Abstract
Purpose
High-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) are frequently used in an attempt to improve outcome in patients with mantle-cell lymphoma (MCL); however, the importance of intensive induction regimens before transplantation is unknown.
Patients and Methods
To address this question, we evaluated baseline characteristics, time to treatment, induction regimen, disease status at the time of transplantation, and MIPI score at diagnosis and their associations with survival in 118 consecutive patients with MCL who received HDT and ASCT at our centers.
Results
The MIPI was independently associated with survival after transplantation in all 118 patients (hazard ratio [HR], 3.5; P < .001) and in the 85 patients who underwent ASCT as initial consolidation (HR, 7.2; P < .001). Overall survival rates were 93%, 60%, and 32% at 2.5 years from ASCT for all patients with low-, intermediate-, and high-risk MIPI, respectively. Low-risk MIPI scores were more common in the intensive induction group than the standard induction group in all patients (64% v 46%, respectively; P = .03) and in the initial consolidation group (66% v 45%, respectively; P = .03). After adjustment for the MIPI, an intensive induction regimen was not associated with improved survival after transplantation in all patients (HR, 0.5; P = .10), the initial consolidation group (HR, 1.1; P = .86), or patients ≤ 60 years old (HR, 0.6; P = .50). Observation of more than 3 months before initiating therapy did not yield inferior survival (HR, 2.1; P = .12) after adjustment for the MIPI in patients receiving ASCT.
Conclusion
An intensive induction regimen before HDT and ASCT was not associated with improved survival after adjusting for differences in MIPI scores at diagnosis.
INTRODUCTION
Mantle-cell lymphoma (MCL) accounts for approximately 6% of all non-Hodgkin's lymphoma, exhibits short remission durations, and carries a poor prognosis, with a median survival of 4 to 5 years.1–3 Phase II and limited phase III trials suggest that outcomes can be improved with the use of high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) once patients achieve their first remission.4–9 Furthermore, single-arm and retrospective studies suggest that patients receiving intensive high-dose cytarabine-containing induction regimens, such as hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (HyperCVAD), experience improved survival.4,10–12 However, methods to compare outcomes between studies using varied induction regimens remain problematic because patients with high-risk disease features but younger age and good performance status may have been offered intensive regimens, whereas older, frail patients or patients with lower risk disease may have been offered less aggressive inductions. Thus, the relative benefit of these intense initial strategies remains uncertain. Finally, single-center data suggest that an initial strategy of observation after diagnosis may be acceptable for certain patients, yet the impact of this approach in patients who are destined for HDT and ASCT is unknown.13
The Mantle Cell Lymphoma International Prognostic Index (MIPI) has been recently generated as a prognostic tool specific for patients with MCL.14 Its scoring is based on a complex mathematical model using four clinical variables. Hoster et al14 developed a simplified point scoring index, the simplified MIPI (sMIPI), that has demonstrated a high concordance with the original MIPI.14,15 The predictive value of the MIPI or the sMIPI in patients with MCL receiving HDT followed by ASCT is not yet defined in an unselected group of patients receiving varied induction regimens. The Nordic Lymphoma Group reported a positive correlation of the MIPI and sMIPI with the overall survival (OS) of patients uniformly treated on the Nordic Lymphoma Group MCL2 protocol followed by HDT and ASCT.15 In contrast, van't Veer et al12 found no prognostic value of MIPI in patients with MCL who were responsive to rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and who received high-dose cytarabine followed by HDT and ASCT.
We hypothesized that if the MIPI was able to predict outcome after HDT and ASCT, then it could be used to provide an adjusted comparison such that the impact of the induction regimen could be assessed in a group of patients receiving varied pretransplantation therapies. Herein, we report the results of this analysis demonstrating the association of the MIPI with survival in a group of unselected patients with MCL undergoing ASCT, and to our knowledge, we are the first to use this score to account for differences in outcomes between patients receiving intensive and standard induction regimens. We also provide the primary data on the MIPI-adjusted outcomes of an initial watch-and-wait approach before induction therapy and ASCT.
PATIENTS AND METHODS
Patient Selection
All patients older than age 18 years with a confirmed diagnosis of MCL receiving HDT and ASCT between May 1993 and May 2010 at the Fred Hutchinson Cancer Research Center, University of Washington Medical Center, and Veterans Affairs Puget Sound Health Care System in Seattle, Washington, were evaluated. Patients who received a planned tandem autologous-allogeneic transplantation or syngeneic transplantation were excluded. The Institutional Review Board of the Fred Hutchinson Cancer Research Center approved this study.
Data Collection and Definitions
Clinical information was reviewed, and baseline characteristics, including factors that make up the MIPI at the time of diagnosis (age, ratio of lactate dehydrogenase to its upper limit of normal, total WBC counts, and Eastern Cooperative Oncology Group performance status), were recorded. The MIPI and sMIPI scores were calculated based on the formula described by Hoster et al.14 Disease progression, OS, and progression-free survival (PFS) were defined by standard criteria16 and measured from the time of transplantation. Patients were considered to have chemotherapy-sensitive disease if they had either a partial response (PR) or complete response to the most recent therapy before the transplantation. Patients were considered to have chemotherapy-resistant disease if they had less than a PR to the most recent therapy before the transplantation. Intensive induction regimens were prespecified as those containing high-dose cytarabine and included HyperCVAD with or without rituximab,6 whereas standard induction regimens included all others (CHOP; R-CHOP; etoposide, doxorubicin, vincristine, prednisone, and cyclophosphamide [ EPOCH]; rituximab plus EPOCH; cyclophosphamide, vincristine, and prednisone [CVP]; rituximab plus CVP; rituximab plus ifosfamide, carboplatin, and etoposide; single-agent rituximab; or fludarabine). Patients who did not receive treatment within 3 months after their disease diagnosis were assigned in the initial observation group. Patients who received HDT and ASCT immediately after their initial induction regimen were included in the initial consolidation group.
Statistical Analysis
Patient characteristics were compared between patients who received intensive and standard induction regimens by t test or χ2 test as appropriate. Kaplan-Meier curves were used to estimate the probabilities of OS and PFS. The statistical significance of differences in event rates was evaluated with the Cox proportional hazards regression model. For comparisons in which no deaths were observed in a group, a generalized Wilcoxon test was applied. Factors considered as potential confounders of the relationships between the MIPI score at diagnosis and induction regimen with OS and PFS included sex, transplantation year, and time from diagnosis to first treatment (≤ or > 3 months). Such factors were retained in the model if their presence influenced either coefficient of interest by ≥ 10%. Reported P values are based on the Wald statistic. Two-sided P < .05 was considered statistically significant. Statistical analyses were performed using SAS Version 9.0 (SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
We identified 118 consecutive patients with MCL who met the previously detailed criteria and had all relevant requisite characteristics available, including the MIPI and sMIPI at diagnosis. The baseline features of these patients are listed in Table 1. At diagnosis of MCL, the median age was 57 years (range, 35 to 70 years), 86% of patients were men, 97% of patients had stage III or IV disease, 9% of patients had blastoid variant pathologic subtype, and 86% of patients had chemotherapy-sensitive disease before transplantation. ASCT was performed as initial consolidation in 85 patients, including 56 patients in first complete response and 26 patients in first PR. With regard to MIPI factors, 97% of patients had an Eastern Cooperative Oncology Group performance score of 0 to 1, the median ratio of lactate dehydrogenase to its upper limit of normal was 0.9 (range, 0.5 to 10.7), and median WBC count was 7.5 × 109/L (range, 1.4 to 132.0 × 109/L). Sixty-three patients at diagnosis had a low-risk MIPI score, 35 patients had an intermediate-risk score, and 20 patients had a high-risk score. Forty percent of patients (n = 47) received HyperCVAD plus rituximab as the induction regimen, whereas 60% received other induction regimens including R-CHOP (n = 45), CHOP (n = 12), and other standard regimens (n = 14). One hundred five patients (89%) received their induction regimen within 3 months of diagnosis (median observation, 1.0 month; range, 0.1 to 3.0 months). Thirteen patients (11%) were observed for at least 3 months before initiation of treatment.
Table 1.
Baseline Demographics and Clinical Characteristics of All Patients at Diagnosis
| Characteristic | All Patients (N = 118) |
Intensive Induction (n = 47) |
Standard Induction (n = 71) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. of Patients | % | No. of Patients | % | ||
| Age, years | .01 | ||||||
| Median | 57 | 54 | 58 | ||||
| Range | 35-70 | 38-69 | 35-70 | ||||
| Sex | .34 | ||||||
| Female | 17 | 14 | 5 | 11 | 12 | 17 | |
| Male | 101 | 86 | 42 | 89 | 59 | 83 | |
| Performance score | .24 | ||||||
| 0 | 83 | 70 | 35 | 74 | 48 | 68 | |
| 1-3 | 35 | 30 | 12 | 26 | 23 | 32 | |
| LDH/ULN ratio | .85 | ||||||
| Median | 0.9 | 0.9 | 0.9 | ||||
| Range | 0.5-10.7 | 0.5-10.7 | 0.5-5.7 | ||||
| WBC, ×109/L | .67 | ||||||
| Median | 7.5 | 7.8 | 7.3 | ||||
| Range | 1.4-132.0 | 2.7-132.0 | 1.4-54.7 | ||||
| MIPI at diagnosis | .03 | ||||||
| Low risk | 63 | 53 | 30 | 64 | 33 | 46 | |
| Intermediate risk | 35 | 30 | 13 | 28 | 22 | 31 | |
| High risk | 20 | 17 | 4 | 9 | 16 | 23 | |
| Induction regimen | |||||||
| R-HyperCVAD | 46 | 39 | 46 | 99 | 0 | 0 | |
| HyperCVAD | 1 | 1 | 1 | 1 | 0 | 0 | |
| R-CHOP | 45 | 38 | 0 | 0 | 45 | 63 | |
| CHOP | 12 | 10 | 0 | 0 | 12 | 17 | |
| Other | 14 | 12 | 0 | 0 | 14 | 20 | |
| Time from diagnosis to treatment, months | .48 | ||||||
| ≤ 3 | 105 | 89 | 43 | 91 | 62 | 87 | |
| > 3 | 13 | 11 | 4 | 9 | 9 | 13 | |
| Blastoid variant | 11 | 9 | 3 | 6 | 8 | 11 | .32 |
| Disease status | .003 | ||||||
| Initial consolidation | |||||||
| First CR | 56 | 47 | 30 | 63 | 26 | 37 | |
| First PR | 26 | 22 | 10 | 21 | 16 | 23 | |
| Primary refractory | 3 | 3 | 1 | 2 | 2 | 3 | |
| Relapsed | 33 | 28 | 6 | 13 | 27 | 38 | |
| Chemotherapy sensitive | .01 | ||||||
| No | 17 | 14 | 2 | 4 | 15 | 21 | |
| Yes | 101 | 86 | 45 | 96 | 56 | 79 | |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CR, complete response; HyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index; PR, partial response; R, rituximab; ULN, upper limit of normal.
The baseline features of the 85 patients undergoing HDT and ASCT as initial consolidation are listed in Table 2. Forty-one patients underwent intensive induction, and 44 received a standard regimen. Patients who received an intensive induction were more likely to have a lower risk as estimated by the MIPI score (P = .03, χ2 test for trend) than patients who received a standard induction regimen.
Table 2.
Baseline Demographic and Clinical Characteristics at Diagnosis of Patients Receiving High-Dose Therapy and Autologous Stem-Cell Transplantation As Initial Consolidation
| Characteristic | Intensive Induction (n = 41) |
Standard Induction (n = 44) |
P | ||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | ||
| Age, years | .003 | ||||
| Median | 54 | 59 | |||
| Range | 38-69 | 35-70 | |||
| Sex | .62 | ||||
| Female | 5 | 12 | 7 | 16 | |
| Male | 36 | 88 | 37 | 84 | |
| Performance score | .41 | ||||
| 0 | 29 | 71 | 29 | 66 | |
| 1-3 | 12 | 29 | 15 | 34 | |
| LDH/ULN ratio | .77 | ||||
| Median | 0.9 | 0.8 | |||
| Range | 0.5-10.7 | 0.5-5.7 | |||
| WBC, ×109/L median (range) | .65 | ||||
| Median | 7.8 | 7.4 | |||
| Range | 2.7-132.0 | 1.4-48.0 | |||
| MIPI at diagnosis | .03 | ||||
| Low risk | 27 | 66 | 20 | 45 | |
| Intermediate risk | 11 | 27 | 15 | 34 | |
| High risk | 3 | 7 | 9 | 20 | |
| Induction regimen | — | ||||
| HyperCVAD ± R | 41 | 100 | 0 | 0 | |
| R-CHOP | 0 | 0 | 33 | 75 | |
| CHOP | 0 | 0 | 5 | 11 | |
| Other | 0 | 0 | 6 | 14 | |
| Time from diagnosis to treatment, months | |||||
| ≤ 3 | 37 | 90 | 40 | 91 | |
| > 3 | 4 | 10 | 4 | 9 | .92 |
| Blastoid variant (n = 37) | 2 | 5 | 2 | 5 | .99 |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; HyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; LDH, lactate dehydrogenase; MIPI, Mantle Cell Lymphoma International Prognostic Index; R, rituximab; ULN, upper limit of normal.
OS and PFS
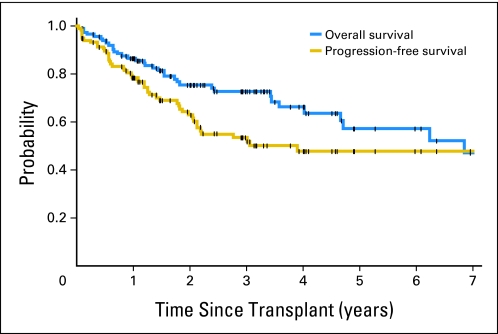
At last contact, 81 (69%) of 118 patients were alive, and 68 patients (58%) were alive and progression free, resulting in an estimated 5-year OS of 57.2% (95% CI, 42.7% to 69.2%) and PFS of 47.9% (95% CI, 36.4% to 58.5%), with a median follow-up time for surviving patients of 2.4 years (range, 0.1 to 12.6 years; Fig 1).
Fig 1.
Overall and progression-free survival of 118 patients with mantle-cell lymphoma with evaluable Mantle Cell Lymphoma International Prognostic Index scores who underwent high-dose therapy and autologous stem-cell transplantation. Tick marks represent censor times.
Association of MIPI With OS and PFS
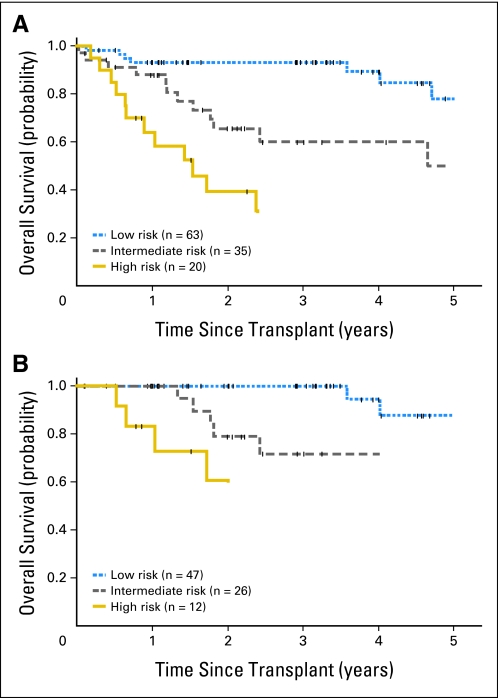
The estimated 2.5-year OS was 93% for patients with low-risk MIPI, 60% for patients with intermediate-risk MIPI (hazard ratio [HR] for death, 2.7; 95% CI, 1.2 to 6.2; P = .02), and 32% for patients with high-risk MIPI (HR for death, 10.1; 95% CI, 4.2 to 24.2; P < .001). Fourteen of 20 patients with high-risk MIPI died within 3.5 years of ASCT (Fig 2A). Similarly, the estimated 1.5-year PFS was 87% for patients with low-risk MIPI, 57% for patients with intermediate-risk MIPI (HR for death or progression, 3.1; 95% CI, 1.5 to 6.3; P = .002), and 31% for patients with high-risk MIPI (HR for death or progression, 8.2; 95% CI, 3.9 to 17.2; P < .001). Multivariable modeling evaluating all 118 patients with MCL undergoing HDT and ASCT identified the MIPI at diagnosis as an independent predictor of OS (HR, 3.5; 95% CI, 2.1 to 6.0; P < .001; Table 3) and PFS (HR, 2.9; 95% CI, 1.9 to 4.6; P < .001). Likewise, the MIPI remained independently associated with OS (HR, 7.2; 95% CI, 3.0 to 17.1; P < .001; Table 3, Fig 2B) and PFS (HR, 3.9; 95% CI, 2.0 to 7.5; P < .001) when limiting this analysis to patients who received HDT and ASCT as initial consolidation.
Fig 2.
(A) Overall survival of patients with mantle-cell lymphoma (MCL; n = 118) undergoing high-dose therapy (HDT) and autologous stem-cell transplantation (ASCT) stratified by the Mantle Cell Lymphoma International Prognostic Index (MIPI) at diagnosis (P < .001). (B) Overall survival of patients with MCL (n = 85) undergoing HDT and ASCT as initial consolidation stratified by the MIPI at diagnosis (P < .001). Tick marks represent censor times.
Table 3.
Multivariable Model of OS and PFS for All Patients and for Patients Who Received Transplantation As Initial Consolidation
| Factor | OS |
PFS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| All patients (N = 118) | ||||||
| MIPI at diagnosis (linear trend) | 3.5 | 2.1 to 6.0 | < .001 | 2.9 | 1.9 to 4.6 | < .001 |
| Induction regimen | ||||||
| Standard* | 1.0 | 1.0 | ||||
| Intensive† | 0.5 | 0.2 to 1.1 | .10 | 0.4 | 0.2 to 0.8 | .01 |
| Time from diagnosis to treatment, months | ||||||
| ≤ 3 | 1.0 | 1.0 | ||||
| > 3 | 2.1 | 0.8 to 5.3 | .12 | 1.6 | 0.7 to 3.7 | .26 |
| Patients with initial consolidation (n = 85) | ||||||
| MIPI at diagnosis (linear trend) | 7.2 | 3.0 to 17.1 | < .001 | 3.9 | 2.0 to 7.5 | < .001 |
| Induction regimen | ||||||
| Standard* | 1.0 | 1.0 | ||||
| Intensive† | 1.1 | 0.3 to 3.6 | .86 | 0.6 | 0.2 to 1.5 | .26 |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; HR, hazard ratio; HyperCVAD, hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MIPI, Mantle Cell Lymphoma International Prognostic Index; OS, overall survival; PFS, progression-free survival; R, rituximab.
CHOP or other regimen with or without R, but without high-dose cytarabine.
HyperCVAD/methotrexate/high-dose cytarabine with or without R.
Association of Induction Regimen With OS and PFS
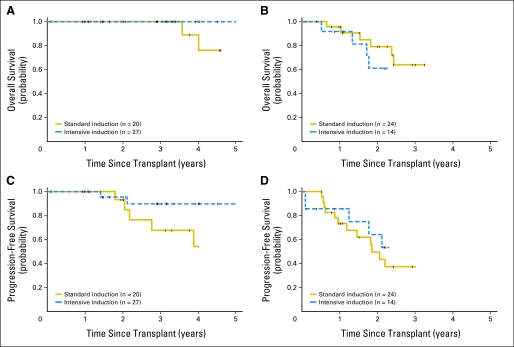
We next evaluated whether using an intensive induction regimen yielded an improved OS and PFS in all patients and patients undergoing HDT and ASCT as initial consolidation. Unadjusted comparisons suggested an improved survival in the group receiving the intensive regimen for all patients (HR, 0.4; 95% CI, 0.2 to 0.9; P = .03) and a trend toward improved survival in patients who received ASCT as initial consolidation (HR, 0.5; 95% CI, 0.2 to 1.5; P = .23). Because this outcome could have been impacted by imbalances between the induction regimen groups identified in Table 2, we performed a multivariable analysis of factors collected from all 118 patients with MCL as well as patients who received HDT and ASCT as initial consolidation to assess the impact of induction regimen on survival. Adjustment for MIPI reduced the difference in OS associated with induction regimen in all 118 patients (HR, 0.5; 95% CI, 0.2 to 1.1; P = .10) and, most importantly, the 85 patients who received ASCT as initial consolidation (HR, 1.1; 95% CI, 0.3 to 3.6; P = .86) regardless of the intensity of their first therapy (Table 3). However, an intensified induction regimen was associated with improved PFS in all patients, even after adjustment for MIPI (HR, 0.4; 95% CI, 0.2 to 0.8; P = .01), although this was not seen when limited to patients who received ASCT as initial consolidation (HR, 0.6; 95% CI, 0.2 to 1.5; P = .26; Table 3). OS and PFS grouped by MIPI score for patients receiving intensive and standard induction regimens before ASCT as initial consolidation are shown in Figure 3.
Fig 3.
Overall survival (OS) and progression-free survival (PFS) of patients with mantle-cell lymphoma undergoing autologous stem-cell transplantation as initial consolidation grouped by induction regimen. (A) OS of patients with low-risk Mantle Cell Lymphoma International Prognostic Index (MIPI) score (P = .09). (B) OS of patients with intermediate/high-risk MIPI score (P = .64). (C) PFS of patients with low-risk MIPI score (P = .10). (D) PFS of patients with intermediate/high-risk MIPI score (P = .42). Standard induction involved cyclophosphamide, doxorubicin, vincristine, and prednisone or other regimen with or without rituximab, but without high-dose cytarabine. Intensive induction involved hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with methotrexate and high-dose cytarabine with or without rituximab. Tick marks represent censor times.
To account for the possibility that age influenced this result, we limited the analysis to patients younger than 61 years of age who should have been able to tolerate an intensive induction. Again, the MIPI was associated with OS (HR, 14.9; 95% CI, 4.0 to 55.7; P < .001), but the induction regimen was not (HR, 0.6; 95% CI, 0.2 to 2.4; P = .50).
Impact of Initial Observation Period on Survival
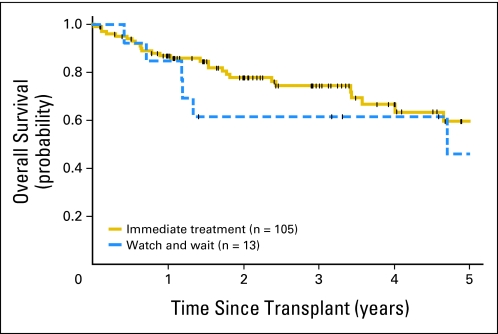
Because many patients may be minimally symptomatic at the time of diagnosis and not clinically require immediate treatment, we evaluated the impact of an initial observation period of more than 3 months on outcome after HDT and ASCT when adjusted for the MIPI score. The median time from diagnosis to initiating therapy for the 13 patients (11%) who started therapy more than 3 months from diagnosis was 5 months (range, 4 to 74 months). Neither the univariate model (HR, 1.3; 95% CI, 0.5 to 3.2; P = .54) nor the MIPI-adjusted multivariable model (HR, 2.1; 95% CI, 0.8 to 5.3; P = .12; Table 3, Appendix Fig A1, online only) suggested a major adverse impact of an initial observation period of more than 3 months.
DISCUSSION
To our knowledge, this is the first study evaluating the MIPI in an unselected population of transplantation patients. It provides the initial data on the use of this tool to account for differences in outcomes based in therapeutic measures. Importantly, we identify the MIPI score at diagnosis as independently associated with OS and PFS of patients with MCL who received HDT and ASCT regardless of the type of induction regimen received and the timing of transplantation. Geisler et al15 have recently shown that both MIPI and sMIPI predicted survival in patients who received a uniform induction regimen on the Nordic Lymphoma Group MCL2 study with augmented CHOP alternating with high-dose cytarabine followed by HDT and ASCT. Damon et al9 have also found that MIPI score was predictive of OS and PFS in patients with MCL who received a homogenous aggressive induction regimen followed by ASCT. Our study confirms and extends these observations by showing that the positive association between the MIPI score and outcome pertains to patients who received different induction regimens including patients who received transplantation after relapse. In contrast, van't Veer et al12 found no predictive value of the MIPI score in their phase II study using an intensive regimen containing high-dose cytarabine followed by ASCT in patients with R-CHOP–sensitive MCL, potentially because of a limited range of MIPI scores masking the impact of this tool.
Prior unadjusted data from our center and others have suggested that results after intensive HyperCVAD-based induction regimens before ASCT in first remission yield improved outcomes.17,18 These observations have led to prospective phase II data also indicating that patients uniformly treated with intensive high-dose cytarabine–containing induction regimens may experience improved long-term outcomes after ASCT in first remission when compared with historical controls.4,9,10 Corroborating these findings, a recent phase III trial by the European MCL Network19 has shown longer time to treatment failure in patients receiving a high-dose cytarabine–containing induction and transplantation conditioning regimen. Our data also indicate that after adjustment for the MIPI, an intensive induction regimen was associated with an improvement in PFS in all 118 patients, although this was not evident when only the initial consolidation patients were evaluated. Unfortunately, survival, arguably the most relevant end point in a noncurable entity such as MCL, was not improved with a high-dose cytarabine–containing regimen in our MIPI-adjusted data set or in the European MCL Network trial, to date.
Our study also highlights the challenge of interpreting such data comparing outcomes between various trials and potentially imbalanced treatment groups. One could hypothesize that patients with higher risk disease features, younger age, or fewer comorbidities would be more likely to receive a more intensive induction, whereas older, frailer, or lower risk patients would be more likely to receive a more standard approach. We found that in the patients who received transplantation at our centers, the lower risk patients more often received intensive inductions. Once this imbalance was taken into account, there seemed to be no obvious survival benefit to the augmented induction across all risk groups as a whole and, most notably, no improved survival in patients who received transplantation as initial consolidation (P = .86). This observation also held true when limited to younger patients who could have received an intensive induction regimen (P = .5).
Our data also allow us to address the question of an initial watch-and-wait approach for patients who are destined to undergo HDT and ASCT. Martin et al13 identified such a group of patients with MCL who had superior OS despite not receiving early treatment, although only 3% received intensive induction regimens and no formal adjustment for baseline features was made. In our study, we found comparable survival in patients who did not receive systemic induction treatment within 3 months of diagnosis when compared with patients who received early initiation of treatment, even after adjusting for the MIPI to account for the potential that disease risk may have impacted the decision to delay initial therapy. Because survival in our study was measured from the time of receiving ASCT, the relative benefit of early initiation of therapy in OS from diagnosis is likely further diminished. Therefore, our data support the notion that early treatment of asymptomatic patients with MCL may not translate into superior survival even when one intends to use ASCT in first remission.
Despite the potentially meaningful implications of these results, our conclusions must be tempered by the fact that we were only able to evaluate patients who received transplantation and were not able to capture patients who had planned to undergo transplantation as initial consolidation but did not because of disease progression, toxicity, or patient choice. However, the impact of this limitation is likely minor because most prospective trials suggest that 87% to 90% of patients with MCL intended for consolidative ASCT receive it (Geisler et al,4 90%; Damon et al,9 86%; Dreyling et al,5 87%). Further evaluation of our hypotheses will likely require mature survival results of ongoing prospective randomized comparisons of intensive versus standard inductions stratified by MIPI.
In conclusion, we report that the MIPI score at diagnosis reliably predicts OS and PFS after ASCT for an unselected group of patients with MCL and that adjustment for this score minimizes any apparent survival benefit of high-dose cytarabine–based induction regimens before HDT and ASCT. These data also emphasize the potential influence of prognostic factors and patient selection on outcomes and highlight the need for randomized controlled studies stratifying patients by the MIPI to determine the true contributions of different treatments.
Appendix
Fig A1.
Overall survival of patients undergoing initial observation versus immediate therapy. Tick marks represent censor times.
Footnotes
Supported by National Institutes of Health Grant No. PO1CA44991, the Lymphoma Research Foundation Mantle Cell Lymphoma Initiative, Specialized Center of Research Grant No. 7040 from the Leukemia and Lymphoma Society, the Mary A. Wright Memorial Research Fund, and a donation from Frank and Betty Vandermeer. L.E.B. is a Special Fellow in Clinical Research of the Leukemia and Lymphoma Society. A.K.G. is a Scholar in Clinical Research of the Leukemia and Lymphoma Society.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Lihua E. Budde, Ajay K. Gopal
Financial support: Ajay K. Gopal
Administrative support: Ajay K. Gopal
Provision of study materials or patients: Lihua E. Budde, Oliver W. Press, Thomas R. Chauncey, John M. Pagel, Steven H. Petersdorf, William I. Bensinger, Leona A. Holmberg, Andrei R. Shustov, Damian J. Green, David G. Maloney, Ajay K. Gopal
Collection and assembly of data: Lihua E. Budde, Katherine A. Guthrie, Brian G. Till, John M. Pagel, Ajay K. Gopal
Data analysis and interpretation: Lihua E. Budde, Katherine A. Guthrie, Brian G. Till, Oliver W. Press, Thomas R. Chauncey, John M. Pagel, Steven H. Petersdorf, William I. Bensinger, Leona A. Holmberg, Andrei R. Shustov, Damian J. Green, David G. Maloney, Ajay K. Gopal
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fisher RI, Dahlberg S, Nathwani BN, et al. A clinical analysis of two indolent lymphoma entities: Mantle cell lymphoma and marginal zone lymphoma (including the mucosa-associated lymphoid tissue and monocytoid B-cell subcategories)—A Southwest Oncology Group study. Blood. 1995;85:1075–1082. [PubMed] [Google Scholar]
- 2.Hiddemann W, Unterhalt M, Herrmann R, et al. Mantle-cell lymphomas have more widespread disease and a slower response to chemotherapy compared with follicle-center lymphomas: Results of a prospective comparative analysis of the German Low-Grade Lymphoma Study Group. J Clin Oncol. 1998;16:1922–1930. doi: 10.1200/JCO.1998.16.5.1922. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann A, Hoster E, Zwingers T, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol. 2009;27:511–518. doi: 10.1200/JCO.2008.16.8435. [DOI] [PubMed] [Google Scholar]
- 4.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: Results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF, Romaguera J, Kantarjian H, et al. Hyper-CVAD and high-dose methotrexate/cytarabine followed by stem-cell transplantation: An active regimen for aggressive mantle-cell lymphoma. J Clin Oncol. 1998;16:3803–3809. doi: 10.1200/JCO.1998.16.12.3803. [DOI] [PubMed] [Google Scholar]
- 7.Dreger P, Martin S, Kuse R, et al. The impact of autologous stem cell transplantation on the prognosis of mantle cell lymphoma: A joint analysis of two prospective studies with 46 patients. Hematol J. 2000;1:87–94. doi: 10.1038/sj.thj.6200007. [DOI] [PubMed] [Google Scholar]
- 8.Vandenberghe E, Ruiz de Elvira C, Loberiza FR, et al. Outcome of autologous transplantation for mantle cell lymphoma: A study by the European Blood and Bone Marrow Transplant and Autologous Blood and Marrow Transplant Registries. Br J Haematol. 2003;120:793–800. doi: 10.1046/j.1365-2141.2003.04140.x. [DOI] [PubMed] [Google Scholar]
- 9.Damon LE, Johnson JL, Niedzwiecki D, et al. Immunochemotherapy and autologous stem-cell transplantation for untreated patients with mantle-cell lymphoma: CALGB 59909. J Clin Oncol. 2009;27:6101–6108. doi: 10.1200/JCO.2009.22.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evens AM, Winter JN, Hou N, et al. A phase II clinical trial of intensive chemotherapy followed by consolidative stem cell transplant: Long-term follow-up in newly diagnosed mantle cell lymphoma. Br J Haematol. 2008;140:385–393. doi: 10.1111/j.1365-2141.2007.06908.x. [DOI] [PubMed] [Google Scholar]
- 11.Fayad L, Thomas D, Romaguera J. Update of the M. D. Anderson Cancer Center experience with hyper-CVAD and rituximab for the treatment of mantle cell and Burkitt-type lymphomas. Clin Lymphoma Myeloma. 2007;8(suppl 2):S57–S62. doi: 10.3816/clm.2007.s.034. [DOI] [PubMed] [Google Scholar]
- 12.van't Veer MB, de Jong D, MacKenzie M, et al. High-dose Ara-C and beam with autograft rescue in R-CHOP responsive mantle cell lymphoma patients. Br J Haematol. 2009;144:524–530. doi: 10.1111/j.1365-2141.2008.07498.x. [DOI] [PubMed] [Google Scholar]
- 13.Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol. 2009;27:1209–1213. doi: 10.1200/JCO.2008.19.6121. [DOI] [PubMed] [Google Scholar]
- 14.Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood. 2008;111:558–565. doi: 10.1182/blood-2007-06-095331. [DOI] [PubMed] [Google Scholar]
- 15.Geisler CH, Kolstad A, Laurell A, et al. The Mantle Cell Lymphoma International Prognostic Index (MIPI) is superior to the International Prognostic Index (IPI) in predicting survival following intensive first-line immunochemotherapy and autologous stem cell transplantation (ASCT) Blood. 2010;115:1530–1533. doi: 10.1182/blood-2009-08-236570. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 17.Till BG, Gooley TA, Crawford N, et al. Effect of remission status and induction chemotherapy regimen on outcome of autologous stem cell transplantation for mantle cell lymphoma. Leuk Lymphoma. 2008;49:1062–1073. doi: 10.1080/10428190801923725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vose J, Loberiza F, Bierman P, et al. Mantle cell lymphoma (MCL): Induction therapy with HyperCVAD/high-dose methotrexate and cytarabine (M-C) (±rituximab) improves results of autologous stem cell transplant in first remission. J Clin Oncol. 2006;24(suppl):424s. abstr 7511. [Google Scholar]
- 19.Hermine O, Hoster E, Walewski J, et al. Alternating courses of 3x CHOP and 3x DHAP plus rituximab followed by a high-dose ARA-C containing myeloablative regimen and autologous stem cell transplantation (ASCT) is superior to six courses of CHOP plus rituximab followed by myeloablative radiochemotherapy and ASCT in mantle cell lymphoma: Results of the MCL younger trial of the European Mantle Cell Lymphoma Network (MCLnet) Blood. 2010;116:110. abstr. [Google Scholar]