Abstract
Purpose
Cancer and Leukemia Group B conducted a randomized phase II trial to investigate two novel chemotherapy regimens in combination with concurrent thoracic radiation therapy (TRT).
Patients and Methods
Patients with unresectable stage III non–small-cell lung cancer (NSCLC) were randomly assigned to carboplatin (area under the curve, 5) and pemetrexed (500 mg/m2) every 21 days for four cycles and TRT (70 Gy; arm A) or the same treatment with cetuximab administered concurrent only with TRT (arm B). Patients in both arms received up to four cycles of pemetrexed as consolidation therapy. The primary end point was the 18-month overall survival (OS) rate; if the 18-month OS rate was ≥ 55%, the regimen(s) would be considered for further study.
Results
Of the 101 eligible patients enrolled (48 in arm A and 53 in arm B), 60% were male; the median age was 66 years (range, 32 to 81 years); 44% and 35% had adenocarcinoma and squamous carcinoma, respectively; and more patients enrolled onto arm A compared with arm B had a performance status of 0 (58% v 34%, respectively; P = .04). The 18-month OS rate was 58% (95% CI, 46% to 74%) in arm A and 54% (95% CI, 42% to 70%) in arm B. No significant difference in OS between patients with squamous and nonsquamous NSCLC was observed (P = .667). The toxicities observed were consistent with toxicities associated with concurrent chemoradiotherapy.
Conclusion
The combination of pemetrexed, carboplatin, and TRT met the prespecified criteria for further evaluation. This regimen should be studied further in patients with locally advanced unresectable nonsquamous NSCLC.
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality in the United States for both men and women, and 87% of lung cancers are non–small-cell lung cancer (NSCLC).1,2 Approximately one third of patients with NSCLC will present with unresectable stage III disease.3 Concurrent administration of chemotherapy with thoracic radiation therapy (TRT) is the standard of care for appropriate patients.4 The standard therapy used in this setting involves systemic doses of cisplatin and etoposide concurrent with TRT.5,6 It has been challenging to administer systemically active doses of docetaxel, gemcitabine, or vinorelbine in combination with TRT.7–9 A novel chemotherapy regimen that could be administered in systemically active doses yet tolerable in combination with TRT would be of interest because a majority of relapses after treatment with concurrent chemotherapy and TRT are distant. Pemetrexed was initially approved for the treatment of metastatic NSCLC in the second-line setting10 and was subsequently approved for patients with nonsquamous NSCLC as first-line and maintenance therapy.11,12
Systemic doses of carboplatin and pemetrexed and TRT were delivered with acceptable toxicities in a phase I trial.13 Phase II studies in advanced NSCLC suggested improved outcomes with the addition of cetuximab, a monoclonal antibody against the epidermal growth factor receptor, to platinum-based chemotherapy.14–16 A phase III trial in patients with squamous cancer of the head and neck revealed a significant improvement in overall survival (OS) with cetuximab and radiation therapy compared with radiation therapy alone.17 Cancer and Leukemia Group B (CALGB) conducted a randomized phase II study (CALGB 30407) that incorporated [18F]fluorodeoxyglucose (FDG) positron emission tomography (PET) staging before therapy and three-dimensional conformal TRT with the intention of developing a novel systemic therapy regimen to be used concurrent with TRT (ClinicalTrials.gov identifier: NCT00117962). We elected to use carboplatin-based therapy because the phase I trial and recent CALGB chemoradiotherapy trials have used carboplatin, and we decided not to use any induction chemotherapy based on our previous experience.13,18,19 The fact that pemetrexed is inactive in squamous NSCLC was not known at the time the study was developed.11,12
PATIENTS AND METHODS
Eligibility
Patients were required to have histologic or cytologic diagnosis of NSCLC, inoperable stage IIIA or stage IIIB disease, measurable disease according the Response Evaluation Criteria in Solid Tumors (RECIST),20 an Eastern Cooperative Oncology Group performance status of 0 to 1, age ≥ 18 years, and weight loss ≤ 10% in the past 3 months. Laboratory requirements were as follows: an absolute neutrophil count (ANC) of ≥ 1,500/μL, platelets ≥ 100,000 μL, a calculated creatinine clearance (CrCl) of ≥ 45 mL/min, bilirubin 1.5× upper limit of normal (ULN), AST and ALT less than 3× ULN, and alkaline phosphatase less than 3× ULN. Patients were required to undergo FDG-PET imaging, computed tomography (CT) scan of the chest and abdomen, and pulmonary function tests before enrollment. Patients were required to have a mediastinal lymph node (LN) of ≥ 2 cm by CT scans; if the mediastinal LN was less than 2 cm, then biopsy confirmation of malignant involvement in mediastinal LN was required (regardless of the results of PET imaging). Patients with contralateral mediastinal LN disease (N3) were eligible if disease could be encompassed within a single radiation field. Patients with scalene, supraclavicular, and contralateral hilar LN involvement based on assessment of the treating physician; direct invasion of vertebral body; or exudative, bloody, or cytologically malignant pleural effusions were not eligible. Prior chemotherapy for NSCLC, chest irradiation therapy, or therapy directed at the epidermal growth factor receptor pathway was not allowed. Patients who were pregnant or nursing; with known hypersensitivity to carboplatin, pemetrexed, or a monoclonal antibody; or who were HIV positive were ineligible. This trial was approved by the institutional reviews boards of the participating institutions, and patients were required to provide informed consent before enrollment.
Chemotherapy Treatment Plan
The first 19 patients in arms A and B received carboplatin area under the curve (AUC) of 6 using the Calvert equation21 over 30 minutes and pemetrexed 500 mg/m2 over 10 minutes every 21 days for four cycles. Patients randomly assigned to arm B received cetuximab 400 mg/m2 over 120 minutes on day 1 of week 1 and then 250 mg/m2 over 60 minutes weekly for 6 weeks during the radiation therapy (for a total of 7 weeks). Radiation therapy started on day 1 of therapy. After 19 patients had been treated, the trial was amended, and the dose of carboplatin was reduced to an AUC of 5 because five patients had experienced grade 4 thrombocytopenia. In the absence of disease progression or unacceptable toxicity, patients in both arms were treated with four additional cycles of pemetrexed 500 mg/m2 administered every 21 days. Patients received folic acid, vitamin B12, and dexamethasone as recommended in the pemetrexed package insert.22 Patients received diphenhydramine 50 mg intravenously 30 to 60 minutes before the first dose of cetuximab, and dose could be repeated before subsequent doses.
Patients received carboplatin and pemetrexed if the ANC was ≥ 1,500/μL and platelet count was ≥ 100,000/μL; if ANC or platelet counts were less than these levels, the treatment was held, and a CBC was checked on a weekly basis. Febrile neutropenia resulted in a dose reduction of both agents. Both agents were held for a CrCl of less than 45 mL/min, and if CrCl remained less than 45 mL/min after 1 week, the protocol therapy was discontinued. Patients who experienced a grade ≥ 3 infusion reaction as a result of carboplatin were removed from protocol therapy. Patients who experienced grade ≥ 3 infusion reactions as a result of cetuximab discontinued cetuximab and continued other protocol therapy. Dermatologic toxicity related to cetuximab was managed according to the package insert.23 For the consolidation therapy, guidelines for dose adjustment were similar to those used during initial concurrent chemoradiotherapy. For patients experiencing radiation-related esophagitis of grade ≥ 3, chemotherapy was held, patients were re-evaluated on a weekly basis, and therapy was resumed when dysphagia had improved to grade less than 3; carboplatin and pemetrexed were reduced one dose level for subsequent cycles. Radiation was held for grade 4 esophagitis and then resumed when dysphagia had improved to grade ≤ 2.
Radiation Treatment Plan
All patients were assigned to receive a cumulative dose of 70 Gy in 35 daily fractions of 2 Gy. Three-dimensional conformal TRT planning was required, although the use of intensity-modulated radiotherapy was not allowed. Photon beam energies of 4 to 25 MV could be used, and tissue heterogeneity factors were used for bone, soft tissue, and lung in dose calculations. The gross tumor volume included the primary lung tumor on the planning CT scan and staging FDG-PET, and LN metastases included LNs that were pathologically proven, were greater than 1.0 cm in short axis measurement on CT or magnetic imaging, had a necrotic center, or demonstrated activity on FDG-PET. PET imaging guidelines were included in the protocol, but determination of FDG-PET–active disease was left to the discretion of the treating radiation oncologist, and a specific standardized uptake value cutoff point was not specified. The clinical target volume included the primary tumor volume and nodal volume with a 1.0-cm margin, except at the tumor/lung interface, where a 0.5-cm margin was used. The planning target volume (PTV) included the clinical target volume with a minimum margin of 0 to 0.5 cm and was adjusted for respiration visualized under treatment simulation. Clinically uninvolved LNs were not targeted. A single PTV was used for the entire treatment course, and the 95% isodense line was required to encompass the entire PTV. Normal tissue constraints limited the volume of lung receiving 20 Gy to 40% (calculated based on lung volume not involved with tumor), and the maximum spinal cord point dose allowed was 50 Gy. Specific esophageal dose constraints were not mandated by protocol. Radiotherapy data, including treatment planning CT images, portal images, and the three-dimensional data set, were reviewed by Quality Assurance Review Center during the first week of treatment, and a final review of all radiotherapy data was performed by the radiotherapy study chair in conjunction with Quality Assurance Review Center. The use of image-guided radiotherapy or respiratory gating was not specified. An analysis of the radiation treatment planning parameters and toxicity is pending and will be published separately.
Efficacy and Toxicity Evaluation
The response was assessed according to RECIST after every two cycles while on therapy; after completion of therapy, disease status was assessed every 4 months for 1 year, and then every 6 months for the next 2 years, and then annually for the next 3 years. Toxicity assessments according to National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0) were performed weekly for the first four cycles, and then every 3 weeks during single-agent pemetrexed.
Statistical Methods
This was a single-stage randomized phase II trial with the primary objective of estimating the OS for patients treated with carboplatin and pemetrexed with or without cetuximab in combination with TRT of 70 Gy. On the basis of the estimated 18-month OS probability of 35% from the previous CALGB trial, it was determined that if an 18-month OS probability of ≤ 35% was observed, then the combination was not worthy of further investigation. If the 18-month OS probability was ≥ 55%, the combination would be worthy of further investigation. Using a one-sided binomial exact test with a significance level of P = .10, the study, with 50 patients in each arm, had 90% power to differentiate the hypotheses. The probability of erroneously concluding that the treatment regimen was worthy of further investigation when the survival probability was truly ≤ 35% was 0.071, and the probability of erroneously concluding the treatment was not worthy of further investigation when the survival probability was ≥ 55% was 0.078. The trial was not designed to have adequate power to compare the two treatment arms.
OS is defined as the time from patient random assignment until death from any cause. The Kaplan-Meier24 product-limit estimator was used to estimate the median OS and the OS probability at 18 months, as well as the 95% CIs. A similar analytic method was used to characterize the failure-free survival, which is the time between patient random assignment and a failure event, defined as disease progression or death from any cause (whichever occurred first). The proportion of patients who experienced a response (partial or complete) to each combination was estimated, and binomial 95% CIs were calculated. Toxicity was assessed for both combinations. For each type of toxicity, a patient's worst treatment-related toxic episode was used to summarize distribution of toxicity grade experienced.
Patient registration and data collection were managed by the CALGB Statistical Center. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairman. CALGB statisticians performed statistical analyses.
As part of the quality assurance program of the CALGB, members of the Audit Committee visit all participating institutions at least once every 3 years to review source documents. The auditors verify compliance with federal regulations and protocol requirements, including those pertaining to eligibility, treatment, adverse events, tumor response, and outcome in a sample of protocols at each institution. Such on-site review of medical records was performed for a subgroup of 27 patients (25%) of the 109 patients enrolled onto this study.
RESULTS
Patients
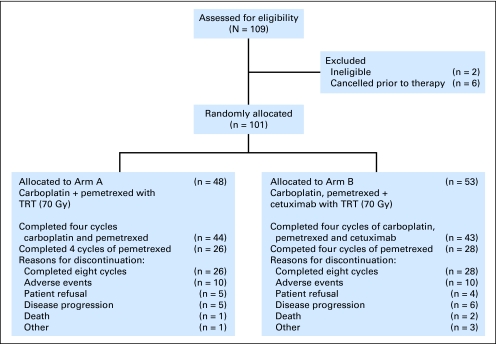
The trial was activated in September 2005, and 109 patients were registered between November 2005 and January 2008 (Fig 1). Of the 109 patients enrolled, six patients were cancelled before receiving any protocol-related therapy as a result of myocardial infarction (n = 1), lack of adequate radiation planning equipment (n = 1), physician decision (n = 1), and determination of ineligibility after enrollment but before starting protocol therapy (n = 3); two patients were found to be ineligible after starting protocol therapy. The patient characteristics are listed in Table 1. An imbalance in performance status was observed between the two treatment arms.
Fig 1.
CONSORT diagram. TRT, thoracic radiation therapy.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Arm A (n = 48) |
Arm B (n = 53) |
Overall (N = 101) |
P | |||
|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| Sex | .42 | ||||||
| Male | 27 | 56 | 24 | 64 | 61 | 60 | |
| Female | 21 | 44 | 19 | 36 | 40 | 39 | |
| Age, years | .74 | ||||||
| Median | 65 | 66 | 66 | ||||
| Range | 41-79 | 32-81 | 32-81 | ||||
| Race | .04 | ||||||
| White | 39 | 81 | 50 | 94 | 89 | 88 | |
| Black | 8 | 17 | 2 | 4 | 10 | 10 | |
| Asian | 0 | 0 | 1 | 2 | 1 | 1 | |
| Unknown | 1 | 2 | 0 | 0 | 1 | 1 | |
| Histology | .78 | ||||||
| Adenocarcinoma | 22 | 46 | 22 | 42 | 44 | 44 | |
| Squamous | 17 | 35 | 18 | 34 | 35 | 35 | |
| NSCLC, undifferentiated | 8 | 17 | 10 | 19 | 18 | 18 | |
| Large cell | 0 | 0 | 2 | 4 | 2 | 2 | |
| Missing | 1 | 2 | 1 | 2 | 2 | 2 | |
| Performance status | .01 | ||||||
| 0 | 28 | 58 | 18 | 34 | 46 | 46 | |
| 1 | 20 | 42 | 35 | 66 | 55 | 54 | |
| Stage | .34 | ||||||
| IIIA | 29 | 60 | 27 | 51 | 56 | 55 | |
| IIIB | 18 | 38 | 24 | 45 | 42 | 42 | |
| Missing | 1 | 2 | 2 | 4 | 3 | 2 | |
Abbreviation: NSCLC, non–small-cell lung cancer.
Treatment Administration
Forty-eight patients initiated chemoradiotherapy in arm A, and 26 patients completed the eight cycles (Table 2). The most common reasons for treatment discontinuation before completion in arm A were adverse events (AEs; n = 10), disease progression (n = 5), patient refusal (n = 5), and death (n = 1; Fig 1). Fifty-three patients initiated chemoradiotherapy in arm B, and 28 patients completed the eight cycles. The most common reasons for treatment discontinuation before completion in arm B were AEs (n = 10), disease progression (n = 6), patient refusal (n = 4), and death (n = 2). The median three-dimensional conformal TRT for all patients with initiated radiotherapy was 70 Gy (range, 10.53 to 76.92 Gy); 14 patients experienced a radiation treatment interruption, and the median number of days of treatment interruption was 2 (range, 1 to 9 days).
Table 2.
Treatment Administration
| Treatment | No. of Patients |
|
|---|---|---|
| Arm A: Carboplatin + Pemetrexed(n = 48) | Arm B: Carboplatin + Pemetrexed + Cetuximab(n = 53) | |
| Completed all planned treatment | 26 | 28 |
| Chemoradiotherapy | ||
| Completed 1 cycle | 1 | 3 |
| Completed 2 cycles | 2 | 5 |
| Completed 3 cycles | 1 | 2 |
| Completed 4 cycles | 44 | 43 |
| Postchemoradiation chemotherapy | ||
| Completed 0 cycles | 7 | 16 |
| Completed 1 cycle | 3 | 2 |
| Completed 2 cycles | 8 | 6 |
| Completed 3 cycles | 4 | 1 |
| Completed 4 cycles | 26 | 28 |
Toxicity
In arms A and B, 50 and 53 patients, respectively, were evaluable for toxicity. The most common grade 3 and 4 hematologic AEs observed in arms A and B are listed in Table 3. The common (≥ 10%) grade 3 to 5 nonhematologic toxicities observed in arms A and B (Table 3) were esophagitis, dysphagia, fatigue, pneumonitis, dehydration, and nausea/vomiting. The rates of observed grade 3 and 4 hematologic AEs were 42% and 28%, respectively, in arm A and 38% and 32%, respectively, in arm B. No grade 5 hematologic AEs were observed in either arm. The rates of observed grade 3 and 4 nonhematologic AEs were 46% and 6%, respectively, in arm A and 53% and 9%, respectively, in arm B. Two patients in arm A experienced grade 5 AEs (pulmonary hemorrhage and pneumonitis), and three patients in arm B experienced grade 5 AEs (two experienced pneumonitis, and one experienced grade 3 pneumonitis complicated by intrathoracic recurrence and pulmonary embolism leading to respiratory failure).
Table 3.
Grade ≥ 3 Toxicities Observed on Cancer and Leukemia Group B Trial 30407
| Toxicity | Arm A: Carboplatin + Pemetrexed(n = 50) |
Arm B: Carboplatin + Pemetrexed + Cetuximab(n = 53) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Hematologic toxicity | ||||
| Anemia | 9 | 18 | 7 | 13 |
| Neutropenia | 21 | 42 | 25 | 47 |
| Thrombocytopenia | 18 | 36 | 18 | 34 |
| Febrile neutropenia | 4 | 8 | 3 | 6 |
| Maximum hematologic | 35 | 70 | 37 | 70 |
| Nonhematologic toxicity occurring at ≥ 10% in one or both treatment arms | ||||
| Dehydration | 6 | 12 | 5 | 9 |
| Dysphagia | 8 | 16 | 6 | 11 |
| Dyspnea | 5 | 10 | 3 | 6 |
| Esophagitis | 8 | 16 | 7 | 13 |
| Fatigue | 11 | 22 | 9 | 17 |
| Hypokalemia | 0 | 0 | 6 | 11 |
| Nausea/vomiting | 4 | 8 | 5 | 9 |
| Pneumonitis | 6 | 12 | 6 | 11 |
| Rash (acneiform) | 0 | 0 | 7 | 13 |
| Maximum adverse events* | 38 | 76 | 45 | 85 |
Two treatment-related deaths were observed on arm A (one as a result of pneumonitis and one as a result of pulmonary hemorrhage), and three were observed on arm B (two as a result of pneumonitis and one as a result of grade 3 respiratory toxicity complicated by pulmonary embolism and intrathoracic recurrence).
Efficacy
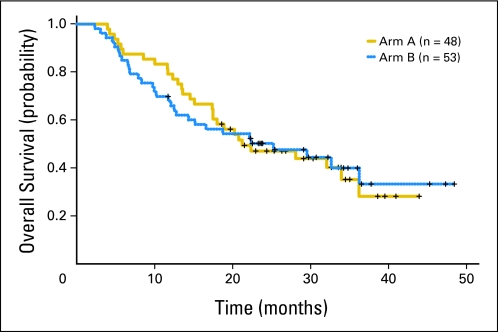
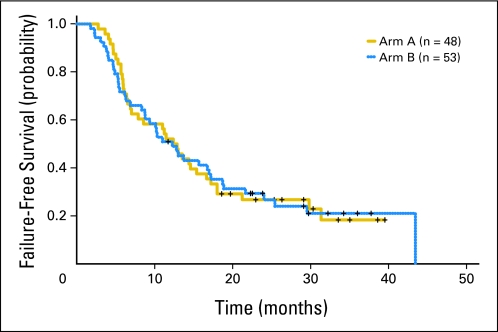
The efficacy data are listed in Table 4 and Appendix Table A1 (online only). With a median follow-up of 32 months (range, 11.7 to 48.4 months), 59 of 101 patients have died; the 18-month OS rates observed in arms A and B were 58% (95% CI, 46% to 74%) and 54% (95% CI, 42% to 70%), respectively (Table 4; Fig 2). The median failure-free survival times observed in arms A and B were 12.6 months (95% CI, 7.9 to 17.2 months) and 12.3 months (95% CI, 8.8 to 18.7 months), respectively (Fig 3).
Table 4.
Efficacy of Treatment Arms A and B
| Efficacy | Arm A: Carboplatin + Pemetrexed (n = 48) |
Arm B: Carboplatin + Pemetrexed + Cetuximab (n = 53) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Best Response | ||||
| Complete response | 4 | 8 | 2 | 4 |
| Partial response | 33 | 69 | 36 | 68 |
| Stable response | 11 | 23 | 12 | 23 |
| Progressive disease | 0 | 0 | 2 | 4 |
| Not evaluable | 0 | 0 | 1 | 2 |
| Overall response rate, % | 77 | 72 | ||
| 95% CI | 63 to 88 | 58 to 83 | ||
| Median failure-free survival, months | 12.6 | 12.3 | ||
| 95% CI | 7.9 to 17.2 | 8.8 to 18.7 | ||
| 18-month failure-free survival, % | 29 | 33 | ||
| 95% CI | 19 to 45 | 23 to 49 | ||
| Median overall survival, months | 21.2 | 25.2 | ||
| 95% CI | 17.5 to NA | 14.4 to NA | ||
| 18-month overall survival, % | 58 | 54 | ||
| 95% CI | 46 to 74 | 42 to 70 | ||
Abbreviation: NA, not available.
Fig 2.
Kaplan-Meier curve of overall survival by treatment arm.
Fig 3.
Kaplan-Meier curve of failure-free survival by treatment arm.
An unplanned analysis of OS by histology revealed that among patients with squamous and nonsquamous histology in both arms, the median OS was 22.2 months (95% CI, 12.1 months to not available) and 22.4 months (95% CI, 16.6 to 36.2 months), respectively; no significant difference in OS between the squamous and nonsquamous patients was observed (P = .667). The efficacy results of patients with squamous and nonsquamous histology in treatment arms A and B are listed in Table 5.
Of 101 patients, 59 patients have experienced progression. Fourteen patients (24%) experienced local and distant progression, 19 patients (32%) experienced local disease progression alone, 26 patients (44%) experienced distant disease progression alone, and 12 patients developed brain metastases.
DISCUSSION
The OS observed in this study is significantly better than many of our previous CALGB studies in patients with locally advanced unresectable NSCLC.18,25 It is tempting to attribute these results to the novel chemotherapy regimen and higher doses of TRT, but a number of others factors could have contributed to the results. The mandatory use of FDG-PET scans for staging purposes and the exclusion of patients with significant pretreatment weight loss may have contributed to the selection of patients with a better prognosis than those enrolled in CALGB 39801. The use of a higher dose of TRT could have contributed to the improved survival. The CALGB 30105 study of high-dose TRT produced a median survival similar to CALGB 30407.19
The toxicities observed were similar to previous CALGB trials, although rates of severe esophagitis were lower in this study presumably related to absence of radiation to the uninvolved mediastinal nodes.18 The fact that the efficacy of pemetrexed is limited to patients with nonsquamous histology was not known when this study was designed and conducted. The OS among patients with squamous and nonsquamous histology was similar, but the number of patients in each of the histologic subgroups was small, which reduced our ability to detect an established treatment interaction. The ongoing international phase III study sponsored by Eli Lilly is investigating pemetrexed and cisplatin in combination with TRT to 66 Gy compared with cisplatin, etoposide, and TRT in patients with nonsquamous NSCLC.26
In metastatic NSCLC, a phase III trial of cisplatin and vinorelbine with or without cetuximab revealed an improvement in OS with the addition of cetuximab.27 The addition of cetuximab to paclitaxel, carboplatin, and radiation resulted in an encouraging median survival of 23 months in a single-arm phase II study conducted by the Radiation Therapy Oncology Group (RTOG).28 It is not clear whether the observed improvement in survival (compared with prior RTOG studies) is a result of the addition of cetuximab or patient selection. We would like to emphasize that CALGB 30407 was not designed to compare the two different systemic therapy regimens (arm A without cetuximab and arm B with cetuximab) but was designed only to identify the arm(s) that would meet a predefined end point for further exploration. Unlike arm A, arm B narrowly missed the predefined criterion for further study. The ongoing RTOG phase III trial 0617 will address the utility of cetuximab in addition to the role of an escalated dose of TRT in the management of patients with unresectable stage III NSCLC.
We should await the results of these two large trials before incorporating pemetrexed or cetuximab in routine clinical practice for the treatment of patients with locally advanced NSCLC.
Appendix
The following institutions and members participated in this study: Christiana Care Health Services, Community Clinical Oncology Program (CCOP), Wilmington, DE–Stephen Grubbs, MD, supported by Grant No. CA45418; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by Grant No. CA47577; Hematology-Oncology Associates of Central New York CCOP, Syracuse, NY–Jeffrey Kirshner, MD, supported by Grant No. CA45389; Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO–Rakesh Gaur, MD; Missouri Baptist Medical Center, St. Louis, MO–Alan P. Lyss, MD, supported by Grant No. CA114558-02; Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John A. Ellerton, MD, supported by Grant No. CA35421; New Hampshire Oncology-Hematology PA, Concord, NH–Douglas J. Weckstein; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by Grant No. CA08025; Southeast Cancer Control Consortium CCOP, Goldsboro, NC–James N. Atkins, MD, supported by Grant No. CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by Grant No. CA21060; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by Grant No. CA77658; University of California at San Diego, San Diego, CA–Barbara A. Parker, MD, supported by Grant No. CA11789; University of Chicago, Chicago, IL–Hedy L. Kindler, MD, supported by Grant No. CA41287; University of Iowa, Iowa City, IA–Daniel A. Vaena, MD, supported by Grant No. CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, MD, supported by Grant No. CA31983; University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, MD, supported by Grant No. CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, MD, supported by Grant No. CA47559; University of Vermont, Burlington, VT–Steven M. Grunberg, MD, supported by Grant No. CA77406; Washington University School of Medicine, St Louis, MO–Nancy Bartlett, MD, supported by Grant No. CA77440; and University of Minnesota, Minneapolis, MN– Robert Kratzke, MD, supported by Grant No. CA16450.
Table A1.
Efficacy by Histology and Treatment Arms
| Efficacy | Arm A: Carboplatin + Pemetrexed (n = 48) |
Arm B: Carboplatin + Pemetrexed + Cetuximab (n = 53) |
||
|---|---|---|---|---|
| Squamous (n = 17) | Nonsquamous (n = 31) | Squamous (n = 18) | Nonsquamous (n = 35) | |
| ORR, % | 76 | 77 | 83 | 66 |
| 95% CI | 50 to 93 | 59 to 90 | 59 to 96 | 48 to 81 |
| Median FFS, months | 15 | 11 | 9 | 13 |
| 95% CI | 6 to NA | 6 to 17 | 5 to 17 | 9 to 19 |
| 18-month FFS, % | 29 | 29 | 28 | 36 |
| 95% CI | 11 to 51 | 15 to 45 | NA | 21 to 52 |
| Median OS, months | NA* | 21 | 17 | 25 |
| 95% CI | — | 14 to 34 | 7 to NA | 14 to NA |
| 18-month OS, % | 59 | 58 | 44 | 56 |
| 95% CI | 33 to 78 | NA | 22 to 65 | 38 to 71 |
Abbreviations: FFS, failure-free survival; NA, not available; ORR, overall response rate; OS, overall survival.
The median OS among patients with squamous histology in arm A has not been reached but is estimated to be greater than 20 months.
Footnotes
Supported, in part, by grants from the National Cancer Institute to the Cancer and Leukemia Group B (CALGB; Monica M. Bertagnolli, MD, Chair; Grant No. CA31946) and to the CALGB Statistical Center (Daniel Sargent, PhD; Grant No. CA33601). See Appendix (online only) for grants provided to participating institutions.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00117962.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ramaswamy Govindan, Eli Lilly (C) Stock Ownership: None Honoraria: Thomas Stinchcombe, Eli Lilly Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Ramaswamy Govindan, Jeffrey Bogart, Xiaofei Wang, Robert Kratzke, Everett E. Vokes
Administrative support: Thomas Stinchcombe
Provision of study materials or patients: Ramaswamy Govindan, Thomas Stinchcombe, Jennifer Garst, Timothy Brotherton, Everett E. Vokes
Collection and assembly of data: Ramaswamy Govindan, Thomas Stinchcombe, Xiaofei Wang, Lydia Hodgson, Robert Kratzke, Jennifer Garst
Data analysis and interpretation: Ramaswamy Govindan, Jeffrey Bogart, Thomas Stinchcombe, Xiaofei Wang, Lydia Hodgson, Robert Kratzke, Timothy Brotherton, Everett E. Vokes
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: Experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small cell lung cancer guideline: Update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non–small-cell lung cancer: The Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 6.Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet. 2009;374:379–386. doi: 10.1016/S0140-6736(09)60737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vokes EE, Herndon JE, 2nd, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non–small-cell lung cancer: Cancer and Leukemia Group B study 9431. J Clin Oncol. 2002;20:4191–4198. doi: 10.1200/JCO.2002.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Segawa Y, Kiura K, Takigawa N, et al. Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non–small-cell lung cancer: OLCSG 0007. J Clin Oncol. 2010;28:3299–3306. doi: 10.1200/JCO.2009.24.7577. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto N, Nakagawa K, Nishimura Y, et al. Phase III study comparing second- and third-generation regimens with concurrent thoracic radiotherapy in patients with unresectable stage III non–small-cell lung cancer: West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol. 2010;28:3739–3745. doi: 10.1200/JCO.2009.24.5050. [DOI] [PubMed] [Google Scholar]
- 10.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non–small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 11.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: A randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non–small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 13.Seiwert TY, Connell PP, Mauer AM, et al. A phase I study of pemetrexed, carboplatin, and concurrent radiotherapy in patients with locally advanced or metastatic non-small cell lung or esophageal cancer. Clin Cancer Res. 2007;13:515–522. doi: 10.1158/1078-0432.CCR-06-1058. [DOI] [PubMed] [Google Scholar]
- 14.Thienelt CD, Bunn PA, Jr, Hanna N, et al. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non–small-cell lung cancer. J Clin Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 15.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 16.Robert F, Blumenschein G, Herbst RS, et al. Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non–small-cell lung cancer. J Clin Oncol. 2005;23:9089–9096. doi: 10.1200/JCO.2004.00.1438. [DOI] [PubMed] [Google Scholar]
- 17.Bonner JA, Harari PM, Giralt J, et al. Cetuximab prolongs survival in patients with locoregionally advanced squamous cell carcinoma of head and neck: A phase III study of high-dose radiation therapy with and without cetuximab. J Clin Oncol. 2004;22(suppl):489s. abstr 5507. [Google Scholar]
- 18.Vokes EE, Herndon JE, 2nd, Kelley MJ, et al. Induction chemotherapy followed by chemoradiotherapy compared with chemoradiotherapy alone for regionally advanced unresectable stage III non–small-cell lung cancer: Cancer and Leukemia Group B. J Clin Oncol. 2007;25:1698–1704. doi: 10.1200/JCO.2006.07.3569. [DOI] [PubMed] [Google Scholar]
- 19.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non–small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–2463. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Calvert A, Newell D, Gumbrell L, et al. Carboplatin dosage: Prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 22.US Food and Drug Administration. Pemetrexed package insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/021462ppilbl.pdf.
- 23.ImClone Systems, Bristol-Myers Squibb. Erbitux (cetuximab) package insert. http://www.erbitux.com/index.aspx.
- 24.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Akerley W, Herndon JE, Jr, Lyss AP, et al. Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small-cell lung cancer: A limited-access study—CALGB 9534. Clin Lung Cancer. 2005;7:47–53. doi: 10.3816/CLC.2005.n.021. [DOI] [PubMed] [Google Scholar]
- 26.Vokes EE, Senan S, Treat JA, et al. PROCLAIM: A phase III study of pemetrexed, cisplatin, and radiation therapy followed by consolidation pemetrexed versus etoposide, cisplatin, and radiation therapy followed by consolidation cytotoxic chemotherapy of choice in locally advanced stage III non-small-cell lung cancer of other than predominantly squamous cell histology. Clin Lung Cancer. 2009;10:193–198. doi: 10.3816/CLC.2009.n.027. [DOI] [PubMed] [Google Scholar]
- 27.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 28.Blumenschein GR, Moughan J, Curran W, et al. A phase II study of cetuximab (C225) in combination with chemoradiation: B3-07. J Thorac Oncol. 2007;2(suppl 4):S342–S343. [Google Scholar]