Abstract
Purpose
In light of inverse relationships reported in observational studies of vitamin D intake and serum 25-hydroxyvitamin D levels with risk of nonmelanoma skin cancer (NMSC) and melanoma, we evaluated the effects of vitamin D combined with calcium supplementation on skin cancer in a randomized placebo-controlled trial.
Methods
Postmenopausal women age 50 to 79 years (N = 36,282) enrolled onto the Women's Health Initiative (WHI) calcium/vitamin D clinical trial were randomly assigned to receive 1,000 mg of elemental calcium plus 400 IU of vitamin D3 (CaD) daily or placebo for a mean follow-up period of 7.0 years. NMSC and melanoma skin cancers were ascertained by annual self-report; melanoma skin cancers underwent physician adjudication.
Results
Neither incident NMSC nor melanoma rates differed between treatment (hazard ratio [HR], 1.02; 95% CI, 0.95 to 1.07) and placebo groups (HR, 0.86; 95% CI, 0.64 to 1.16). In subgroup analyses, women with history of NMSC assigned to CaD had a reduced risk of melanoma versus those receiving placebo (HR, 0.43; 95% CI, 0.21 to 0.90; Pinteraction = .038), which was not observed in women without history of NMSC.
Conclusion
Vitamin D supplementation at a relatively low dose plus calcium did not reduce the overall incidence of NMSC or melanoma. However, in women with history of NMSC, CaD supplementation reduced melanoma risk, suggesting a potential role for calcium and vitamin D supplements in this high-risk group. Results from this post hoc subgroup analysis should be interpreted with caution but warrant additional investigation.
INTRODUCTION
Skin cancer is the most common malignancy in the United States, with more than 1 million new diagnoses each year.1 Over the past 40 years, there has been a dramatic increase in the incidence of both nonmelanoma skin cancer (NMSC) and melanoma, especially among women,2–4 and the significant morbidity and mortality in advanced stages provide compelling reasons to identify novel chemopreventive agents.5 Although the etiology of NMSC (basal cell and squamous cell carcinomas) and melanoma is not completely understood, sun exposure is a primary risk factor.6–9
Currently, there is considerable interest in possible chemopreventive effects of vitamin D, which has been associated with reduced risk of colon, prostate, and breast cancers.10–13 In observational studies, calcium supplementation has also been associated with a reduced risk of developing colon polyps,14 and one small randomized controlled trial showed a significant reduction in total all-cancer incidence with calcium plus vitamin D (CaD) supplementation.15
Vitamin D may be important in skin cancer development. Mice lacking the vitamin D receptor have increased numbers of NMSC,16 and vitamin D treatment decreases the growth of NMSC and melanoma cells in vitro and in mouse models.17–21 There is evidence in humans that higher serum 25-hydroxyvitamin D (25[OH]D) levels are associated with reduced risk of NMSC22 and with thinner melanomas and improved survival from melanoma.23 However, evidence from randomized controlled trials of vitamin D for skin cancer risk is lacking.
The Women's Health Initiative (WHI) CaD trial, designed to test the hypotheses that dietary CaD supplementation would reduce hip fractures and colorectal cancer in postmenopausal women,24–26 provided the opportunity to investigate whether CaD supplementation reduces risk of NMSC and melanoma within a randomized placebo-controlled trial.
METHODS
Study Design
Detailed CaD trial methods have been published previously.24–27 In brief, postmenopausal women age 50 to 79 years enrolled as participants in the WHI dietary modification (DM) and/or hormone therapy (HT) trials were invited to join the CaD supplementation trial at their first and second annual follow-up visits. Key outcomes of the CaD trial were hip fracture (primary end point)24 and colorectal cancer (secondary end point).25 To enroll onto the WHI trials, participants had to have a life expectancy of at least 3 years and no history of cancer, except nonmelanoma skin cancer, within the past decade. WHI participants with history of hypercalcemia, kidney stones, corticosteroid, or calcitriol use were ineligible for the CaD trial.
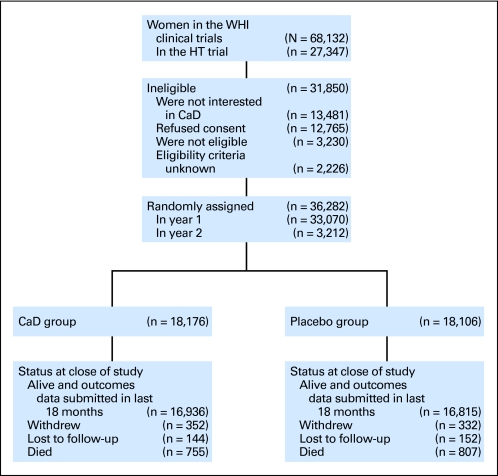
The CaD trial enrolled 36,282 WHI participants between 1995 and 2000; 25,210 (69%) were participating in the DM trial, 16,089 (44%) were in one of the HT trials, and 5,017 (14%) were in both DM and HT trials24 (Fig 1). Participants were randomly allocated using a permuted block algorithm that assigned them in a double-blind fashion to receive either a twice daily supplement containing 500 mg of elemental calcium and 200 IU of vitamin D3 (n = 18,716) or a placebo identical in appearance (n = 18,106; GlaxoSmithKline, Middlesex, United Kingdom), stratified by age and clinical center. Participants were instructed to take the tablets with meals, ideally morning and evening, to improve absorption. Personal CaD supplementation was also allowed, initially limited to a 600 IU total daily intake of vitamin D but raised to a maximum of 1,000 IU daily in 1999, after 1997 Institute of Medicine recommendations were released.28 Serum 25(OH)D levels measured 2 years after randomization were 28% higher in a subset of 227 women assigned to receive active supplements compared with 221 assigned to receive placebo pills.24 Participants' total CaD dietary intake was evaluated using a food frequency questionnaire assessing intake of fortified dairy products, oily fish, and supplements; this was completed at time of randomization in the CaD trial, if available; otherwise, data collected at time of randomization in the HT or DM trials (1 to 2 years before) were used. HT use was also determined at CaD trial initiation; all other baseline variables were obtained at baseline visits for the HT/DM trials. The protocol was approved by the review board of each participating institution. Written informed consent was obtained for each participant at the CaD randomization visit.
Fig 1.
CONSORT diagram of the Women's Health Initiative (WHI) randomized trial of calcium and vitamin D (CaD). HT, hormone therapy. Data adapted.12
Retention, Adherence, and Follow-Up
Adherence to study medication was measured by weighing returned pill bottles at annual visits and defined as use of 80% or more of study medication. Adherence rates in the first 3 years of follow-up ranged from 60% to 63%, with another 13% to 21% of participants taking at least 50% of their study pills. By the end of the study, 76% of participants were still taking study medications, with 59% taking 80% or more of their daily pills.24 Participants were observed for major outcomes, regardless of adherence to study protocol, until death, loss to follow-up, or study closure. Over the course of the study, 980 participants (2.7%) withdrew or were lost to follow-up, and 1,551 participants (4.3%) died.24 Mean duration of follow-up was 7.0 years, with 16,936 participants (93%) in the CaD group and 16,815 participants (93%) in the placebo group in active follow-up at the end of the trial.
Determining Incident Cases of Skin Cancers
Women were mailed annual questionnaires to report hospitalizations and other health outcomes, including self-reported incident NMSC and melanomas. Reports of incident melanoma were confirmed by physician-adjudicated medical record review, including pathology reports, and coded as invasive, in situ, or borderline.29 Per protocol, women diagnosed with melanoma fewer than 10 years before enrollment in the HT/DM trials were excluded. In the period between the start of the HT/DM trials and initiation of the CaD trial, 15 women were diagnosed with melanoma; these 15 women are included among women with history of melanoma in our analysis.
Statistical Analysis
Descriptive characteristics and potential risk factors for skin cancer, including race, body mass index (BMI), smoking, geographic region, physical activity (outdoor walking), multivitamin use, and history of skin cancer were compared between the CaD and placebo groups using χ2 tests with a two-sided P value of less than .05 defined as significant. Modeling analyses used time-to-event methods according to the intention-to-treat principle. Incidences of NMSC and melanoma were compared between the randomization groups using hazard ratios (HRs) with corresponding 95% CIs and Wald statistic P values from Cox proportional hazards models (Figs 2, 3). Kaplan-Meier estimates were used to describe event rates over time. An adherence analysis was also performed as a corollary to the intention-to-treat analysis.
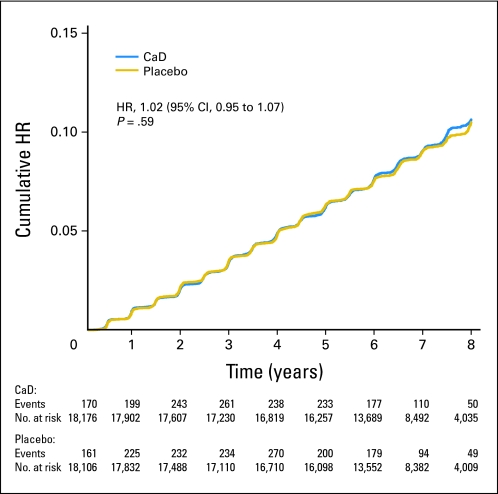
Fig 2.
Kaplan-Meier estimates of the cumulative hazard ratio (HR) for nonmelanoma skin cancer with supplemental calcium plus vitamin D (CaD) as compared with placebo.
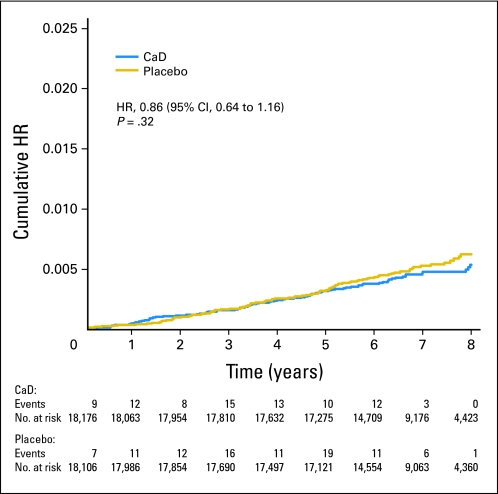
Fig 3.
Kaplan-Meier estimates of the cumulative hazard ratio (HR) for melanoma with supplemental calcium plus vitamin D (CaD) as compared with placebo.
To assess whether the effect of CaD supplementation on NMSC and melanoma risk varied according to baseline risk factors for both types of skin cancer, Cox proportional hazards models were extended to include the variable of interest and interaction with group assignment. HRs for CaD versus placebo within each subgroup are presented along with the P value for the CaD by subgroup interaction. Eight prespecified subgroup analyses were conducted for both NMSC and melanoma outcomes: one, age (50 to 59, 60 to 69, and 70 to 79 years); two, BMI less than 25 kg/m2, 25 to 29.9 kg/m2, and 30 kg/m2 or more; three, low versus high ultraviolet (UV) exposure (as measured by mean annual solar radiation in Langleys and defined as ≤ 375 Langleys, low UV exposure v > 375 Langleys, high UV exposure30); four, history of NMSC; five, vitamin D intake (≥ 400 v < 400 IU); six, history of melanoma; seven, history of cancer; and eight, white versus black/Hispanic/Asian or Pacific Islander/Native American or Alaskan native/other race. There were too few NMSC and melanoma cases in the nonwhite race categories to conduct subgroup analyses of race/ethnicity. Although subgroups were prespecified before this secondary data analysis, skin cancer was not a primary or secondary end point. Thus, all analyses of skin cancer are considered post hoc. A total of 16 subgroups were examined; thus, approximately one statistically significant interaction test (P < .05) might be expected by chance alone. P values are presented without adjustment for multiple testing. All proportional hazards models were also adjusted for age, assignment in the HT (active, placebo, not randomly assigned) and DM (intervention, comparison, not randomly assigned) trials, and outcome prevalent condition (melanoma or NMSC). Statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). The WHI investigators and National Institutes of Health sponsors all contributed to the study design and execution. Statistical analyses and data management were conducted at the WHI Clinical Coordinating Center at the Fred Hutchinson Cancer Research Center (Seattle, WA).
RESULTS
Demographics of Participants
Table 1 shows the baseline characteristics of participants in the active CaD (n = 18,176) and placebo (n = 18,106) groups. Demographics, health behaviors, and diet were balanced between randomization groups, as were specified skin cancer risk factors (eg, age, smoking, nonsteroidal anti-inflammatory drug use, history of skin cancer, and sun exposure [measured via regional solar radiation, geography, and outdoor walking]). Total vitamin D intake of 400 IU or more per day was reported by 41.8% of women in the CaD arm and 42.3% of women assigned to placebo (P = .37). At year 6, off-protocol CaD supplement use was similar in both randomization groups (CaD arm, 52.0%; placebo group, 52.8%).24
Table 1.
Descriptive Characteristics of Participants at Baseline by Random Assignment
| Characteristic | CaD |
Placebo |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total No. of participants | 18,176 | 18,106 | |||
| Age, years | .997 | ||||
| 50-59 | 6,726 | 37.0 | 6,696 | 37.0 | |
| 60-69 | 8,276 | 45.5 | 8,243 | 45.5 | |
| 70-79 | 3,174 | 17.5 | 3,167 | 17.5 | |
| Race/ethnicity | .446 | ||||
| White | 15,051 | 82.8 | 15,104 | 83.4 | |
| Black | 1,680 | 9.2 | 1,635 | 9.0 | |
| Hispanic | 785 | 4.3 | 717 | 4.0 | |
| American Indian | 77 | 0.4 | 72 | 0.4 | |
| Asian/Pacific Islander | 370 | 2.0 | 351 | 1.9 | |
| Unknown | 213 | 1.2 | 227 | 1.3 | |
| Education | .986 | ||||
| High school/GED or lower | 4,286 | 23.6 | 4,290 | 23.7 | |
| School after high school | 7,217 | 39.7 | 7,156 | 39.5 | |
| College degree or higher | 6,557 | 36.1 | 6,545 | 36.1 | |
| Body mass index, kg/m2 | .334 | ||||
| < 25 | 4,745 | 26.1 | 4,834 | 26.7 | |
| 25 to < 30 | 6,476 | 35.6 | 6,487 | 35.8 | |
| ≥ 30 | 6,870 | 37.8 | 6,692 | 37.0 | |
| Smoking | .504 | ||||
| Never | 9,325 | 51.3 | 9,428 | 52.1 | |
| Past | 7,255 | 39.9 | 7,133 | 39.4 | |
| Current | 1,405 | 7.7 | 1,356 | 7.5 | |
| NSAID use | .303 | ||||
| Yes | 6,249 | 34.4 | 6,132 | 33.9 | |
| No | 11,927 | 65.6 | 11,974 | 66.1 | |
| Total vitamin D intake (dietary plus supplements), IU | .372 | ||||
| < 200 | 6,842 | 37.6 | 6,678 | 36.9 | |
| 200 to < 400 | 3,373 | 18.6 | 3,419 | 18.9 | |
| 400 to < 600 | 4,180 | 23.0 | 4,293 | 23.7 | |
| ≥ 600 | 3,426 | 18.8 | 3,363 | 18.6 | |
| Total calcium intake (dietary plus supplements), mg | .740 | ||||
| < 400 | 1,318 | 7.3 | 1,273 | 7.0 | |
| 400 to < 800 | 4,790 | 26.4 | 4,732 | 26.1 | |
| 800 to < 1,200 | 4,712 | 25.9 | 4,654 | 25.7 | |
| ≥ 1,200 | 7,001 | 38.5 | 7,094 | 39.2 | |
| Solar radiation of region, Langleys | .999 | ||||
| 300-325 | 5,366 | 29.5 | 5,351 | 29.6 | |
| 350 | 3,920 | 21.6 | 3,880 | 21.4 | |
| 375-380 | 2,012 | 11.1 | 2,009 | 11.1 | |
| 400-430 | 3,018 | 16.6 | 3,015 | 16.7 | |
| 475-500 | 3,860 | 21.2 | 3,851 | 21.3 | |
| US region | .996 | ||||
| Northeast | 4,018 | 22.1 | 4,001 | 22.1 | |
| South | 4,327 | 23.8 | 4,304 | 23.8 | |
| Midwest | 4,429 | 24.4 | 4,399 | 24.3 | |
| West | 5,402 | 29.7 | 5,402 | 29.8 | |
| Total outdoor walking energy expenditure, METs/wk | .964 | ||||
| None | 6,081 | 33.5 | 6,056 | 33.4 | |
| ≤ 3.5 | 3,491 | 19.2 | 3,481 | 19.2 | |
| 3.6-7.0 | 3,241 | 17.8 | 3,233 | 17.9 | |
| > 7.0 | 3,733 | 20.5 | 3,678 | 20.3 | |
| History of cancer | .235 | ||||
| Yes | 745 | 4.1 | 698 | 3.9 | |
| No | 17,431 | 95.9 | 17,408 | 96.1 | |
| History of melanoma cancer | .293 | ||||
| Yes | 92 | 0.5 | 78 | 0.4 | |
| No | 18,084 | 99.5 | 18,028 | 99.6 | |
| History of nonmelanoma skin cancer | .300 | ||||
| Yes | 1,086 | 6.0 | 1,129 | 6.2 | |
| No | 17,090 | 94.0 | 16,977 | 93.8 | |
| Hormone use (CaD baseline)* | .237 | ||||
| Never user | 5,810 | 32.0 | 5,685 | 31.4 | |
| Past user | 3,007 | 16.5 | 2,937 | 16.2 | |
| Current user | 9,359 | 51.5 | 9,484 | 52.4 | |
| HT intervention assignment | .740 | ||||
| Not randomly assigned | 10,122 | 55.7 | 10,071 | 55.6 | |
| Active | 4,039 | 22.2 | 4,078 | 22.5 | |
| Placebo | 4,015 | 22.1 | 3,957 | 21.9 | |
| DM intervention assignment | .299 | ||||
| Not randomly assigned | 5,582 | 30.7 | 5,490 | 30.3 | |
| Intervention | 4,767 | 26.2 | 4,878 | 26.9 | |
| Comparison | 7,827 | 43.1 | 7,738 | 42.7 | |
Abbreviations: CaD, calcium and vitamin D; DM, dietary modification; GED, general educational development; HT, hormone therapy; MET, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug.
HT variables calculated for time of CaD randomization and include hormone use from Women's Health Initiatve HT trial.
NMSCs
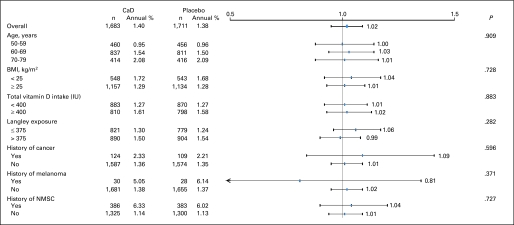
After a mean follow-up of 7.0 years, there was no difference between treatment groups in self-reported NMSCs, with a total of 1,683 NMSC cases reported in the active CaD group and 1,655 cases in the placebo group (HR, 1.02; 95% CI, 0.95 to 1.07; Fig 2). Nor did CaD supplementation affect NMSC outcomes within any of the subgroups (ie, by age, BMI, total vitamin D intake, solar radiation [Langleys], history of cancer, history of melanoma, or history of NMSC; Fig 4).
Fig 4.
Estimated effects of supplemental calcium with vitamin D (CaD) on risk of nonmelanoma skin cancer (NMSC), according to selected baseline characteristics. All models were adjusted for age, assignment in Women's Health Initiative (WHI) hormone therapy trial (active, placebo, not randomly assigned), assignment in WHI dietary modification trial (intervention, comparison, not randomly assigned), and outcome prevalent condition (NMSC). BMI, body mass index; Langley exposure, average regional solar radiation.
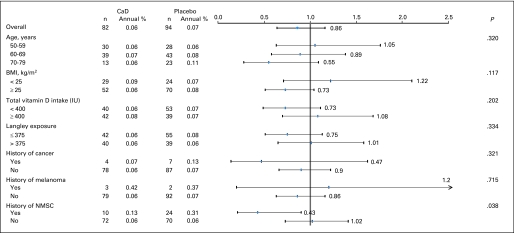
Melanoma
After the mean 7.0-year follow-up, there was no difference in melanoma incidence between groups, with 82 melanomas diagnosed in the active group and 94 in the placebo group (HR, 0.86; 95% CI, 0.64 to 1.16; Fig 3). In prespecified subgroup analysis, women who reported a history of NMSC had 57% fewer melanomas when assigned to active CaD versus placebo (HR, 0.43; 95% CI, 0.21 to 0.90; P = .026; Pinteraction = .038; Fig 5). This effect was not seen in women without history of NMSC (HR, 1.02; 95% CI, 0.73 to 1.41). CaD supplementation did not affect melanoma within any of the other subgroups. There were no significant differences in subtypes of melanomas (invasive v in situ), whether comparing women randomly assigned to receive CaD versus placebo or based on skin cancer history (Table 2).
Fig 5.
Estimated effects of supplemental calcium with vitamin D (CaD) on risk of melanoma, according to selected baseline characteristics. All models were adjusted for age, assignment in Women's Health Initiative (WHI) hormone therapy trial (active, placebo, not randomly assigned), assignment in WHI dietary modification trial (intervention, comparison, not randomly assigned), and outcome prevalent condition (melanoma). BMI, body mass index; Langley exposure, regional solar radiation; NMSC, nonmelanoma skin cancer.
Table 2.
Characteristics of Melanomas by Study Group
| Melanoma Subtype | CaD (n = 82) |
Placebo (n = 94) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total invasive melanomas | 44 | 54 | 58 | 62 |
| Total in situ melanomas | 38 | 46 | 36 | 38 |
| History of NMSC (total) | 10 | 24 | ||
| Invasive | 5 | 50 | 12 | 50 |
| In situ | 5 | 50 | 12 | 50 |
| No history of NMSC (total) | 72 | 70 | ||
| Invasive | 39 | 54 | 46 | 66 |
| In situ | 33 | 46 | 24 | 34 |
| History of melanoma (total) | 3 | 2 | ||
| Invasive | 2 | 67 | 2 | 100 |
| In situ | 1 | 33 | 0 | 0 |
| No history of melanoma (total) | 79 | 92 | ||
| Invasive | 42 | 53 | 56 | 61 |
| In situ | 37 | 47 | 36 | 39 |
Abbreviations: CaD, calcium and vitamin D; NMSC, nonmelanoma skin cancer.
Sensitivity Analyses
In analyses restricted to adherent participants (ie, women who took ≥ 80% of study pills during the trial), there was no effect of CaD supplementation on NMSC or melanoma (data not shown).
DISCUSSION
Daily supplementation with 1,000 mg of calcium and 400 IU of vitamin D had no effect on NMSC or melanoma skin cancer incidence in this large randomized double-blinded placebo-controlled trial. One possible reason for the overall null results is that a daily 400-IU dose of vitamin D is inadequate for reducing cancer risk. Since the report of no reduction in colorectal cancer—the key secondary outcome of the CaD trial—at the dose tested,25 some31,32 have recommended higher intakes of vitamin D for reducing colorectal and other cancers. The daily 400-IU dose of vitamin D3 is estimated to have raised mean serum 25(OH)D levels by approximately 4 ng/mL, which may be inadequate to observe a clinical difference in skin cancers. Indeed, 400 IU per day is 33% lower than the daily adequate intake level of 600 IU recommended in a recent Institute of Medicine consensus statement (November 2010).33 However, this dose was considered adequate to aid calcium absorption at the inception of the trial in the early 1990s and thus appropriate for testing the primary CaD trial hypothesis that CaD supplementation would reduce hip fracture. Although hip fracture incidence was not, in fact, reduced in the WHI cohort of women age 50 to 79 years at baseline, significant reduction was seen in women age 60 years and older.24
Another possible explanation for the null results regarding skin cancer is that the study design allowed off-protocol calcium and/or vitamin D supplementation; women were allowed to take up to 600 IU of vitamin D daily initially and up to 1,000 IU daily from 1999 onward. Therefore, some in the placebo group were taking more vitamin D than some women in the intervention group. However, there is some evidence that active CaD-arm participants had higher overall vitamin D intake than those assigned to receive placebo; in a subset of 227 women assigned to receive active pills versus 221 assigned to receive placebo, serum 25(OH)D levels in women taking active pills were 28% higher than those in women taking placebo pills 2 years after randomization.24 These 448 women with baseline and year-2 serum 25(OH)D levels were randomly selected and were at least 50% adherent to study medication. The 28% difference in 25(OH)D levels between the two groups is likely to be generalizable to the entire trial, because 84% of all participants were more than 50% adherent to study medications. Although the difference in 25(OH)D levels is modest, this substudy confirmed that 400 IU of vitamin D did raise 25(OH)D levels in this trial.
Whether the trial dose was too low or the difference in intake between treatment groups was too small to see an effect of CaD on NMSC, it is of interest that in subgroup analysis, CaD supplementation reduced risk of melanoma by 57% in women with history of NMSC. Additionally, CaD supplementation tended to reduce risk of melanoma in women with higher BMIs (> 25 kg/m2), with lower baseline vitamin D intake (< 400 IU), and from areas with less sun exposure (< 375 Langleys), although there were no statistically significant interactions of CaD treatment with any of these variables. In the subgroup of women with history of NMSC, the absolute number of melanoma cases in both arms was small: 10 in the CaD arm and 24 in the placebo arm. Thus, this finding may have resulted by chance, particularly because we examined 16 subgroups in these analyses. However, there is some evidence that vitamin D may affect development of melanoma. Vitamin D reduces UV-induced DNA damage in mice and has antiproliferative and prodifferentiative effects on melanoma cell lines in vitro.19,34 In humans, previous studies have reported an association between higher 25(OH)D levels and lower Breslow thickness at melanoma diagnosis as well as decreased risk of relapse and death23 and metastasis.35 Others have noted a positive relationship between signs of sun damage (and presumably higher vitamin D levels) and decreased death from melanoma.36 Finally, some37 but not all38 studies have shown that higher dietary or supplemental intake of vitamin D is associated with reduced risk of melanoma. Previous WHI and other cohort studies have reported that women with history of NMSC are 2.4 times more likely to develop melanoma than those without history of NMSC.39–43 Therefore, NMSC may be a marker for participants with more UV-induced DNA damage6,44–47 and thus higher risk for melanoma.
The possibility that CaD supplementation, or either vitamin D or calcium alone, might prevent melanoma in this high-risk group has important public health implications. Although we cannot rule out a role for calcium in preventing melanoma, we have some evidence that vitamin D may be an important factor in our findings. In exploratory patient case–control analyses of serum 25(OH)D levels measured in 4,868 women for previously reported fracture and breast and colorectal cancer patient case–control studies,25,26 higher 25(OH)D levels were associated with 50% reduction in melanoma (manuscript in preparation). We hypothesize that raising vitamin D levels may prevent development of melanoma in women at high risk. A possible role for vitamin D supplementation in preventing melanoma in women with a history of NMSC warrants further investigation.
This study has several limitations. First, the WHI CaD trial was designed to examine the effect of a specific dose of CaD supplementation on hip fracture and colorectal cancer. Post hoc analyses of other malignancies may lack statistical power to detect differences between active and placebo groups, and it may be that any potential benefit of CaD supplementation on cancer incidence takes more than 7 years to appear. Second, NMSC outcome was based on particpant self-report, and cases were not adjudicated; however, several studies have found self-reported skin cancer to be highly accurate.48,49 We lacked information on specific types of NMSC in this study; thus, we cannot determine if there was a differential effect of CaD supplementation on basal cell versus squamous cell carcinoma risk. Additionally, we lacked systematic measurements of 25(OH)D achieved after intervention and cannot determine the relationship between 25(OH)D levels after CaD supplementation and skin cancer risk. Even though women were randomly assigned to receive 400 IU of vitamin D, serum 25(OH)D levels could have varied as a result of genetic or environmental factors affecting vitamin D metabolism.50,51 Finally, the finding that CaD supplementation reduced risk of incident melanomas in women with a history of NMSC must be interpreted with caution, because the P value is modest (Pinteraction = .038) and may reflect a chance finding given the multiple subgroups tested.
Strengths of this study include its randomized double-blinded design, large well-characterized study population, and long follow-up duration. This study controlled for many potential confounding factors, including diverse geographic regions with differing solar radiation, physical activity, smoking and alcohol status, education, socioeconomic status, and nutrient intake.
Despite the large study population, the number of incident melanomas was small (n = 176), illustrating the size of the cohort needed to detect a chemopreventive effect of any agent in a 7-year period. At this time, it seems unlikely that a randomized trial of the scope needed to examine the effect of calcium or vitamin D (or combined) supplementation on skin cancer risk will be performed, and this trial may provide the best data with which to assess this issue in the near future.
In conclusion, daily supplementation of calcium (1,000 mg) and vitamin D (400 IU) did not reduce incidence of NMSC or melanoma in a large cohort of postmenopausal women age 50 to 79 years. Therefore, our results do not support use of these supplements, at these doses, for preventing skin cancer in older women. However, CaD supplementation seems to have reduced the incidence of melanoma in women with history of NMSC, suggesting a possible role for either vitamin D or calcium, or their combination, in reducing melanoma in this high-risk group. Additional investigations of CaD supplementation to prevent melanoma in women with history of NMSC are warranted.
Acknowledgment
We thank Susan Swetter, MD, of Stanford University for her help in reviewing and editing this article.
Footnotes
Supported by Contract No. N01-WH-32108 with the Women's Health Initiative Program, funded by the National Heart, Lung and Blood Institute, US Department of Health and Human Services; in part by Grants No. 1K23AR056736-01 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases and KL2 RR024130 from the National Center for Research Resources (J.Y.T.); and in part by the Stanford Medical School Medical Scholars Research Fellowship (T.F.).
Presented in abstract form at the Annual Meeting of the American Academy of Dermatology, February 4-8, 2011, New Orleans, LA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00000611.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jean Y. Tang, Teresa Fu, Marcia L. Stefanick
Collection and assembly of data: Jean Y. Tang, Joseph Larson
Data analysis and interpretation: Jean Y. Tang, Erin S. LeBlanc, Joann E. Manson, David Feldman, Eleni Linos, Nathalie Zeitouni, Joseph Larson, Marcia L. Stefanick
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 1994;30:774–778. doi: 10.1016/s0190-9622(08)81509-5. [DOI] [PubMed] [Google Scholar]
- 2.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–690. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 3.Purdue MP, Freeman LE, Anderson WF, et al. Recent trends in incidence of cutaneous melanoma among US Caucasian young adults. J Invest Dermatol. 2008;128:2905–2908. doi: 10.1038/jid.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen JG, Fleischer AB, Jr, Smith ED, et al. Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg. 2001;27:1035–1038. doi: 10.1046/j.1524-4725.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 6.Epstein JH. Photocarcinogenesis, skin cancer, and aging. J Am Acad Dermatol. 1983;9:487–502. doi: 10.1016/s0190-9622(83)70160-x. [DOI] [PubMed] [Google Scholar]
- 7.Rosso S, Zanetti R, Pippione M, et al. Parallel risk assessment of melanoma and basal cell carcinoma: Skin characteristics and sun exposure. Melanoma Res. 1998;8:573–583. doi: 10.1097/00008390-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Rivers JK. Is there more than one road to melanoma? Lancet. 2004;363:728–730. doi: 10.1016/S0140-6736(04)15649-3. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 11.Flood A, Peters U, Chatterjee N, et al. Calcium from diet and supplements is associated with reduced risk of colorectal cancer in a prospective cohort of women. Cancer Epidemiol Biomarkers Prev. 2005;14:126–132. [PubMed] [Google Scholar]
- 12.Garland C, Garland F, Gorham E, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 14.Wallace K, Baron JA, Cole BF, et al. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96:921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 15.Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 16.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 17.Bijlsma MF, Spek CA, Zivkovic D, et al. Repression of smoothened by patched-dependent (Pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: The presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 19.Seifert M, Rech M, Meineke V, et al. Differential biological effects of 1, 25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol. 2004:375–379. doi: 10.1016/j.jsbmb.2004.03.002. 89–90. [DOI] [PubMed] [Google Scholar]
- 20.Yudoh K, Matsuno H, Kimura T. 1alpha,25-dihydroxyvitamin D3 inhibits in vitro invasiveness through the extracellular matrix and in vivo pulmonary metastasis of B16 mouse melanoma. J Lab Clin Med. 1999;133:120–128. doi: 10.1016/s0022-2143(99)90004-5. [DOI] [PubMed] [Google Scholar]
- 21.Tang JY, Xiao T, Wu A, et al. Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev Res (Phila) 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang J, Wu A, Paarmi N, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387–391. doi: 10.1007/s10552-009-9470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newton-Bishop JA, Beswick S, Randerson-Moor J, et al. Serum 25-hydroxyvitamin D3 levels are associated with Breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27:5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 25.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 26.Chlebowski RT, Johnson KC, Kooperberg C, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100:1581–1591. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson RD, LaCroix AZ, Cauley JA, et al. The Women's Health Initiative calcium-vitamin D trial: Overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S98–S106. doi: 10.1016/s1047-2797(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 28.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine: Vitamin D, in Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington, DC: National Academies Press; 1999. pp. 250–287. [Google Scholar]
- 29.Curb JD, McTiernan A, Heckbert SR, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 30.Garland CF, Garland FC. Do sunlight and vitamin D reduce the likelihood of colon cancer? Int J Epidemiol. 1980;9:227–231. doi: 10.1093/ije/9.3.227. [DOI] [PubMed] [Google Scholar]
- 31.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Cancer Society: Canadian Cancer Society announces vitamin D recommendations. http://www.cancer.ca/canada-wide/about%20us/media%20centre/cw-media%20releases/cw-2007/canadian%20cancer%20society%20announces%20vitamin%20d%20recommendation.aspx.
- 33.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board Institute of Medicine: Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2010. pp. 291–340. [Google Scholar]
- 34.Evans SR, Houghton AM, Schumaker L, et al. Vitamin D receptor and growth inhibition by 1,25-dihydroxyvitamin D3 in human malignant melanoma cell lines. J Surg Res. 1996;61:127–133. doi: 10.1006/jsre.1996.0092. [DOI] [PubMed] [Google Scholar]
- 35.Nurnberg B, Graber S, Gartner B, et al. Reduced serum 25-hydroxyvitamin D levels in stage IV melanoma patients. Anticancer Res. 2009;29:3669–3674. [PubMed] [Google Scholar]
- 36.Berwick M, Armstrong BK, Ben-Porat L, et al. Sun exposure and mortality from melanoma. J Natl Cancer Inst. 2005;97:195–199. doi: 10.1093/jnci/dji019. [DOI] [PubMed] [Google Scholar]
- 37.Millen AE, Tucker MA, Hartge P, et al. Diet and melanoma in a case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:1042–1051. [PubMed] [Google Scholar]
- 38.Weinstock MA, Stampfer MJ, Lew RA, et al. Case-control study of melanoma and dietary vitamin D: Implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98:809–811. doi: 10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg CA, Khandekar J, Greenland P, et al. Cutaneous melanoma in postmenopausal women after nonmelanoma skin carcinoma. Cancer. 2006;106:654–663. doi: 10.1002/cncr.21627. [DOI] [PubMed] [Google Scholar]
- 40.Efird JT, Friedman GD, Habel L, et al. Risk of subsequent cancer following invasive or in situ squamous cell skin cancer. Ann Epidemiol. 2002;12:469–475. doi: 10.1016/s1047-2797(01)00276-9. [DOI] [PubMed] [Google Scholar]
- 41.Levi F, La Vecchia C, Te VC, et al. Incidence of invasive cancers following basal cell skin cancer. Am J Epidemiol. 1998;147:722–726. doi: 10.1093/oxfordjournals.aje.a009516. [DOI] [PubMed] [Google Scholar]
- 42.Marghoob AA, Slade J, Salopek TG, et al. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma: Screening implications. Cancer. 1995;75:707–714. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 43.Levi F, Randimbison L, La Vecchia C, et al. Incidence of invasive cancers following squamous cell skin cancer. Am J Epidemiol. 1997;146:734–739. doi: 10.1093/oxfordjournals.aje.a009349. [DOI] [PubMed] [Google Scholar]
- 44.Hsu J, Forbes PD, Harber LC, et al. Induction of skin tumors in hairless mice by a single exposure to UV radiation. Photochem Photobiol. 1975;21:185–188. doi: 10.1111/j.1751-1097.1975.tb06650.x. [DOI] [PubMed] [Google Scholar]
- 45.Freeman SE, Hacham H, Gange RW, et al. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci U S A. 1989;86:5605–5609. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitasa BC, Taylor HR, Strickland PT, et al. Association of nonmelanoma skin cancer and actinic keratosis with cumulative solar ultraviolet exposure in Maryland watermen. Cancer. 1990;65:2811–2817. doi: 10.1002/1097-0142(19900615)65:12<2811::aid-cncr2820651234>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 47.Kraemer KH, Lee MM, Andrews AD, et al. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer: The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 48.Ming ME, Levy RM, Hoffstad OJ, et al. Validity of patient self-reported history of skin cancer. Arch Dermatol. 2004;140:730–735. doi: 10.1001/archderm.140.6.730. [DOI] [PubMed] [Google Scholar]
- 49.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 50.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: The Women's Health Initiative calcium plus vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Engelman CD, Fingerlin TE, Langefeld CD, et al. Genetic and environmental determinants of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D levels in Hispanic and African Americans. J Clin Endocrinol Metab. 2008;93:3381–3388. doi: 10.1210/jc.2007-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]