Abstract
Purpose
Trastuzumab resistance has been linked to activation of the phosphoinositol 3-kinase (PI3K) pathway. Phosphatase and tensin homolog (PTEN) is a dual phosphatase that counteracts the PI3K function; PTEN loss leads to activation of the Akt cascade and the downstream mammalian target of rapamycin (mTOR). Preclinical studies demonstrated that mTOR inhibition sensitized the response to trastuzumab in mice with HER2 overexpressing and PTEN-deficient breast xenografts. Our trial evaluated the safety and efficacy of the combination of everolimus and trastuzumab in women with HER2-overexpressing metastatic breast cancer (MBC) that progressed on trastuzumab-based therapy.
Patients and Methods
This represents a pooled analysis (n = 47), stemming from two trials that occurred concurrently in The University of Texas MD Anderson Cancer Center, Beth Israel Deaconess Medical Center, and Dana-Farber Cancer Institute. Patients with HER2-overexpressing MBC who had progressed on trastuzumab-based therapy received trastuzumab every 3 weeks in combination with daily everolimus.
Results
Among 47 patients, the combination of everolimus and trastuzumab provided partial responses in seven patients (15%) and persistent stable disease (lasting 6 months or longer) in nine patients (19%), resulting in a clinical benefit rate of 34%. The median progression-free survival (PFS) was 4.1 month. Fatigue, infection, and mucositis were the predominant nonhematologic toxicities. Trastuzumab did not have significant influence on the pharmacokinetic profile of everolimus. Patients with PTEN loss demonstrated decreased overall survival (P = .048). However, PFS was not affected by PTEN loss.
Conclusion
Inhibition of mTOR results in clinical benefit and disease response in patients with trastuzumab-resistant HER2-overexpressing MBC.
INTRODUCTION
HER2 overexpression or gene amplification, which occurs in approximately 25% of all breast cancers, is associated with decreased disease-free survival and overall survival (OS).1 Trastuzumab binds with high affinity to the extracellular domain of the HER2 receptor. Addition of trastuzumab to chemotherapy, in the first-line setting, has resulted in a significantly increased objective response, time to disease progression, and OS.2 However, 25% to 30% of patients do not respond to trastuzumab-based therapy in the metastatic setting.2 Among those who respond, the majority of patients eventually develop progressive disease (PD) while receiving trastuzumab-based regimens.3,3a
Trastuzumab resistance has been linked to activation of the phosphoinositol 3-kinase (PI3K) pathway.4,5 Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene that converts PI (3,4,5)P3 to PIP2 and antagonizes the PI3K cascade. PTEN deficiency occurs in 40% to 50% of breast cancers.6 Loss of PTEN leads to constitutive activation of Akt, resulting in activation of mammalian target of rapamycin (mTOR). Preclinical studies have demonstrated that mTOR inhibition reduces tumor formation/growth in mice with PTEN-deficient tumors and sensitizes response to trastuzumab in mice bearing HER2-overexpressing and PTEN-deficient breast tumor xenografts.7,8 Everolimus binds to FKB-12, and the resulting complex inhibits mTOR.9 A phase I study demonstrated that the combination of everolimus and paclitaxel resulted in stable disease (SD) in 11 of 16 patients with trastuzumab-resistant tumors.10 Pooled analysis of two phase I/II studies, in which 138 patients received six cycles of everolimus, trastuzumab, and either paclitaxel or vinorelbine, followed by an extension phase in which the cytotoxic agent could be discontinued, demonstrated that, among trastuzumab-resistant and taxane-pretreated patients, five patients had a complete response (CR), 10 patients had partial response (PR), and 16 patients had SD.11
We hypothesized that, in patients with PTEN deficiency, mTOR inhibition with everolimus should result in abrogation of trastuzumab resistance. As levels of P-Akt and p70S6K-T389-P in breast cancers reflect PI3K/Akt/mTOR kinase pathway activation, we postulated that trastuzumab and everolimus treatment would reduce the levels of P-Akt and p70S6K-T389-P in breast tumors.5 Thus, we determined expression levels of total and phosphorylated mTOR and p70S6K-T389-P as well as relevant downstream signaling components (eg, S6, 4E-BP1) in pre- and post-treatment tumor samples.
PATIENTS AND METHODS
Study Design
Two phase I/II trials were conducted under separate investigational new drug applications (INDs) at MD Anderson Cancer Center (MDACC; IND 69277), Dana-Farber Cancer Institute (DFCI), and Beth Israel Deaconess Medical Center (BIDMC; IND 76179). Results were combined in 2009, with approval by the US Food and Drug Administration, in order to complete the trial with adequate power. As results were pooled for analytic purposes, the BIDMC/DFCI protocol was amended to match the MDACC protocol. Pertinent differences between the trials are discussed throughout this article.
This open-label phase I/II study was approved by the local institutional review board at each institution. All participants provided written informed consent. If a patient was on trastuzumab at time of registration, the loading dose of trastuzumab was deferred, and she received the maintenance dose (6 mg/kg every 3 weeks). If the last trastuzumab dose was given ≥ 1 week (for patients receiving 2 mg/kg/wk), or ≥ 3 weeks before registration (for patients receiving 6 mg/kg every 3 weeks), the patient received a loading dose (8 mg/kg) followed by the maintenance dose. Institution-specific study designs are specified as follows.
MDACC Study Design
The MDACC phase I dosing schema for everolimus is detailed below; the 10 mg dose was used in the phase II portion.
At study inception, the optimal dose of everolimus in combination with trastuzumab was not known. Therefore, we performed a dose-finding study to evaluate safety of two dose levels of daily everolimus (5 and 10 mg). At MDACC, the first patient was treated at 10 mg (dose level 1). Patients underwent clinical evaluation every 3 weeks (one cycle) and radiologic evaluations every 6 weeks. After the second cycle, patients underwent a radiologic evaluation using the same imaging technique used at initial evaluation (ie, computed tomography or magnetic resonance imaging). If the patient exhibited PR or SD, radiologic evaluations occurred every 6 weeks. Provided the tumor was stable or smaller, and the patient had recovered to grade 1 or lower treatment-related toxicity, she began another cycle. At MDACC, if the patient had treatment-related toxicity higher than grade 1 at the time of clinical evaluation (3 weeks), treatment was held for 1 week. If the toxicity had not resolved, treatment was held for a second week. In instances in which weekly evaluation was needed, patients returned to MDACC weekly to be assessed. If treatment-related toxicities recovered to grade 1 or lower after 2 weeks of withholding therapy on cycle 1, the dose of everolimus remained unchanged. If toxicities recovered to grade 1 or lower after 2 weeks of withholding therapy on subsequent cycles, the everolimus dose was decreased to 5 mg, if the patient was on dose level 1 (10 mg). If the patient was on dose level −1 (5 mg) and the treatment-related toxicities recovered to grade 1 or lower after 2 weeks of withholding therapy, the patient resumed treatment at the same dose. The trastuzumab dose was not modified. If treatment-related toxicities higher than grade 1 persisted after 2 weeks of withholding therapy, the patient was taken off protocol. Treatment continued indefinitely as long as there were no unacceptable toxicities and no tumor progression.
DFCI/BIDMC Study Design
At DFCI/BIDMC, a 3+3 study design was utilized in the phase I portion. Furthermore, if patients developed grade 3 or 4 neutropenia or thrombocytopenia, therapy was delayed ≤ 3 weeks until absolute neutrophil count ≥ 1,500 and platelets ≥ 100,000. If recovery occurred within 3 weeks after treatment was held, dose was reduced to 5 mg (if patient was taking 10 mg). If the patient was taking 5 mg, she was withdrawn from the study. If recovery did not occur within 3 weeks, the patient was withdrawn from study. For all other nonhematologic grade 1 or 2 adverse events, treatment was continued, but persistent and intolerable grade 2 toxicity warranted dose delay/reduction or withdrawal from the study, at the discretion of the treating physician.
Patient Eligibility
Eligible women were ≥ 18 years of age with history of biopsy-proven HER2-overexpressing breast cancer (determined by immunohistochemistry [score, 3+] or by fluorescence in situ hybridization [her-2/neu to chromosome 17 ratio > 2]) and radiographic evidence of metastatic breast cancer (MBC). Patients were required to have an Eastern Cooperative Oncology Group performance status of ≤ 2; ≥ 1 measurable lesion according to Response Evaluation Criteria in Solid Tumors, and could not receive investigational agents within 15 days of enrollment. Eligible patients had adequate hematologic, renal, hepatic, and cardiac function.
Patients were required to have PD after ≥ 1 trastuzumab-based therapy for MBC. At MDACC, each patient could not have received more than two prior trastuzumab-based regimens and one lapatinib-based regimen for MBC. Patients who developed MBC within 12 months of adjuvant or neoadjuvant trastuzumab were eligible. The DFCI/BIDMC trial placed no limits on number of prior chemotherapy or trastuzumab-containing regimens.
Study End Points
Objectives of the two parallel trials closely resembled each other. At MDACC, the primary objectives were to: identify the optimal dose of everolimus in combination with trastuzumab, and determine the efficacy of everolimus plus trastuzumab in patients with HER2-expressing tumors with resistance to trastuzumab-based therapy for MBC. Efficacy was measured by the clinical benefit response rate (CBR), defined as confirmed CR plus PR at any time plus persistent SD (pSD). Confirmed CR was defined as disappearance of all target lesions at the time of radiographic evaluation; pSD was defined as SD lasting ≥ 24 weeks.
Secondary objectives were to: determine pharmacokinetics of everolimus in combination with trastuzumab; determine nature and degree of toxicity of everolimus in combination with trastuzumab; determine whether PTEN, Akt, p70S6, Src protein expression, and PIK3CA mutations in breast cancer tissue correlate with CBR from everolimus/trastuzumab therapy; and determine whether changes in fluorodeoxyglucose uptake and changes in circulating tumor cells (CTCs) predict clinical benefit in this population. Discussion of pharmacokinetic, CTCs, and positron emission tomography/computed tomography studies is found in the Data Supplement.
At DFCI/BIDMC, the primary objective was to assess safety and tolerability of everolimus in combination with trastuzumab in HER2-positive MBC. The secondary objectives were to: evaluate the activity of everolimus plus trastuzumab in patients with progression on a trastuzumab-containing regimen; evaluate changes in signaling molecules in response to trastuzumab and everolimus in CTCs and tumor tissue; and to evaluate pharmacokinetics of everolimus in combination with trastuzumab.
Safety Assessments
Left ventricular ejection function was assessed by echocardiogram or multiple-gated acquisition scan at baseline, every 3 months while the patient was on study, and at the time the patient was taken off study. Adverse events assessments, graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 3.0, were performed at 3-week intervals and at treatment completion. Highest grade of toxicity was recorded for each patient.
Biomarker Studies
Paraffin-embedded tissue and/or fresh-frozen tissue from the original tumors were collected. In consenting patients, we obtained biopsies of metastatic tumors. Biomarker studies were performed in the laboratory of Dihua Yu (MDACC) and Myriad Genetics (Salt Lake City, UT). We evaluated expression and/or phosphorylation status of PTEN, mTOR, Akt, and p70S6 kinase by immunohistochemistry. The phospho-P70S6 (P7056-P, Thr389 site) antibody was obtained from Millipore (Billerica, MA; 04-392, 1:100 dilution). Antibodies to P-Akt-Ser473 (736E, #3787, 1:50 dilution), Src (36D10, #2109, 1:1,400 dilution), and P-Src-Tyr416 (#2101, 1:400 dilution) were received from Cell Signaling Technology (Danvers, MA). Tissue samples were fixed in 4% buffered formalin and embedded in paraffin. Antigen retrieval was performed in sodium citrate buffer (pH 6.0) via pressure cooker. Immunostaining was performed using the Dako Autostain plus System (Carpinteria, CA). Positive and negative control slides (without primary antibody) were included within each batch for staining.
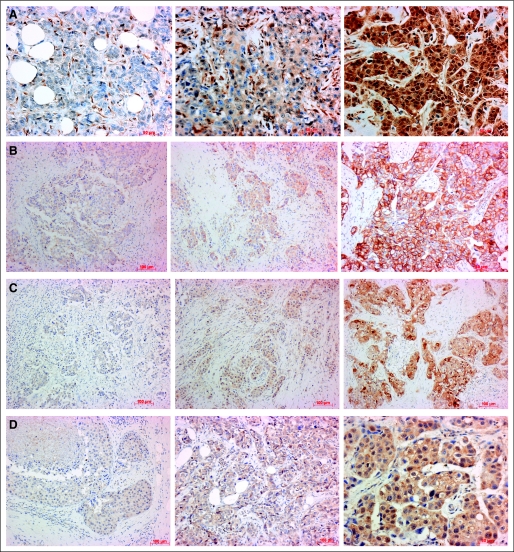
For the immunohistochemistry analysis, the expression levels were semiquantified using immunoreactive scores, which were calculated by multiplying percentage of positive cells (scored 0 to 4) with staining intensity (1 to 3). Score range was 0 to 12. A score of 0 to 3 was considered negative. Positive scoring was evaluated as 1+ (score of 4 to 6), 2+ (score of 7 to 9), or 3+ (score of 10 to 12; Fig 1A).
Fig 1.
Patterns of (A) phosphatase and tensin homolog, (B) P-Src, (C) P-Akt, and (D) P-P70S6 expression in breast tumors.
In consenting patients, we evaluated primary tumors and metastatic tumors for alterations in expression and/or phosphorylation status of these biomarkers during progression of disease and/or by treatment (Figs 1B to 1D). Specifically, levels of P-Akt and p70S6K-P in breast cancers reflected PI3K/Akt/mTOR kinase pathway activation. P70S6 kinase expression was determined as previously described.14 Finally, PIK3CA gene was sequenced to determine whether PTEN mutations correlated with response to therapy.
Statistical Analysis
In the phase I portion of this trial, dose-finding was conducted using a continuous reassessment model, which relies on a simple Bayesian one parameter model of the dose-toxicity curve (DTC). After each patient was treated and outcome observed (toxicity or no toxicity), distribution of the parameter was updated and the next dose level was selected based on the predicted toxicity. The target toxicity probability was 20%, with a planned maximum of 16 patients to be accrued. The estimated DTC was updated after each outcome was observed, so that each patient's dose was based on information about how previous patients tolerated the treatment. Using the updated DTC, the best estimate of the optimal dose (dose most closely associated with a toxicity rate of 0.20) was determined.
MDACC data were combined with data from BIDMC/DFCI for both phase I and II components of the trial. Because no dose-limiting toxicity was observed with everolimus 10 mg daily, this became the phase II dose. Therefore, all patients treated at MDACC received everolimus 10 mg daily. At DFCI/BIDMC, the first three patients were involved in the phase I portion, the remainder were involved in the phase II trial. Best clinical response was dichotomized as PD (nonresponders) versus PR/SD (responders). Fisher's exact test was used to investigate the effect of dichotomous factors (eg, E542K mutation, PTEN expression) on best clinical response. OS and progression-free survival (PFS) distribution functions were estimated by Kaplan-Meier method. Survival distribution differences were evaluated by log-rank test. Analyses were conducted using the SAS software release 9.2 (SAS Institute, Cary, NC); statistical significance was defined as P < .05.
RESULTS
Patient Characteristics
Between May 2006 and May 2009, 50 patients were recruited from both institutions. Three patients were ineligible (n = 2 for left ventricular ejection fraction < 50%; n = 1 for brain metastases). Table 1 lists the baseline characteristics of the 47 eligible patients. Most patients (83%) had visceral disease. Six patients (13%) had never received previous chemotherapy for MBC, whereas nineteen patients (40%) had received two or more regimens of chemotherapy for MBC.
Table 1.
Demographic and Baseline Clinical Characteristics
| Characteristic | Patients (N = 47) |
|
|---|---|---|
| No. | % | |
| Age, years | ||
| Median | 50 | |
| Range | 28-66 | |
| Hormone receptor status | ||
| ER or PR positive | 28 | 60 |
| ER and PR negative | 19 | 40 |
| Histology | ||
| Ductal | 42 | 89 |
| Lobular | 1 | 2 |
| Other | 4 | 9 |
| Visceral disease | 39 | 83 |
| Prior lines of chemotherapy for MBC | ||
| 0 | 7 | 15 |
| 1 | 23 | 49 |
| 2 | 15 | 32 |
| 3 | 1 | 2 |
| > 3 | 1 | 2 |
| Prior lapatinib therapy | 9 | 19 |
| Prior lines of trastuzumab-containing chemotherapy for MBC | ||
| 0 | 10 | 21 |
| 1 | 29 | 62 |
| 2 | 8 | 17 |
| Relapsed within 1 year of adjuvant trastuzumab | 20 | 42 |
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; MBC, metastatic breast cancer.
Clinical Outcomes
The combination of everolimus and trastuzumab provided PRs in seven patients (15%) and pSD in nine patients (19%), resulting in a CBR of 34%. Among the 16 patients who demonstrated evidence of clinical benefit, nine patients (56%) had relapsed within 1 year of adjuvant trastuzumab therapy, six patients (38%) had received two or more lines of chemotherapy for MBC, and two patients (13%) had received prior lapatinib therapy. Median PFS was 4.1 months.
Toxicity
Forty-seven patients were evaluable for toxicity. Common toxicities, grade 2 or higher, are listed in Table 2 . Fatigue, infection, and mucositis were the predominant nonhematologic toxicities. Grade 2 hematologic toxicity was common, accounting for 13% to 17% of patients. No significant cardiovascular toxicity was noted. There were no treatment related deaths.
Table 2.
Grade 2 or Higher Adverse Events
| Toxicity | Toxicity by Grade (% patients) |
||
|---|---|---|---|
| 2 | 3 | 4 | |
| Hematologic | |||
| Anemia | 17 | 4 | 0 |
| Lymphopenia | 13 | 13 | 0 |
| Neutropenia | 13 | 9 | 0 |
| Thrombocytopenia | 6 | 4 | 0 |
| Nonhematologic | |||
| Diarrhea | 2 | 9 | 0 |
| Fatigue | 23 | 9 | 0 |
| Hyperglycemia | 9 | 11 | 2 |
| Hypokalemia | 6 | 6 | 0 |
| Hyperlipidemia | 9 | 4 | 0 |
| Infection | 19 | 4 | 0 |
| Mucositis | 25 | 9 | 0 |
| Rash | 9 | 0 | 0 |
| Transaminitis | 9 | 2 | 0 |
| Thrombosis/embolism | 2 | 0 | 2 |
Dose reductions/delays occurred in 25 patients (53%). The most common causes for alterations in dosing schedule were (in order of frequency): mucositis, rash, and infection. Among the patients who required dose delays, 10 (40%) required ≥ 2 dose delays while being treated on the trial; average length of dose delay was 9 days. One patient discontinued therapy due to persistent grade 3 stomatitis.
Biomarker Analysis
Twenty-six pretreatment specimens (from tissue samples of original tumors) and one pretreatment biopsy specimen (obtained before treatment) were evaluated. Post-treatment specimens, representing biopsies from metastatic sites of eight patients, were evaluated. Metastatic sites included: liver, chest wall, lung, dura, peritoneum, and lymph nodes. Of the eight patients in whom both pre- and post-treatment samples were available, only one patient demonstrated a change in biomarker status after treatment. Analysis for four predominant mutations in the PIK3CA pathway (E542K, E545K, H1047R, and H1047L) demonstrated that E542K mutations occurred in 3% of samples, E545K mutations occurred in 11.8% of samples, and H1047R or H1047L mutations occurred in 20.5% of samples. However, presence of these mutations, when analyzed individually as well as collectively, did not correlate with response or lack of response (P = not significant).
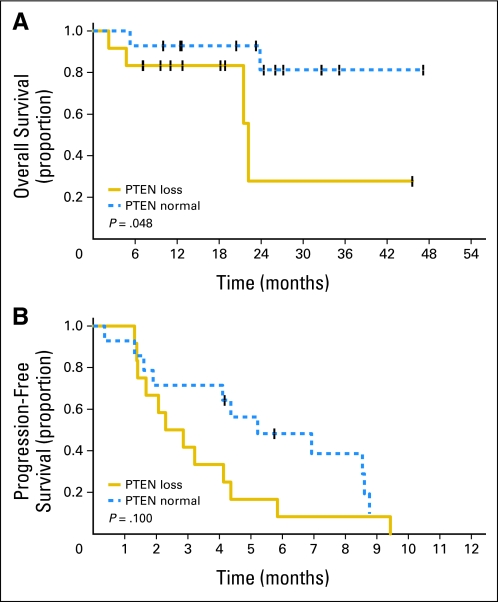
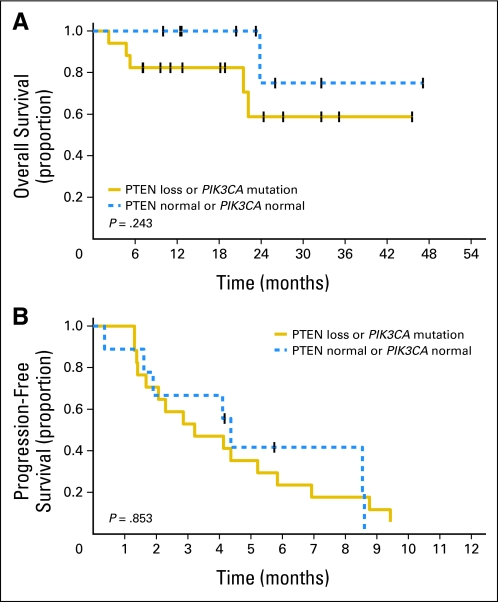
Furthermore, we evaluated the expression and effect of PTEN loss and/or PIK3CA mutations on OS and PFS. When compared with patients without PTEN deficiency, patients with PTEN loss demonstrated a statistically decreased OS (P = .048; Fig 2A). However, PFS was not significantly affected by PTEN loss (Fig 2B). PFS and OS were not significantly affected by mutations in PIK3CA. Patients with either PTEN loss or PIK3CA mutation demonstrated no statistically significant decline in OS or PFS (Fig 3A and 3B).
Fig 2.
Effect of phosphatase and tensin homolog (PTEN) loss on (A) overall survival and (B) progression-free survival.
Fig 3.
Effect of the presence of phosphatase and tensin homolog (PTEN) loss or PIK3CA mutation (A) on overall survival and (B) progression-free survival.
Increased phosphorylation of P70S6 kinase occurred in 17 (53%) of 32 samples and did not correlate with response (P = .27). Elevated expression of P-Akt occurred in half of samples; differences in the level of P-Akt were not predictive of response (P = .7). Overexpression of Src and P-Src occurred in 83% of samples, but these levels did not correlate with response (P = 1.0).
DISCUSSION
This study demonstrated that the combination of everolimus and trastuzumab is a feasible and biologically active regimen in patients with HER2-overexpressing MBC that progressed on prior trastuzumab-based therapy, in the adjuvant and/or metastatic setting. The CBR of 34% is clinically important in this population because many patients demonstrated a high burden of visceral disease and had received ≥ 2 chemotherapy regimens for MBC.
In addition, this study supports the findings of two recent randomized trials that examined the benefit of continuation of trastuzumab beyond progression. Blackwell et al17 noted that, when patients with HER2-positive MBC who had demonstrated progression on prior trastuzumab-based therapy were randomly assigned to lapatinib alone versus lapatinib in combination with trastuzumab, the combination arm demonstrated improvement in PFS. In addition, interim analysis of the Trastuzumab Beyond Progression study demonstrated a trend toward improvement in time to progression in the trastuzumab-containing arm.18
The overall safety profile of this regimen was acceptable, in this pretreated population. Incidence of stomatitis, infection, and hematologic toxicity was significantly higher with the addition of everolimus to trastuzumab. However, the majority of the adverse events were grade 1 or 2, and most events resolved without need for dose modification.
Biomarker analysis of the available tumors demonstrated that PTEN loss was associated with poorer OS, confirming PTEN loss enables activation of downstream cascades that promotes tumorigenesis and progression. However, the finding that PFS was not significantly affected by PTEN loss and/or PIK3CA mutation suggests that the addition of everolimus may mitigate tumor progression through inhibition of mTOR. This clinical result supports preclinical data that demonstrated that human cell lines with mutations in PIK3CA (H1047R or E545K) had increased sensitivity to everolimus.19,20
Our trial demonstrated a novel approach involving utilization of the combination of everolimus and trastuzumab, two targeted therapies that inhibit different functional domains in cancer cells, to overcome trastuzumab resistance in patients with HER2-positive MBC, in the absence of cytotoxic therapy. This regimen offers a targeted, nonchemotherapy option for patients with trastuzumab-resistant MBC. While it has potential toxicities, these are balanced by the ability to offer pretreated patients a chemotherapy-free, biologically active regimen. These results support the known preclinical activity of everolimus in combination with trastuzumab, and they help to validate the ability of everolimus to overcome PTEN-mediated trastuzumab resistance through inhibition of the mTOR pathway. This hypothesis is being tested in ongoing randomized studies evaluating the role of combining everolimus with trastuzumab and chemotherapy in the first- and second-line treatment of MBC.
Supplementary Material
Acknowledgment
We thank Steve Stone and Zaina Sangale (Myriad Genetics) for their contributions in phosphatase and tensin homolog immunohistochemistry and PIK3CA mutational analysis.
Footnotes
See accompanying editorial on page 3111
Supported by Grant No. 1 P50 CA116199-01 from the National Cancer Institute (NCI) Breast Cancer SPORE (Specialized Program of Research Excellence) and Novartis Pharmaceuticals (to The University of Texas MD Anderson Cancer Center); and Grant No. 1P50 CA089393 NCI Breast Cancer SPORE, the AVON foundation, and Novartis Pharmaceuticals (to Beth Israel Deaconess Medical Center, Dana-Farber Cancer Institute, and Brigham and Women's Hospital).
Presented in abstract format at the 46th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 4-8, 2010.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00317720.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ian E. Krop, Genentech (U), Novartis (U); Gabriel N. Hortobagyi, Novartis (C), Genentech (C) Stock Ownership: None Honoraria: None Research Funding: Phuong Khanh Morrow, Novartis; Ian E. Krop, Genentech; Eric P. Winer, Genentech; Gabriel N. Hortobagyi, Novartis; Francisco J. Esteva, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Phuong K. Morrow, Gerburg M. Wulf, Joe Ensor, Ian E. Krop, Eric P. Winer, David W. Kindelberger, Gabriel N. Hortobagyi, Dihua Yu, Francisco J. Esteva
Administrative support: Phuong K. Morrow, Gerburg M. Wulf, Gabriel N. Hortobagyi, Francisco J. Esteva
Provision of study materials or patients: Phuong K. Morrow, Gerburg M. Wulf, Daniel J. Booser, Ian E. Krop, Eric P. Winer, Gabriel N. Hortobagyi, Dihua Yu, Francisco J. Esteva
Collection and assembly of data: Phuong K. Morrow, Gerburg M. Wulf, Julia A. Moore, Peter R. Flores, Yan Xiong, Ian E. Krop, David W. Kindelberger, Jeanna Coviello, Rodolfo Nuñez, Francisco J. Esteva
Data analysis and interpretation: Phuong K. Morrow, Gerburg M. Wulf, Joe Ensor, Yan Xiong, Siyuan Zhang, Ian E. Krop, Eric P. Winer, David W. Kindelberger, Aysegul A. Sahin, Rodolfo Nuñez, Gabriel N. Hortobagyi, Dihua Yu, Francisco J. Esteva
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 3.Nahta R, Yu D, Hung MC, et al. Mechanisms of disease: Understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–280. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 3a.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2–overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 4.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Pandolfi PP. Breast cancer: Loss of PTEN predicts resistance to treatment. N Engl J Med. 2004;351:2337–2338. doi: 10.1056/NEJMcibr043143. [DOI] [PubMed] [Google Scholar]
- 7.Squarize CH, Castilho RM, Gutkind JS. Chemoprevention and treatment of experimental Cowden's disease by mTOR inhibition with rapamycin. Cancer Res. 2008;68:7066–7072. doi: 10.1158/0008-5472.CAN-08-0922. [DOI] [PubMed] [Google Scholar]
- 8.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 9.Heitman J, Movva NR, Hall MN. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science. 1991;253:905–909. doi: 10.1126/science.1715094. [DOI] [PubMed] [Google Scholar]
- 10.Campone M, Levy V, Bourbouloux E, et al. Safety and pharmacokinetics of paclitaxel and the oral mTOR inhibitor everolimus in advanced solid tumours. Br J Cancer. 2009;100:315–321. doi: 10.1038/sj.bjc.6604851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jerusalem GH, Fasolo A, Massacesi C, et al. Maintenance with everolimus (RAD001) and trastuzumab (T) after discontinuation of chemotherapy in patients (pts) with heavily pretreated HER2-positive metastatic breast cancer (MBC): Pooled data of extension cohorts of phase Ib/II studies. J Clin Oncol. 2010;28:124s. abstr 1041. [Google Scholar]
- 12. Reference deleted.
- 13. Reference deleted.
- 14.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status: Association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reference deleted.
- 16. Reference deleted.
- 17.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G VP, Schmidt M, Eidtmann H, et al. Trastuzumab treatment beyond progression in patients with HER-2 positive metastatic breast cancer the TBP study (GBG 26/BIG 3-05).. Presented at San Antonio Breast Cancer Symposium; December 8-12, 2010; San Antonio, TX. abstr 4056. [Google Scholar]
- 20.Di Nicolantonio F, Arena S, Tabernero J, et al. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 120:2858–2866. doi: 10.1172/JCI37539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohseni M, Park BH. PIK3CA and KRAS mutations predict for response to everolimus therapy: Now that's RAD001. J Clin Invest. 120:2655–2658. doi: 10.1172/JCI44026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.