Abstract
Purpose
This phase II trial was designed to assess the efficacy and safety of cetuximab, gemcitabine, and oxaliplatin followed by cetuximab, capecitabine, and radiation therapy in locally advanced pancreatic cancer (LAPC).
Patients and Methods
Treatment-naive eligible patients (n = 69) received intravenous gemcitabine (1,000 mg/m2) and oxaliplatin (100 mg/m2) every 2 weeks for four doses, followed by radiation (50.4 Gy to the gross tumor only) with concurrent capecitabine (825 mg/m2 twice daily on radiation treatment days). Cetuximab (500 mg/m2) was started on day 1 of chemotherapy and was continued every 2 weeks during chemotherapy and chemoradiotherapy. Diagnostic cytology specimens were immunostained for Smad4(Dpc4) expression.
Results
Median overall survival time was 19.2 months (95% CI, 14.2 to 24.2 months), and 1-year, 2-year, and 4-year actuarial overall survival rates were 66.0%, 25.02%, and 11.3%, respectively. Acneiform rash correlated with improved survival (P = .001), but initial CA19-9, borderline resectable initial stage, and surgical resection (n = 7) did not. The 1-year and 2-year radiographic local progression rates were 22.8% and 61.0%, respectively. The worst acute toxic effects were GI toxicity (32% and 10% for grades 2 and 3, respectively); fatigue (26% and 6% for grades 2 and 3, respectively); sensory neuropathy (9% and 1% for grades 2 and 3, respectively); and acneiform rash (54% and 3% for grades 2 and 3, respectively). Smad4(Dpc4) expression correlated with a local rather than a distant dominant pattern of disease progression (P = .016).
Conclusion
This regimen appears effective and has acceptable toxicity. The primary end point (1-year overall survival rate > 45%) was met, with encouraging survival duration. Smad4(Dpc4) immunostaining correlated with the pattern of disease progression. Prospective validation of Smad4(Dpc4) expression in cytology specimens as a predictive biomarker is warranted and may lead to personalized treatment strategies for patients with localized pancreatic cancer.
INTRODUCTION
Locally advanced pancreatic cancer is incurable with standard therapies, and the overall survival rate is typically less than 1 year. Although recent trials comparing gemcitabine alone to chemoradiotherapy followed by gemcitabine have produced discordant results,1,2 both chemotherapy and chemoradiotherapy are considered standard initial treatment options, although they have significant limitations. The sequencing of chemotherapy followed by chemoradiotherapy is thought to optimally incorporate standard therapies by selecting the most appropriate patients for consolidation with chemoradiotherapy.3,4
Cetuximab showed promising results in combination with gemcitabine in a phase II trial for patients with advanced pancreatic cancer5 and with radiation therapy in preclinical6 and clinical studies.7 The addition of oxaliplatin to gemcitabine was found to improve median survival in patients with advanced pancreatic cancer.8
Smad4(Dpc4) is a tumor suppressor gene that is inactivated in 53% of pancreatic cancers. It encodes a transcription factor that is involved in the regulation of expression of a broad set of genes; it has been implicated in the regulation of tumor microenvironment, and it has been correlated clinically with prognosis9 and the pattern of disease spread.10
We designed a phase II trial to evaluate the efficacy and safety of gemcitabine, oxaliplatin, and cetuximab followed by chemoradiotherapy with concurrent cetuximab in patients with locally advanced pancreatic cancer. We subsequently developed a correlative study hypothesis that Smad4(Dpc4) immunostaining of diagnostic cytology specimens was feasible and would correlate with the pattern of disease spread on the basis of a rapid autopsy study10 that was reported after the trial completed accrual.
PATIENTS AND METHODS
Eligibility Criteria
Treatment-naive patients with biopsy-proven, locally advanced (ie, T4 disease occlusion of the portal venous confluence or advanced regional adenopathy) or medically inoperable pancreatic adenocarcinoma with Eastern Cooperative Oncology Group performance status 0 or 1 and hematologic parameters that indicated adequate bone marrow, renal, and hepatic function were eligible. Patients could not have a history of metastatic cancer, prior history of radiotherapy to the abdomen, be younger than 18 years of age, have severe peripheral neuropathy, or be pregnant. All patients signed a study-specific consent form. This study was reviewed, approved, and monitored by the Institutional Review Boards of The University of Texas M.D. Anderson Cancer Center and Brown University Oncology Group.
Initial Evaluation
A medical oncologist and a radiation oncologist independently performed physical examinations and recorded the medical history of each patient before study enrollment. Serum hemoglobin, hematocrit, WBC, and platelet levels were measured, and serum chemistries were analyzed for each patient. A biopsy confirmed diagnosis of pancreatic adenocarcinoma was required. When necessary, biliary patency was established by using endobiliary stenting. Pancreatic protocol computed tomography (CT) and chest CT were performed in all patients. The baseline CA 19-9 level was measured, and treatment was initiated after bilirubin normalized.
Study Design and Treatment Plan
The study was designed to administer gemcitabine (1,000 mg/m2 given over 100 minutes) and oxaliplatin (100 mg/m2) every 2 weeks for four doses and cetuximab (400 mg/m2 loading dose) on day 1 of chemotherapy and then weekly (250 mg/m2). For patients without disease progression, chemotherapy was followed by chemoradiotherapy that consisted of 50.4 Gy and concurrent capecitabine (825 mg/m2 orally twice daily on days of radiation therapy). Weekly cetuximab was continued until restaging at 1 month after chemoradiotherapy. After the first 37 patients were accrued, the cetuximab dosage was changed to 500 mg/m2 every 2 weeks to make it possible for patients traveling a long distance to participate.
Radiation Therapy Technique
All patients underwent treatment simulation by using a CT simulator. Only the gross primary tumor and regional lymph nodes greater than 1 cm were targeted. The planning target volume margin was 1.5 cm in the radial direction and 2.5 cm in the cranial and caudal directions. The dose of 50.4 Gy in 28 fractions over 5.5 weeks was prescribed with 15- or 18-MV photons. A three- or four-field technique was used that had customization of beam angles and weighting.
Dose Modifications for Toxicity
Toxic effects were scored according to the National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0. When multiple treatment-related adverse events of the same type occurred in the same patient, only the worst was reported. Capecitabine was withheld for occurrences of grade 2 or greater hand-foot syndrome, mucositis, or GI toxicities unresponsive to medical management and was restarted after recovery to grade 1 or better. The dose was adjusted according to the number of occurrences of grade 2 or greater events: after a first occurrence, 75% of starting dose was used; after a second occurrence, 50% of the starting dose was used. Cetuximab was discontinued after the third occurrence. Cetuximab and radiation therapy were interrupted for grade 3 or greater toxicity believed to be related to the drug until the toxicity resolved to grade 1; then the treatments continued without adjustment. Cetuximab was discontinued permanently for grade 3 infusion reactions or if toxicity recurred.
Monitoring During Chemotherapy and Chemoradiotherapy
A medical oncologist recorded the history and performed a physical examination of each patient every 2 weeks during protocol therapy. A radiation oncologist independently performed the same evaluation weekly during chemoradiotherapy. Serum hemoglobin, hematocrit, WBC, platelet levels, and serum chemistries were evaluated every 2 weeks during chemotherapy and weekly during chemoradiotherapy. Capecitabine pill diaries were monitored weekly.
Follow-Up Imaging and Additional Therapy
Restaging with pancreatic protocol CT, chest radiography, and CA19-9 level was performed 1 week after the last dose of oxaliplatin, 5 weeks after the completion of chemoradiotherapy, and every 2 months during maintenance chemotherapy until progression. At the completion of protocol-based chemoradiotherapy, all patients with responding or stable disease were given the option of receiving any other available treatment or continuing on maintenance chemotherapy with cetuximab and gemcitabine. Surgery was considered in patients whose tumors were considered technically resectable at any time during follow-up.
Response Criteria and Statistical Design
The primary end point was the 1-year overall survival rate. Secondary end points were safety, tumor response 5 to 6 weeks after the completion of chemoradiotherapy, and pattern of progression. Radiographic tumor response was assessed by using standard response criteria.11 Minor response was defined as any tumor reduction less than 50%. A positive study result was defined as a 1-year survival rate greater than the null hypothesis value of 45% when using one-sided P value ≤ .05. The calculated sample size of 69 patients yielded a power of 87% to detect an improvement to a 1-year survival rate of 60% with a one-sided significance level of .044. An intermediate examination of the data was planned when one quarter of the number of expected deaths (ie, 11 of 44 predicted) had been observed. Study termination was required if the 1-year survival rate at intermediate analysis was less than 45%. Survival duration was estimated from the date of diagnosis until death as a result of any cause by using Kaplan-Meier actuarial methodology, and univariate significance was evaluated by using the log-rank test (SPSS for Windows, version 11.5; SPSS, Chicago, IL). Local and distant progression rates were determined and recorded independently.
Smad4(Dpc4) Immunostaining of Diagnostic Specimens
Specimens from 48 patients were examined, and 41 of those contained adequate tumor cellularity for analysis. Slides from each patient were then examined to determine which slides contained the highest number of tumor cells. Coverslips were removed. Slides then underwent destaining with 1% acid alcohol and subsequently were submitted for Smad4(Dpc4) staining. Smad4(Dpc4) immunostaining (Fig 1) was carried out with a Bond Max instrument (Leica Microsystems, Milton Keynes, United Kingdom) and with the Novocastra lyophilized mouse monoclonal antibody to Smad4(Dpc4) Locus 4 Protein (clone JM 56; Leica Microsystems). Each slide was labeled with a 1:5 dilution of the antibody (for 15 minutes). Slides then were counterstained with Bond 0.02% hematoxylin (for 8 minutes) and were coverslipped. Each specimen slide then was scored as Smad4(Dpc4) positive or Smad4(Dpc4) negative by a single board-certified cytopathologist.
Fig 1.
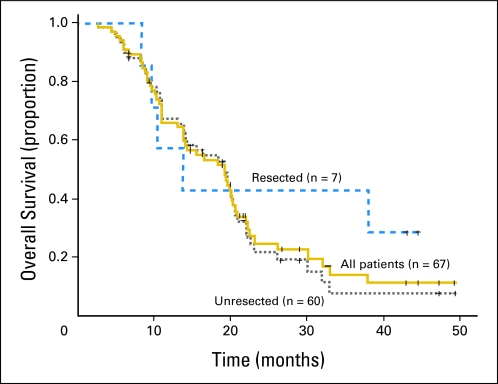
Median survival duration was 19.2 months (95% CI, 14.6 to 23.8 months) and 1-year, 2-year, and 4-year actuarial overall survival rates were 67.1%, 27.2%, and 12.4%, respectively. The survival duration was similar among the patients who did not undergo surgical resection.
RESULTS
Patient Characteristics
The first patient was accrued on October 3, 2005. Full accrual of 69 patients was reached on June 24, 2009. Sixty patients were accrued at The M.D. Anderson Cancer Center, and nine were accrued by Brown University Oncology Group. Fifty-one (74%) had unresectable tumors; 16 (23%) had borderline resectable tumors on the basis of the involvement of less than 180 degrees of the superior mesenteric artery or involvement of the hepatic artery within 1 cm of the celiac axis; and two (3%) had borderline resectable tumors on the basis of advanced regional adenopathy. Median follow-up was 20.9 months (range, 5.2 to 51.5 months) for the 20 living patients and was 16.3 months (range, 2.7 to 49.7 months) for all patients. Patient characteristics are listed in Table 1.
Table 1.
Patient Demographic and Clinical Tumor Characteristics
| Characteristic | No. of Patients (N = 69) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 62 | |
| Range | 39-75 | |
| Sex | ||
| Male | 36 | 52.2 |
| Female | 33 | 47.8 |
| Ethnicity | ||
| White | 61 | 88.4 |
| Hispanic | 5 | 7.2 |
| Black | 2 | 2.9 |
| Indian | 1 | 1.4 |
| CA 19-9 at diagnosis, U/mL | ||
| Median | 269.6 | |
| Range | < 1.0-15,116.1 | |
| ECOG performance status | ||
| 0 | 48 | 69.6 |
| 1 | 21 | 30.4 |
| Radiographic stage | ||
| T4: tumor encasement of the celiac axis or superior mesenteric artery | 51 | 73.9 |
| T4: tumor abutment (< 180 degrees) of the superior mesenteric artery or proximity to the celiac axis | 16 | 23.1 |
| T3: no arterial involvement; advanced regional adenopathy; node positive | 2 | 2.9 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Protocol Compliance
Nine patients (13%) were taken off study before chemoradiotherapy, four as a result of progressive disease, three as a result of poor tolerance (ie, pancreatitis, anaphylaxis, grade 3 fatigue), and two as a result of withdrawn consent for other reasons after initiating chemotherapy but before chemoradiotherapy. The remaining 60 patients (87%) completed planned chemotherapy and chemoradiotherapy according to the protocol.
Treatment-Related Toxicity
The most common toxic events at any time during therapy were nonhematologic and were GI (31.8% for grade 2 and 10.1% for grade 3), fatigue (26.0% for grade 2 and 5.8% for grade 3), sensory neuropathy (14.5% for grade 1, 8.6% for grade 2 and 1.4% for grade 3), and acneiform rash (5.7% for grade 1, 53.6% for grade 2 and 2.9% for grade 3). Six patients (8.7%) had infusion reactions to cetuximab, and two of these were grade 3 (Table 2).
Table 2.
Worst Treatment-Related Toxicity
| Toxicity Grade by Treatment Phase | Toxicity |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heme |
GI |
Fatigue |
Neuropathy |
Infusion Reaction |
HFS |
Acneiform Rash |
Other |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Induction chemotherapy phase (n = 69) | ||||||||||||||||
| Grade 2 | — | 19 | 28 | 14 | 30 | 6 | 9 | 2 | 3 | — | 36 | 52 | 6 | 9 | ||
| Grade 3 | 7 | 10 | 7 | 10 | 2 | 3 | 1 | 1 | 2 | 3 | — | 2 | 3 | — | ||
| Grade 4 | 2 | 3 | — | — | — | — | — | — | — | |||||||
| Total grades 3 and 4 | 9 | 13 | 7 | 10 | 2 | 3 | 1 | 1 | 2 | 3 | — | 2 | 3 | — | ||
| Chemoradiation phase (n = 60) | ||||||||||||||||
| Grade 2 | 1 | 2 | 14 | 23 | 8 | 13 | — | — | 3 | 5 | 22 | 37 | — | |||
| Total grades 3 and 4 | 1 | 2 | 2 | 3 | — | — | — | — | 1 | 2 | ||||||
| Induction or chemoradiation phase (n = 69) | ||||||||||||||||
| Grade 2 | — | 22 | 32 | 18 | 26 | 6 | 9 | 2 | 3 | 3 | 5 | 37 | 54 | 6 | 9 | |
| Grade 3 | 7 | 10 | 7 | 10 | 4 | 6 | 1 | 1 | 2 | 3 | — | 2 | 3 | — | ||
| Grade 4 | 2 | 3 | — | — | — | — | — | — | — | |||||||
| Total grades 3 and 4 | 9 | 13 | 7 | 10 | 4 | 6 | 1 | 1 | 2 | 3 | — | 2 | 3 | — | ||
Abbreviations: Heme, hematologic toxicity; HFS, hand-foot syndrome.
The most common events during induction chemotherapy were fatigue (30.4% for grade 2 and 2.9% for grade 3), GI (27.5% for grade 2 and 10.4% for grade 3), and sensory neuropathy (8.6% for grade 2 and 1.4% for grade 3). During chemoradiotherapy, 23.3% of patients developed grade 2 GI events, and 1.6% developed grade 3 GI events; 13.3% developed grade 2 fatigue, and 3.3% developed grade 3 fatigue; and 5.0% developed grade 2 hand-foot syndrome (Table 2).
Hematologic Toxicity
There was minimal hematologic toxicity. Two patients developed transient grade 4 neutropenia, and seven patients developed grade 3 uncomplicated neutropenia during induction chemotherapy. There was no significant hematologic toxicity during chemoradiotherapy.
Hospitalization
Five patients (7.2%) required hospitalization for adverse events related to treatment (n = 4 for dehydration, and n = 1 for anaphylaxis). In addition, 11 patients (15.9%) required hospitalization for reasons judged to be unrelated to treatment (n = 6 for cholangitis secondary to stent obstruction, n = 1 each for pulmonary embolism and infection, for pneumonia, for pain, for pancreatitis, and for hyperglycemia). None of the five hospitalizations that occurred during chemoradiotherapy were related to treatment.
Dose Interruptions and Reductions
Overall, gemcitabine and oxaliplatin was reduced or held because of toxicity in 12 patients (17.4%). Four patients during induction chemotherapy and three patients during chemoradiotherapy had at least one dose of cetuximab held. Doses of capecitabine were reduced, missed, or held in nine patients (15.0%) for grade 2 or greater nonhematologic toxicity. There were no delays of radiation treatment as a result of toxicity.
Chemoradiotherapy Compliance
All patients completed the prescribed 28 fractions of radiation therapy. All 60 patients who underwent chemoradiotherapy completed capecitabine pill diaries. Forty-two patients (73%) completed all capecitabine doses exactly as prescribed. Of the 18 patients who missed doses, nine missed only one dose (ie, compliant with 98.7% of doses), eight missed between two and six doses, and one missed 36 doses.
Radiographic Response
Twelve patients (18%) had a partial response, 11 (16%) had a minor response, 29 (43%) had stable disease, 16 (24%) had progressive disease, 11 had distant progression, and one had local progression at 5 to 6 weeks after chemoradiotherapy. Two patients had local progression before chemoradiotherapy, two patients had distant progression before chemoradiotherapy, and one patient was not evaluable for response.
Surgical Resection
Nine patients with borderline resectable tumors underwent surgical resection. Histologic evaluation revealed that all nine procedures were R0 resections in which less than 50% viable tumor cells were present.12 Two tumors were found to originate in nonpancreatic sites (ie, duodenum and bile duct), and they were excluded; 67 patients remained in the survival analysis. Two patients died as a result of perioperative complications; one died as a result of complications related to a hepatic artery aneurysm, and the other died as a result of perioperative portal vein thrombosis and multiorgan failure. None of the five remaining patients with resected tumors are alive without evidence of disease.
Maintenance Chemotherapy and Off-Protocol Therapy
Twenty-seven (58.7%) of the 46 patients who had stable or responding disease after initial restaging received maintenance cytotoxic chemotherapy for a median of 4 months (range, 1 to 20 months). Fifteen patients received gemcitabine and cetuximab, six received gemcitabine and erlotinib, five received other gemcitabine-based chemotherapy, and one received single-agent capecitabine. In addition, 28 (58.3%) of the 48 patients with progressive disease received cytotoxic chemotherapy after progression. Four patients received a boost dose to the primary tumor (one each of 10.8, 18.0, 19.8, or 20.0 Gy) at 1.8 or 2.0 Gy per fraction.
Overall Survival
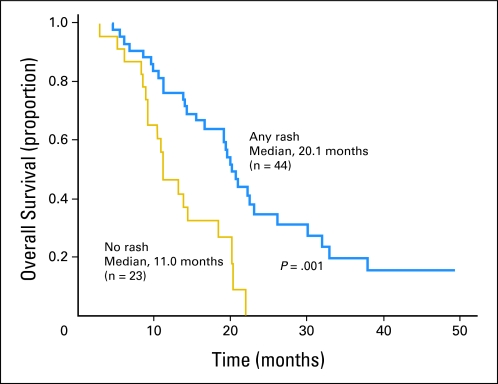
The median survival duration was 19.2 months (95% CI, 14.2 to 24.2 months), and the 1-year, 2-year, and 4-year actuarial overall survival rates were 66.0%, 25.0%, and 11.3%, respectively (Fig 2). Survival duration was greater among the patients who developed any grade acneiform rash during cetuximab administration (P = .001; Fig 3). Initial CA 19-9 level less than 100 (P = .184), borderline resectable initial stage (P = .879), and surgical resection (P = .310; Fig 2) did not significantly influence survival. The median survival duration was 19.2 months (95% CI, 14.7 to 23.7 months) and the 1-year, 2-year, and 4-year actuarial overall survival rates were 67.2%, 22.2%, and 7.6% among the 60 patients who did not undergo surgical resection (Fig 2).
Fig 2.
There was a statistically significant prolongation in survival duration among the patients that developed any grade acneiform rash as a result of cetuximab.
Fig 3.
Local tumor progression was uncommon before 1 year. Isolated local tumor progression leading to death occurred in seven patients between 16.1 and 31.2 months, representing a significant cause of late disease-related mortality.
Pattern of Progression
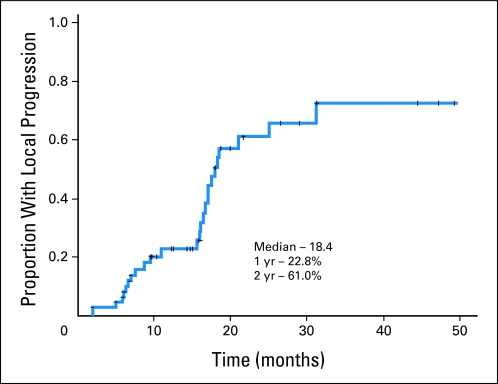
Forty-eight (69.6%) patients had progressive disease. The median progression-free survival duration was 12.5 months. The first site of progression was distant in 26 patients (54%), local in 14 patients (29%), and synchronous distant and local in eight patients (17%). Among the 15 patients with isolated local progression, seven occurred after 16 months, which resulted in a median progression time of 18.0 months and 1-year and 2-year actuarial local progression rates of 22.8%, and 61.0%, respectively (Fig 4). The median progression time was 14.3 months, and the 1-year, and 2-year distant progression rates were 41.0% and 67.7%, respectively. Patients developing acneiform rash had more durable freedom from local progression (P = .015) but not distant progression (P = .512).
Fig 4.
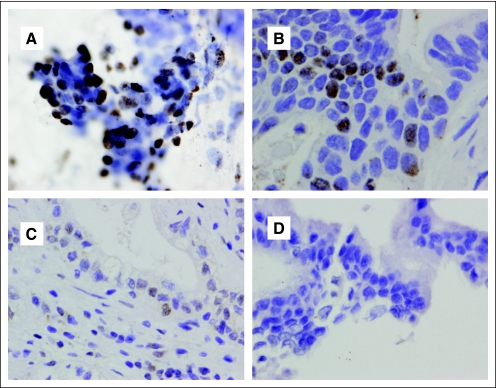
Representative diagnostic cytology specimens that were immunostained for Smad4(Dpc4) expression. Specimens were scored as (A) positive, (B) focal, (C) rare, and (D) negative. Positive and focal were considered to represent intact Smad4(Dpc4) expression, and rare and negative were considered to represent loss of Smad4(Dpc4) expression.
Correlation of Pattern of Progression With Smad4(Dpc4) Expression
Of the 41 patients with adequate tumor specimens for immunostaining, the dominant pattern of progression determined from clinical and radiographic data was local in 15, distant in 14, and indeterminate in eight. Four patients are alive without progression. Indeterminate was defined as progression-free at last follow-up before death. Eleven (73.3%) of 15 patients with intact Smad4(Dpc4) expression had a local dominant pattern of progression, and 10 (71.4%) of 14 patients with Smad4(Dpc4) loss had distant dominant pattern of spread (P = .016). Three patients with intact Smad4(Dpc4) are alive without disease progression at 22.0, 26.1, and 51.5 months; one patient with Smad4(Dpc4) loss is alive without disease progression at 25.5 months.
DISCUSSION
Pancreatic cancer is generally treatment resistant and incurable without surgical resection. Few studies have demonstrated median survival durations longer than 12 months in patients with locally advanced disease. In this trial, the 1-year overall survival rate as the primary end point was met; the median survival duration was 19.2 months and the 4-year survival rate was 7.6% among the 60 patients who were treated without surgical resection. These results compare favorably to similar studies of patients with T4 pancreatic cancer.
The survival duration observed in the patients in this trial was not due to the inclusion of patients with borderline resectable tumors or to the subset of patients who underwent surgical resection. Nevertheless, some caution is appropriate in the interpretation of the impact of the study treatment on the survival duration we observed, because patient selection, aggressive endobiliary stent management, and the individualized use of maintenance and salvage chemotherapy could have contributed to the long survival duration. The median time to local tumor progression in chemoradiotherapy studies has been reported as between 7 and 10 months.13 The 18.4 month median time to local progression in our study suggests that improved local tumor control could have led to improved survival duration. Furthermore, patients who developed acneiform rash related to cetuximab had improved local tumor control and survival duration, as has been reported in other trials,7,14 suggesting a radiosensitizing effect of cetuximab.
The primary end point of this trial was met, which suggests that this regimen should be studied more. Subsequent to the activation of this study, separate phase III trials of oxaliplatin and15 of cetuximab in combination with gemcitabine,16 as well as a phase II trial of gemcitabine, oxaliplatin, and cetuximab,17 have had negative results in studies of patients with metastatic disease. Although these studies were negative, the phase III trials showed a suggestion of benefit of adding these agents to gemcitabine among the patients with ECOG 0 to 1 performance status in unplanned subset analyses. Nevertheless, the weight of the evidence does not support the additional study of this regimen as designed. However, the correlative study results may provide the rationale for future clinical investigation.
In this trial, the pattern of disease progression correlated with Smad4(Dpc4) expression in diagnostic cytology specimens. Patients with intact Smad4(Dpc4) more commonly had a local tumor dominant pattern of progression, and patients with Smad4(Dpc4) loss more commonly had distant disease progression (P = .016). Isolated local tumor progression leading to mortality was common. These observations are consistent with recent evidence from a rapid autopsy series from Johns Hopkins University. Thirty percent of patients in this series died as a result of complications related to their local tumor without metastatic disease, and there was a correlation between the extent of metastatic disease and loss Smad4(Dpc4) expression. For example, only 22% of patients with localized disease had loss of Smad4(Dpc4) expression, compared with 78% of patients with greater than 100 metastases.10 Together, these studies contradict the widespread perception that all patients with pancreatic cancer die as a result of distant metastatic disease, demonstrate that complications of local tumor progression are a significant source of disease-related mortality even after chemoradiotherapy, and these studies suggest the possibility that the a priori pattern of progression may be predictable on the basis of Smad4(Dpc4) expression.
In summary, this phase II clinical trial supports the additional study of epidermal growth factor receptor inhibition in combination with gemcitabine-based chemotherapy and consolidative chemoradiotherapy as a strategy to maximize survival in patients with locally advanced pancreatic cancer and good performance status. The pattern of disease progression (local v distant dominant) appears to correlate with Smad4(Dpc4) expression in cytology specimens. Prospective validation of Smad4(Dpc4) expression as a predictive biomarker is warranted and may lead to personalized treatment strategies.
Footnotes
Supported in part by a Cancer Center Support Grant No. CA16672 from the National Cancer Institute, Department of Health and Human Services, and by a grant from Bristol-Myers Squibb.
Clinical data presented at the 2010 Gastrointestinal Symposium, January 23, 2010. Translational data presented at the 2011 Gastrointestinal Symposium, January 21, 2011.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00338039.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Christopher H. Crane, Bristol Myers-Squibb, Varian Research Funding: Christopher H. Crane, Bristol Myers-Squibb; Prajnan Das, Genentech; Robert A. Wolff, Eli Lilly Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Christopher H. Crane, Gauri R. Varadhachary, Howard Safran, James L. Abbruzzese, Robert A. Wolff
Financial support: Christopher H. Crane
Administrative support: Christopher H. Crane
Provision of study materials or patients: Christopher H. Crane, Gauri R. Varadhachary, Sunil Krishnan, Jason B. Fleming, Jeffrey E. Lee, James L. Abbruzzese, Robert A. Wolff
Collection and assembly of data: Christopher H. Crane, John S. Yordy, Gregg A. Staerkel, Milind M. Javle, Howard Safran, Waqar Haque, Bridgett D. Hobbs
Data analysis and interpretation: Christopher H. Crane, John S. Yordy, Gregg A. Staerkel, Sunil Krishnan, Jason B. Fleming, Prajnan Das, Jeffrey E. Lee, James L. Abbruzzese, Robert A. Wolff
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: Definitive results of the 2000-2001 FFCD/SFRO study. Ann Oncol. 2008;19:1592–1599. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 2.Loehrer P, Powell M, Cardenes H, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201. J Clin Oncol. 2008;26(suppl):214s. doi: 10.1097/COC.0b013e3181e9c103. abstr 4506. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer. 2007;110:47–55. doi: 10.1002/cncr.22735. [DOI] [PubMed] [Google Scholar]
- 4.Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 5.Xiong HQ, Rosenberg A, LoBuglio A, et al. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: A multicenter phase II Trial. J Clin Oncol. 2004;22:2610–2616. doi: 10.1200/JCO.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 6.Milas L, Fan Z, Andratschke NH, et al. Epidermal growth factor receptor and tumor response to radiation: In vivo preclinical studies. Int J Radiat Oncol Biol Phys. 2004;58:966–971. doi: 10.1016/j.ijrobp.2003.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Bonner JA, Harari PM, Giralt J. Cetuximab prolongs survival in patients with locoregionally advanced aquamous cell carcinoma of head and neck: A phase III study of high dose radiation therapy with or without cetuximab. J Clin Oncol. 2004;23(suppl):489s. abstr 5507. [Google Scholar]
- 8.Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: Results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–3516. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Bardeesy N, Cheng KH, Berger JH, et al. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–1813. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 13.Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: A qualitative systematic review. J Clin Oncol. 2009;27:2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 15.Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:3778–3785. doi: 10.1200/JCO.2008.20.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group–directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kullmann F, Hollerbach S, Dollinger MM, et al. Cetuximab plus gemcitabine/oxaliplatin (GEMOXCET) in first-line metastatic pancreatic cancer: A multicentre phase II study. Br J Cancer. 2009;100:1032–1036. doi: 10.1038/sj.bjc.6604983. [DOI] [PMC free article] [PubMed] [Google Scholar]