tubal, and peritoneal carcinomas, the presence of secondary somatic mutations that restore BRCA1/2 in recurrent carcinomas was significantly correlated with response to platinum-based chemotherapy, with 12 (92%) of 13 carcinomas with restored BRCA1/2 proving to be platinum-resistant (95% CI, 66.1% to 98.2%). When treating ovarian carcinomas, oncologists traditionally have used the interval from completion of platinum-based chemotherapy to disease progression to predict platinum sensitivity or resistance. When the treatment-free interval is greater than 6 months before progression, the patient is thought to have a platinum-sensitive recurrence and is often re-treated with platinum-based chemotherapy. In our series, the presence of a secondary mutation restoring BRCA1/2 was a better predictor of platinum resistance than interval since last treatment (Table 1). Of 26 recurrent carcinomas that proved to be platinum resistant, 12 (46.2%) of 26 (95% CI, 28.7% to 64.7%) could have been predicted by detection of a secondary mutation restoring BRCA1/2, as opposed to only four (15.4%) of 26 by treatment-free interval (95% CI, 6.3% to 33.7%; P = .03, Fisher's exact test, two-tailed). Additionally, secondary mutations were significantly more common in recurrent carcinomas sampled after more than one chemotherapy regimen than those sampled after a single chemotherapy regimen. The ability to predict response to platinum chemotherapy is clinically valuable, as it would allow the avoidance of unnecessary toxicity and earlier use of alternate agents. Although our data support the clinical utility of testing for secondary mutations in BRCA1/2-mutated ovarian carcinomas to predict platinum resistance, the clinical implementation may be challenging because this is a technically demanding test, requiring careful microdissection to obtain a pure neoplastic cell population. Additionally, the differentiation of a secondary reversion mutation from contamination with wild-type sequence from non-neoplastic cells requires careful allelic identification with SNP haplotyping or microsatellite testing.
There was a marked association between prior history of breast carcinoma and secondary mutations in either the primary or recurrent ovarian carcinomas, with the breast carcinoma often preceding the ovarian carcinoma by many years. Cumulative chemotherapy exposure is the most likely contributor to these secondary genetic changes. Cyclophosphamide, commonly used in patients with breast carcinoma, is a DNA cross-linking agent and theoretically would induce or select for BRCA1/2 restoration in similar fashion to platinum compounds.21 The occurrence of secondary mutations in ovarian carcinomas more than a decade after chemotherapy for breast cancer is not easily explainable. If breast cancer chemotherapy induced a secondary mutation that restored wild-type sequence in a non-neoplastic ovarian or tubal epithelial cell, such a cell would be homozygous for wild-type BRCA1/2 and presumably be subsequently protected from neoplastic transformation. Alternatively, hypothesizing that the secondary mutation occurs in a cell that is already BRCA1/2 deficient (secondary to deletion of the wild-type allele) implies that the earliest neoplastic cell exists many years before clinical diagnosis. A better understanding of the timing of secondary mutations will shed light on the natural history and molecular progression of hereditary ovarian carcinomas. The association of breast carcinoma with secondary mutations in subsequent ovarian carcinomas could also be unrelated to chemotherapy and perhaps reflects a more severe phenotype in those patients.
The timing of when secondary mutations develop during the treatment of ovarian carcinoma is clinically important. Secondary mutations may be induced by DNA-damaging chemotherapy either for breast carcinoma or primary ovarian carcinoma. Alternatively, secondary mutations could be present in the primary carcinoma due to genomic instability and then selected by chemotherapy. Data from patient UW40 (Fig 3) reveals that secondary mutations can be present in rare cells in the primary carcinoma. Her secondary mutation may have been induced by chemotherapy for breast carcinoma. Whether secondary mutations restoring BRCA1/2 exist in primary ovarian carcinomas in women with no previous chemotherapy exposure is not known. In chronic myelogenous leukemia, BCR-ABL mutations that confer resistance to imatinib therapy in some cases are detectable by PCR in rare neoplastic cells before imatinib exposure.22 If secondary mutations that restore BRCA1/2 are already present in a small percentage of cells in a primary ovarian carcinoma similarly, this could have therapeutic implications. It is possible that the use of PARP inhibitors for prolonged consolidation after primary therapy could accelerate the selection of BRCA1/2-restored neoplastic cells and consequently accelerate the development of platinum resistance after recurrence.
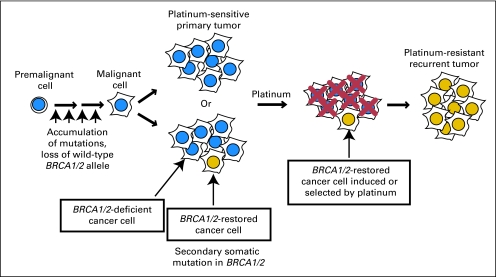
These data add to the current understanding of BRCA1/2 carcinogenesis. Loss of heterozygosity in BRCA1/2 leads to a carcinoma deficient in BRCA1/2 protein. This carcinoma with decreased ability to repair DNA then becomes highly sensitive to DNA cross-linking agents such as platinum drugs. Secondary mutations restoring DNA repair function of BRCA1/2 can occur within the carcinoma, likely as a consequence of prior chemotherapy or perhaps from genomic instability and spontaneous mutation. This restoration of DNA repair can then lead to acquired resistance to platinum and likely resistance to PARP inhibition (Fig 4).
Fig 4.
Model for acquired resistance to platinum chemotherapy by secondary mutations restoring BRCA1/2. Secondary mutation is hypothesized to occur as a result of DNA-damaging chemotherapy, either from primary adjuvant therapy of the ovarian carcinoma or from previous chemotherapy for a separate carcinoma such as breast. This population of cells with restored BRCA1/2 and improved DNA repair is either induced or selected by adjuvant platinum therapy. Adapted with permission from Sakai et al.18
Not all platinum-resistant recurrent carcinomas had detectable secondary somatic mutations, indicating that other mechanisms of acquired resistance occur. Possibly, we missed some larger insertions or deletions that restore the BRCA1/2 reading frame,19 as these alterations would be missed with the relatively short amplicons generated by our PCR of formalin-fixed tissues. Additionally, our retrospective series does not represent all women with BRCA1/2-associated ovarian carcinoma as we selected those cases who had known mutations as opposed to prospectively testing all cases. BRCA1/2 mutation carriers selected in this fashion are likely to include a higher proportion of women with two primary cancers as well as women with better survival who are more likely to undergo clinical genetic testing.
Secondary mutations in BRCA1/2 could also confer resistance to PARP inhibitors. Neoplastic cells with restored BRCA1/2 are resistant to PARP inhibitors,17–19 and platinum-resistant BRCA2-mutated pancreatic cancer clones without secondary BRCA2 mutations remain sensitive to PARP inhibition in vitro.18 Response data from our small number of cases treated with PARP inhibitors support the in vitro data that secondary BRCA1/2 mutations predict PARP inhibitor resistance. However, larger studies are needed to determine whether testing for secondary BRCA1/2 mutations could be used to determine which patients will benefit from PARP inhibition.
Supplementary Material
Acknowledgment
We thank Simon Langdon, PhD, for providing tumor and clinical information for sample PE01. We thank Mary-Claire King, PhD, Piri Welcsh, PhD, and Tom Walsh, PhD, for guidance and assistance.
Appendix
Fig A1.
Distribution of BRCA1 and BRCA2 mutations in primary and recurrent carcinomas by secondary mutation status. (A) Two of 48 BRCA1 mutation carriers with primary carcinomas had secondary mutations restoring BRCA1/2, compared with 0 of 16 BRCA2 mutation carriers (P = 1.00, Fisher's exact test). (B) Six of 31 BRCA1 mutation carriers with recurrent carcinomas had secondary mutations restoring BRCA1/2, compared with six of 13 BRCA2 mutation carriers (P = .13, Fisher's exact test).
Footnotes
Supported by National Institutes of Health Grants No. P50CA83636 and R01CA125636, Marsha Rivkin Center Pilot Study Program Grant, and Howard Hughes Medical Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Barbara Norquist, Toshiyasu Taniguchi, Elizabeth M. Swisher
Financial support: Toshiyasu Taniguchi, Elizabeth M. Swisher
Administrative support: Elizabeth M. Swisher
Provision of study materials or patients: Elizabeth M. Swisher
Collection and assembly of data: Barbara Norquist, Kaitlyn A. Wurz, Christopher C. Pennil, Rochelle Garcia, Jenny Gross, Wataru Sakai, Beth Y. Karlan, Toshiyasu Taniguchi, Elizabeth M. Swisher
Data analysis and interpretation: Barbara Norquist, Toshiyasu Taniguchi, Elizabeth M. Swisher
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pal T, Permuth-Wey J, Betts JA, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 2.Risch HA, McLaughlin JR, Cole DE, et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moynahan ME, Chiu JW, Koller BH, et al. Brca1 controls homology-directed DNA repair. Mol Cell. 1999;4:511–518. doi: 10.1016/s1097-2765(00)80202-6. [DOI] [PubMed] [Google Scholar]
- 4.Moynahan ME, Pierce AJ, Jasin M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol Cell. 2001;7:263–272. doi: 10.1016/s1097-2765(01)00174-5. [DOI] [PubMed] [Google Scholar]
- 5.Neuhausen SL, Marshall CJ. Loss of heterozygosity in familial tumors from three BRCA1-linked kindreds. Cancer Res. 1994;54:6069–6072. [PubMed] [Google Scholar]
- 6.Collins N, McManus R, Wooster R, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995;10:1673–1675. [PubMed] [Google Scholar]
- 7.Gudmundsson J, Johannesdottir G, Bergthorsson JT, et al. Different tumor types from BRCA2 carriers show wild-type chromosome deletions on 13q12-q13. Cancer Res. 1995;55:4830–4832. [PubMed] [Google Scholar]
- 8.Foulkes WD. BRCA1 and BRCA2: Chemosensitivity, treatment outcomes and prognosis. Fam Cancer. 2006;5:135–142. doi: 10.1007/s10689-005-2832-5. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharyya A, Ear US, Koller BH, et al. The breast cancer susceptibility gene BRCA1 is required for subnuclear assembly of Rad51 and survival following treatment with the DNA cross-linking agent cisplatin. J Biol Chem. 2000;275:23899–23903. doi: 10.1074/jbc.C000276200. [DOI] [PubMed] [Google Scholar]
- 10.Yuan SS, Lee SY, Chen G, et al. BRCA2 is required for ionizing radiation-induced assembly of Rad51 complex in vivo. Cancer Res. 1999;59:3547–3551. [PubMed] [Google Scholar]
- 11.Audeh MW, Carmichael J, Penson RT, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 12.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 13.Gien LT, Mackay HJ. The emerging role of PARP inhibitors in the treatment of epithelial ovarian cancer. J Oncol. 2010;2010:151750. doi: 10.1155/2010/151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd J, Sonoda Y, Federici MG, et al. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–2265. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 15.Chetrit A, Hirsh-Yechezkel G, Ben-David Y, et al. Effect of BRCA1/2 mutations on long-term survival of patients with invasive ovarian cancer: The national Israeli study of ovarian cancer. J Clin Oncol. 2008;26:20–25. doi: 10.1200/JCO.2007.11.6905. [DOI] [PubMed] [Google Scholar]
- 16.Cass I, Baldwin RL, Varkey T, et al. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–2195. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 17.Sakai W, Swisher EM, Jacquemont C, et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 2009;69:6381–6386. doi: 10.1158/0008-5472.CAN-09-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakai W, Swisher EM, Karlan BY, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451:1116–1120. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards SL, Brough R, Lord CJ, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451:1111–1115. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 20.Swisher EM, Sakai W, Karlan BY, et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68:2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming RA. An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy. 1997;17:146S–154S. [PubMed] [Google Scholar]
- 22.Roche-Lestienne C, Soenen-Cornu V, Grardel-Duflos N, et al. Several types of mutations of the Abl gene can be found in chronic myeloid leukemia patients resistant to STI571, and they can pre-exist to the onset of treatment. Blood. 2002;100:1014–1018. doi: 10.1182/blood.v100.3.1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.