Abstract
Purpose
We conducted a randomized trial comparing adjuvant treatment with edrecolomab versus observation in patients with resected, low-risk, stage II colon cancer. This study also prospectively studied patient- and tumor-specific markers of treatment outcome.
Patients and Methods
After surgical resection, patients with stage II colon cancer were randomly assigned to either five infusions of edrecolomab at 28-day intervals or observation without adjuvant therapy.
Results
Final accrual included 1,738 patients; 865 patients received edrecolomab, and 873 patients were observed without adjuvant treatment. Median follow-up time was 7.9 years. There were no significant outcome differences between study arms (overall survival [OS], P = .71; disease-free survival, P = .64). The combined 5-year all-cause OS was 0.86 (95% CI, 0.84 to 0.88), and the combined 5-year disease-specific OS was 0.93 (95% CI, 0.91 to 0.94). The relationships between demographic and histopathologic factors and survival differed for all-cause and disease-specific survival outcomes, but no combined prognostic factor model was found to adequately classify patients at higher risk of recurrence or death as a result of colon cancer.
Conclusion
Edrecolomab did not prolong survival. Consequently, this large study with a long duration of follow-up provided unique data concerning the natural history of resected stage II colon cancer. Prognostic factors identified in previous retrospective and pooled analyses were associated with survival outcomes in this stage II patient cohort. Results from ongoing molecular marker studies may enhance our ability to determine the risk profile of these patients.
INTRODUCTION
For patients with colon cancer, prognosis after surgical resection is directly related to the pathologic stage, with relative 5-year survival rates of greater than 90% if the tumor is restricted to the submucosa (T1-2, N0) and less than 10% if distant metastases have developed.1 During the 1990s, adjuvant chemotherapy with fluorouracil (FU) and leucovorin became the standard of care for patients with stage III (node-positive) colon cancer, although the benefit for patients with stage II (node-negative) disease was unclear.2–5 Early intergroup studies documenting the efficacy for patients with stage III disease receiving FU-based combination chemotherapy did not always show the same degree of efficacy among patients with stage II disease.6–10 In addition, no significant differences among patients with stage II disease have been reported from recent trials of oxaliplatin with FU, although trends have been noted.11–13 In 1990, a National Institutes of Health consensus panel recommended against adjuvant therapy for patients with stage II colon cancer.14 Again in 2004, an American Society of Clinical Oncology panel concluded that the routine use of adjuvant chemotherapy for patients with stage II colon cancer was not directly supported by the results of randomized controlled trials. Patient-physician discussion regarding the risks and potential benefits of treatment was recommended, with the suggestion that adjuvant therapy be considered for high-risk patients, such as those presenting with T4 lesions, perforation, peritumoral lymphovascular invasion, poorly differentiated histology, and inadequate lymph node assessment.15 Similar recommendations were made in the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology in 2010.16 However, these recommendations have never been validated in the setting of a prospective clinical trial. Thus, the benefit of adjuvant chemotherapy in stage II colon cancer remains uncertain.17,18
Edrecolomab is a murine immunoglobulin G2a monoclonal antibody directed against a transmembrane glycoprotein preferentially expressed on many adenocarcinomas.19 This agent mediates tumor suppression through antibody-dependent, cell-mediated, and complement-dependent cellular cytotoxicity, and these characteristics allow it to preferentially target and lyse cancer cells.20–25 On the basis of results from an early clinical trial,26 the Cancer and Leukemia Group B (CALGB) initiated a study of edrecolomab versus a no-treatment control for patients with stage II colon cancer whose tumors did not have the high-risk clinical characteristics of obstruction or perforation. Collection of tissue samples to prospectively study prognostic and predictive biomarkers was also an important component of the trial. This article provides the final results of the clinical aspects of the study, with a focus on the characteristics of this prospectively studied, stage II patient cohort illustrating the natural history of stage II colon cancer.
PATIENTS AND METHODS
Trial Conduct
CALGB developed and coordinated this trial. Participants included the Eastern Cooperative Oncology Group, Southwest Oncology Group, North Central Cancer Treatment Group, National Cancer Institute Expanded Participation Project, Cancer Research Clinical Trials Unit, National Cancer Institute of Canada Clinical Trials Group, and European Organisation for Research and Treatment of Cancer. Institutional review board approval and patient informed consent were required at each participating center. Patient registration and data collection were managed by the CALGB Statistical Center. Quarterly electronic reporting to the Clinical Therapy Evaluation Program was made via the Clinical Data Update System. Safety and efficacy data were reviewed by the CALGB Data and Safety Monitoring Board according to CALGB policies and procedures. Data quality was ensured by careful review of data by CALGB Statistical Center staff and by the study chairperson. Statistical analyses were performed by CALGB statisticians.
Eligibility Criteria
Eligible patients had undergone complete en bloc resection of an adenocarcinoma of the colon with no lymph node metastases (stage II, defined as pT3N0 or pT4aN0 lesions excluding pT4bN027,28; modified Astler-Coller stage B2) and no perforation or obstruction. Surgery must have been by open procedure with a minimum of three nodes sampled. Eligible patients were 18 years of age or older, had no prior cancer within 5 years (except nonmelanoma skin cancer or cervical carcinoma in situ), and had no previous radiation or chemotherapy for this malignancy. Eligible patients also had CALGB performance status of 0 (fully active) or 1 (capable of light work), no concurrent treatment with systemic corticosteroids, no prior exposure to murine antibodies, and no uncontrolled or severe cardiovascular disease. Although not a strict eligibility criterion, the protocol recommended initiation of treatment no earlier than 7 days and no later than 42 days after resection.
Statistical Methods
Patients were randomly assigned with equal probability to treatment with edrecolomab or observation, stratified by tumor degree of differentiation, lymphovascular invasion, and preoperative serum carcinoembryonic antigen (CEA) level. The primary end point was overall survival (OS); secondary end points included disease-free survival (DFS) and the relationships between tumor- and patient-specific prognostic factors and OS and DFS. The trial was designed to detect an OS hazard ratio of 1.5 for observation versus treatment with edrecolomab based on the log-rank test (one-sided α = .05). Analyses were performed according to intent to treat.
Survival curves were estimated using the Kaplan-Meier method. The primary hypothesis was tested using the Cox proportional hazards model adjusting for the stratification factors.29,30 The proportional hazards model was also used for multivariable modeling using the methods described by Harrell et al.31,32 The log-rank test was used for univariable comparisons of time-to-event end points.33
The following prognostic factors were studied for association with OS and DFS: sex, race (white v nonwhite), age (actual value and < v ≥ 70 years), tumor differentiation (well or moderate v poor or undifferentiated), lymphovascular invasion (no v yes), performance status (0 v 1) preoperative CEA (< v ≥ 5 ng), tumor location (proximal v distal), number of nodes examined (actual value and < v ≥ 12 nodes), T stage (T1-3 v T4), perineural invasion (no v yes), and peritumoral host lymphoid reaction (no v yes).
An OS event was defined as a death from any cause, and a DFS event was defined as a documented recurrence of primary disease or death from any cause. In additional analyses, disease-specific OS and DFS events were defined as documented death as a result of disease and documented recurrence of primary disease or death as a result of disease, respectively. New primary cancers were considered non–disease-related events. A non–cancer-related OS event was defined as a death documented as attributable to causes other than disease. In the analyses of disease-specific end points, other causes of death were censored. Data completeness for survival was assessed using Wu's criterion.34
The statistical methods proposed by Gray for the analysis of the cumulative incidence of competing risks were used to investigate the impact of prognostic factors on disease-related and other cause–related mortality.35,36 Smoothing splines were used to model the relationships between the log hazard ratios for time-to-event end points and continuous prognostic factors.37 All analyses were conducted using SAS version 9.1.3 (SAS Institute, Cary, NC) or R38 on the study database frozen on December 4, 2009.
Treatment, Dose Modifications, and Adverse Events
Therapy consisted of an initial 2-hour infusion of 500 mg of edrecolomab (cycle 1) followed by 100 mg every 28 days for four additional cycles (a total of approximately 20 weeks of therapy). Any grade 3 or 4 adverse event occurring during or immediately after infusion or between infusions resulted in treatment discontinuation. Data on adverse events were obtained using the CALGB Expanded Common Toxicity Criteria.
Follow-Up
Physical examinations occurred within 2 days before day 1 of each 28-day cycle during treatment with edrecolomab and within 7 days of random assignment and at 3 months and 6 months after random assignment for patients on observation. Patients on both study arms were then observed by physical examinations every 6 months and annual imaging to detect recurrence or second malignancy. For patients on the edrecolomab treatment arm, assessments of adverse events were also conducted. Full data reporting was required at the time of any disease occurrence or recurrence and at death for patients on both arms.
RESULTS
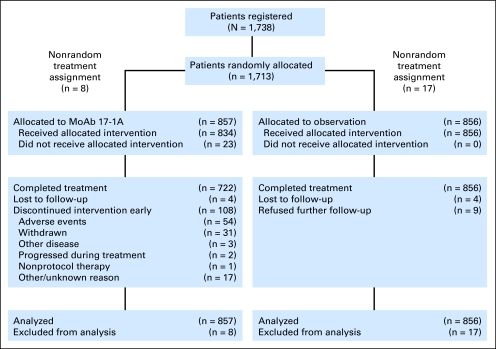
This study was activated on May 31, 1997. On the basis of the initial and disappointing results of studies conducted in stage III colon cancer,39,40 enthusiasm for further investigation of the edrecolomab antibody was severely diminished, and CALGB 9581 was closed to accrual on May 31, 2002 as a result of permanent discontinuation of the drug supply. The final patient accrual was 1,738 of the planned 2,100 patients (edrecolomab, 865 patients; observation, 873 patients). Figure 1 depicts patient flow on study. Because of a computer error in the random assignment algorithm that occurred between January 5, 2000, and February 8, 2000, 25 patients received a treatment assignment but were not randomly assigned. These patients were excluded from the analysis. Thus, the final primary analysis included 1,713 patients, 857 randomly assigned to edrecolomab and 856 randomly assigned to observation. Of the 857 patients assigned to treatment with edrecolomab, 834 patients started treatment and 722 of these patients (86.6%) reported completing treatment.
Fig 1.
Patient flow diagram for Cancer and Leukemia Group B 9581 study. MoAb 17-1A, edrecolomab.
For 834 patients with data available who were randomly assigned and started protocol therapy, the median time between resection and start of treatment was 40 days (range, 0 to 94 days). Patient and tumor characteristics were well balanced between treatment arms (Appendix Table A1, online only).
A maximum of grade 3 toxicity was reported for 242 (29.4%) of 823 participants reporting adverse events on edrecolomab, and 48 of patients (5.8%) experienced a maximum grade 4 toxicity. No individual adverse event was reported in more than 5% of patients. The most prevalent adverse event was diarrhea, with 4.1% of patients experiencing a maximum of grade 3 or 4. One patient died as a result of respiratory arrest secondary to hemoptysis within 30 days of completing treatment with edrecolomab; the death was not attributed to treatment.
Treatment Efficacy
As of December 2009, data completeness for OS is 80% by Wu's criterion.41 Median follow-up time among surviving patients is 7.9 years on both treatment arms. Median times to OS and DFS have not been reached. Total all-cause deaths were 180 on edrecolomab and 186 on observation. Of these 366 patients who died, 203 (55.4%) died without evidence of colon cancer. Overall, 162 deaths (44.2%), 79 on edrecolomab and 83 on observation, were reported as being a result of causes other than the primary colon cancer. Disease-related death was reported for 146 patients (39.8%). Cause of death was not available for 58 patients (15.8%).
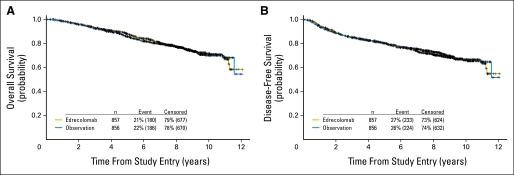
Four hundred fifty-seven treatment failures, defined as disease recurrence or death from any cause, have been reported (233 on the edrecolomab arm and 224 on the observation arm). Ninety-one patients survived with disease recurrence, and 1,256 patients were alive and disease free at the time of analysis. There were no significant survival differences in OS or DFS between treatment arms (Table 1; Fig 2).
Table 1.
Five- and 7-Year All-Cause and Disease-Specific OS and DFS, Overall and by Treatment Arm
| Outcome | 5-Year Results |
7-Year Results |
||||
|---|---|---|---|---|---|---|
| Edrecolomab | Observation | Combined | Edrecolomab | Observation | Combined | |
| All-cause OS | 0.87 | 0.85 | 0.86 | 0.80 | 0.79 | 0.80 |
| 95% CI | 0.85 to 0.90 | 0.82 to 0.88 | 0.84 to 0.88 | 0.77 to 0.83 | 0.76 to 0.82 | 0.77 to 0.82 |
| Disease-specific OS | 0.92 | 0.93 | 0.93 | 0.91 | 0.90 | 0.90 |
| 95% CI | 0.90 to 0.94 | 0.91 to 0.94 | 0.91 to 0.94 | 0.88 to 0.92 | 0.87 to 0.92 | 0.88 to 0.91 |
| All-cause DFS | 0.80 | 0.80 | 0.80 | 0.73 | 0.75 | 0.74 |
| 95% CI | 0.77 to 0.83 | 0.77 to 0.82 | 0.78 to 0.82 | 0.70 to 0.76 | 0.72 to 0.78 | 0.72 to 0.76 |
| Disease-specific DFS | 0.83 | 0.85 | 0.85 | 0.82 | 0.84 | 0.83 |
| 95% CI | 0.80 to 0.86 | 0.83 to 0.88 | 0.83 to 0.87 | 0.79 to 0.85 | 0.81 to 0.87 | 0.81 to 0.85 |
Abbreviations: OS, overall survival; DFS, disease-free survival.
Fig 2.
Kaplan-Meier estimates of (A) overall survival (all-cause death) by treatment (stratified proportional hazards, P = .71) and (B) disease-free survival (documented recurrence of primary colon cancer or death from any cause) by treatment (stratified proportional hazards, P = .64).
Prognostic Factors
Results of univariable analyses based on the primary definitions of OS and DFS are listed in Table 2. Survival estimates at 5 years are listed in Appendix Table A2 (online only). Worse outcomes were associated with male sex, older age, the presence of lymphovascular invasion, performance status less than fully active, T4 tumor stage, preoperative CEA greater than 5 ng (marginally for OS), less than 12 lymph nodes examined, perineural invasion (marginally for DFS), and lack of peritumoral host lymphoid reaction (marginally for DFS). Poor or undifferentiated tumor was significantly associated with worse OS. No other significant differences were observed.
Table 2.
Statistical Significance for OS and DFS by Treatment and Prognostic Factors for Death From All Causes
| Factor | No. of Patients | P* for OS (n = 366 events) | P* for DFS (n = 457 events) |
|---|---|---|---|
| Treatment† | 1,713 | .71 | .64 |
| Sex | 1,713 | < .001 | < .001 |
| Race | 1,707 | .24 | .12 |
| Age‡ | 1,713 | < .001 | < .001 |
| Age (< v ≥ 70 years) | 1,713 | < .001 | < .001 |
| Differentiation (stratification factor) | 1,713 | .48 | .71 |
| Differentiation (well/moderate v poor/undifferentiated) | 1,694 | .04 | .25 |
| Vascular/lymphatic invasion (stratification factor) | 1,713 | .0049 | .005 |
| Performance status | 1,697 | < .001 | < .001 |
| Depth of tumor invasion (T1-3 v T4) | 1,702 | .006 | .013 |
| Preoperative CEA (stratification factor) | 830 | .06 | .019 |
| Tumor location | 1,701 | .77 | .13 |
| No. of nodes examined‡ | 1,702 | .012 | < .001 |
| No. of nodes examined (< v ≥ 12) | 1,702 | < .001 | < .001 |
| Perineural invasion | 1,692 | .025 | .06 |
| Peritumoral host lymphoid reaction | 1,675 | .048 | .06 |
Abbreviations: OS, overall survival; DFS, disease-free survival; CEA, carcinoembryonic antigen.
P values are associated with the log-rank test unless otherwise noted.
Stratified proportional hazards model.
Proportional hazards model.
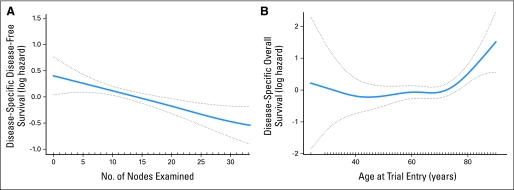
We analyzed outcomes according to disease-specific death, with non–cancer-related causes of death censored (Table 3 and Appendix Fig A1, online only; disease-specific survival estimates at 5 years are listed in Appendix Table A3, online only). Greater depth of tumor invasion, less than 12 nodes examined (categorized at the median; marginal for OS), and perineural invasion remained significant predictors of worse disease-specific OS and DFS. Male sex (marginal), older age, the presence of tumor lymphovascular invasion, and poor or undifferentiated tumor were significant predictors of worse OS. Associations of race (OS and DFS), tumor location (DFS), and number of nodes examined (actual value and categorized; DFS) were also significant predictors of disease-specific outcomes. The log hazard for disease-specific DFS decreased linearly with increasing number of nodes examined, and there seems to be a threshold for increasing log hazard among patients older than age 70 years (Fig 3).
Table 3.
Statistical Significance for Disease-Specific OS and DFS and Non–Cancer-Related OS by treatment and prognostic factors
| Factor | No. of Patients | P* for Disease-Specific OS(n = 146 events) | P* for Disease-Specific DFS(n = 265 events) | P* for Non–Cancer-Related OS(n = 162 events) |
|---|---|---|---|---|
| Treatment† | 1,655 | .91 | .44 | .72 |
| Sex | 1,655 | .05 | .09 | .026 |
| Race | 1,649 | .004 | .002 | .12 |
| Age‡ | 1,655 | .031 | .23 | < .001 |
| Age (< v ≥ 70 years) | 1,655 | .06 | .22 | < .001 |
| Differentiation (stratification factor) | 1,655 | .46 | .71 | .57 |
| Differentiation (well/moderate v poor/undifferentiated) | 1,636 | .004 | .24 | .55 |
| Vascular/lymphatic invasion (stratification factor) | 1,655 | .013 | .09 | .013 |
| Performance status | 1,639 | .35 | .24 | < .001 |
| Depth of tumor invasion (T1-3 v T4) | 1,642 | < .001 | .02 | .21 |
| Preoperative CEA (stratification factor) | 807 | .46 | .04 | .08 |
| Tumor location | 1,643 | .08 | .002 | .14 |
| No. of nodes examined‡ | 1,644 | .17 | .009 | .007 |
| No. of nodes examined (< v ≥ 12) | 1,644 | .06 | .003 | < .001 |
| Perineural invasion | 1,634 | < .001 | .02 | .80 |
| Peritumoral host lymphoid reaction | 1,617 | .26 | .26 | .24 |
NOTE. Analysis of the DFS endpoint includes 12 additional patients who died after recurrence of disease with unknown cause of death.
Abbreviations: OS, overall survival; DFS, disease-free survival; CEA, carcinoembryonic antigen.
P values are associated with the log-rank test unless otherwise noted.
Stratified proportional hazards model.
Proportional hazards model.
Fig 3.
Smoothing splines of (A) the log hazard for disease-specific disease-free survival by number of nodes examined truncated at 32 nodes, representing 95% of the data, and (B) the log hazard for disease-specific overall survival by age at trial entry with 95% confidence bands.
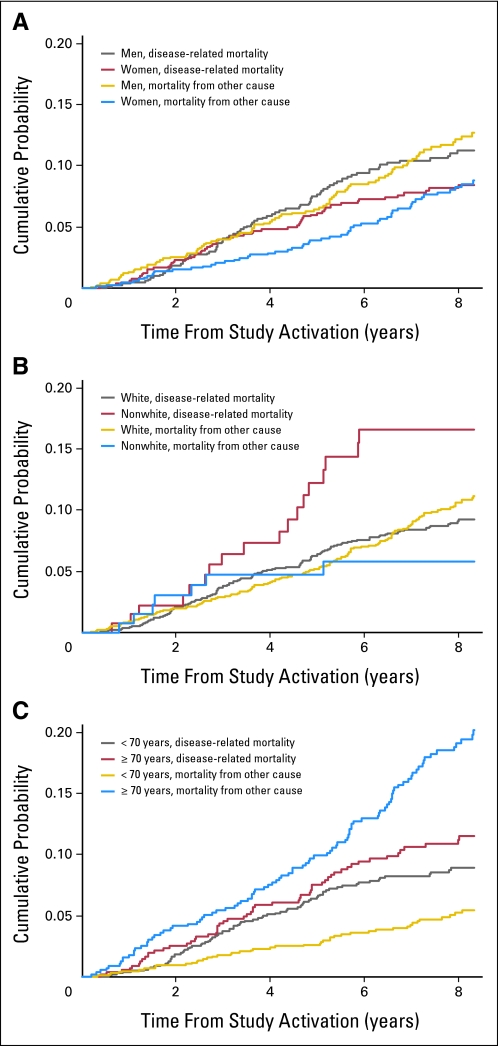
An analysis using a competing risk model for disease-related mortality versus other cause–related mortality (Appendix Table A4 and Fig A2, online only) revealed a significant sex difference, with a higher cumulative probability of death from other causes in men (P = .03). The cumulative probability for disease-related mortality between whites and nonwhites was also significant, with whites having a lower incidence of events (P = .01). In addition, under the cumulative incidence model, patients age 70 years or older at study entry did not experience significantly greater disease-related mortality than younger patients (P = .15); however, the difference in other cause–related mortality was highly significant (P < .001). Significant disease-related OS differences were also found for cumulative incidence by differentiation (P = .005), lymphovascular invasion (P = .02), depth of tumor invasion (P = .001), number of nodes examined (P < .001), and perineural invasion (P < .001). Significant other cause–related OS differences were also found for cumulative incidence by lymphovascular invasion (P = .02), performance status (P < .001), and number of nodes examined (P < .001).
Multivariable Analysis
Regression modeling was used to determine the simultaneous association between study variables and survival outcomes. For disease-specific OS, nonwhite race (P = .006), older age (P = .02), poor tumor differentiation (P = .001), greater depth of tumor invasion (P = .005), distal location (P = .03), and the presence of perineural invasion (P = .001) were simultaneously associated with worse outcome. For non–cancer-related OS, male sex (P = .001), older age (P < .001), poor performance status (P < .001), and less than 12 nodes examined (P = .008) simultaneously predicted for worse outcome. For disease-specific DFS, race (P = .05), depth of tumor invasion (P = .04), the presence of perineural invasion (P = .04), tumor location (P = .02), and fewer number of nodes examined (P = .03) were significantly associated with worse outcome.
Multivariate analyses of the cumulative incidence of disease-related and non–cancer-related mortality produced similar results, except that age was not significant in the model of disease-related OS. No combined prognostic factor model was found to be sufficiently predictive for disease-specific OS and DFS.
DISCUSSION
On the basis of a promising published report of a randomized trial in 189 patients with stage III disease,26 CALGB investigators sought to determine whether the use of edrecolomab, a relatively nontoxic adjuvant therapy, would demonstrate a benefit in a cohort of patients with stage II colon cancer that excluded patients with such high-risk factors as tumor perforation and obstruction. Unfortunately, as the results of C9581 show, this much larger trial of 1,713 patients with stage II disease provides further evidence that the administration of edrecolomab does not improve outcome for such patients. In retrospect, this negative outcome is not surprising. Neither of two randomized trials, initiated in the mid-1990s and conducted in North and South America39 and Europe,40 showed a significant benefit for adding edrecolomab to FU/leucovorin-based therapy for patients with stage III colon cancer.
CALGB 9581 generated an observational data set of uniformly staged and treated patients describing the prognosis of untreated stage II disease.27,28,42 The 5-year all-cause OS in this cohort was 0.86, with a 5-year disease-specific OS of 0.93. With such a remarkable OS and small probability of disease-related death, it would be extremely difficult to demonstrate a benefit for any adjuvant therapy in such a population of patients with stage II colon cancer without accruing thousands of patients.
Our data show that it is important to distinguish between disease-related and other causes of death in this low-risk, older patient population with stage II disease. We found large differences in outcome by sex and age for all-cause mortality that were primarily caused by association of these factors with death as a result of other causes. It is not surprising that study patients older than age 70 years would have more comorbid conditions and a corresponding greater probability of non–cancer-related death than patients less than 70 years of age entered onto this trial. Particularly interesting findings were the differences in treatment outcome according to race, with race being significantly associated with disease-specific OS and DFS and disease-related cumulative incidence in multivariable analyses. The nonwhite category of race comprised a mixture of racial and ethnic minorities that were putatively treated per protocol. No difference in outcome across these subgroups was observed, although sample sizes were small. Differences by race in this study may be partially explained by the apparent poorer survival among nonwhite patients who did not meet the study eligibility criteria (v both whites and nonwhites who did meet study eligibility), although, again, sample sizes were small (six deaths in 19 patients). The results from this study suggest that the racial/ethnic composition of study populations with stage II colon cancer should be considered in the design of future trials. We also found a strongly negative prognosis when fewer nodes were examined, reinforcing previously published results.43,44 Interestingly, there seems to be a continuous linear effect with no threshold.
We used two approaches to analyze these data. First, we defined recurrence or disease-specific death as an event and censored deaths as a result of other causes. A drawback of this approach is that the assumption of independence between the event and the censoring distribution may not hold because of an underlying relationship between DFS and OS. The second method, using analysis of cumulative incidence, allows the two outcome types (disease related and other cause related) to be modeled simultaneously. A limitation of this method is that it unrealistically assumes that the risk set includes patients who have experienced failure from other causes before time t. Cumulative incidence is also sometimes misinterpreted as an estimate of the survival distribution. In both methods, data on cause of death were missing for 15% of patients. Despite these drawbacks, we found that the results of the two analysis methods were generally consistent, and results for the cumulative incidence of other cause–related mortality were also reasonable in most cases. Two exceptions are the significant relationships of lymphovascular invasion and number of nodes examined with non–cancer-related OS and the cumulative incidence of other cause–related OS. Lymphovascular invasion is no longer significant in the multivariable model, reflecting the dominant influence of sex, age, and performance status. Number of nodes examined remained significant; the reason is not apparent but may be a result of confounding with unknown factors.
Given the results obtained in this relatively homogenous patient cohort, regression modeling was also used to potentially develop a predictive model for disease-specific OS and DFS. Unfortunately, no model was sufficiently predictive to propose for validation, illustrating the difficulty of identifying patients at higher risk using known prognostic factors. Analysis of the impact of additional biomarkers and histologic factors at study entry on outcome is under way and should provide new information regarding the risk profile of these patients.
Acknowledgment
We thank Margaret Hall, MS, for her contributions in preparing the trial data for statistical analysis.
Presented, in part, at the 40th Annual Meeting of the American Society of Clinical Oncology, June 5-8, 2004, New Orleans, LA.
Supported in part by National Cancer Institute (NCI) Grant No. CA31946 to the Cancer and Leukemia Group B (CALGB) and NCI Grant No. CA33601 to the CALGB Statistical Center.
Appendix
Table A1.
Baseline Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | Overall (N = 1,713) |
Edrecolomab (n = 857) |
Observation (n = 856) |
|||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |
| Sex | ||||||
| Male | 887 | 51.8 | 454 | 53.0 | 433 | 50.6 |
| Female | 826 | 48.2 | 403 | 47.0 | 423 | 49.4 |
| Age, years | ||||||
| < 40 | 48 | 2.8 | 21 | 2.5 | 27 | 3.2 |
| 40-49 | 138 | 8.1 | 73 | 8.5 | 65 | 7.6 |
| 50-59 | 380 | 22.2 | 184 | 21.5 | 196 | 22.9 |
| 60-69 | 544 | 31.8 | 281 | 32.8 | 263 | 30.7 |
| 70+ | 603 | 35.2 | 298 | 34.8 | 305 | 35.6 |
| Median | 66 | 66 | 66 | |||
| Range | 24-90 | 31-90 | 24-88 | |||
| Race | ||||||
| White | 1,558 | 91.0 | 774 | 90.3 | 784 | 91.6 |
| Nonwhite | 149 | 8.7 | 80 | 9.3 | 69 | 8.1 |
| Hispanic American | 20 | 11 | 9 | |||
| African American | 85 | 46 | 39 | |||
| Asian | 31 | 17 | 14 | |||
| American Indian or Alaska Native | 4 | 1 | 3 | |||
| Indian Subcontinent | 4 | 2 | 2 | |||
| Other | 5 | 3 | 2 | |||
| Unknown | 6 | 0.4 | 3 | 0.4 | 3 | 0.4 |
| Performance status | ||||||
| 0 (fully active) | 1,216 | 71.0 | 609 | 71.1 | 607 | 70.9 |
| 1 (ambulatory) | 481 | 28.1 | 243 | 28.4 | 238 | 27.8 |
| 2 (in bed < 50% of time) | 5 | 0.3 | 2 | 0.2 | 3 | 0.4 |
| Unknown | 11 | 0.6 | 3 | 0.4 | 8 | 0.9 |
| Stratification variables | ||||||
| Differentiation | ||||||
| Well | 1,324 | 77.3 | 664 | 77.5 | 660 | 77.1 |
| Moderately well | 231 | 13.5 | 113 | 13.2 | 118 | 13.8 |
| Poor | 158 | 9.2 | 80 | 9.3 | 78 | 9.1 |
| Vascular/lymphatic invasion | ||||||
| No | 1,522 | 88.8 | 759 | 88.6 | 763 | 89.1 |
| Yes | 191 | 11.2 | 98 | 11.4 | 93 | 10.9 |
| Preoperative CEA, ng | ||||||
| < 5 | 631 | 36.8 | 319 | 37.2 | 312 | 36.4 |
| ≥ 5 | 199 | 11.6 | 99 | 11.6 | 100 | 11.7 |
| Unknown | 883 | 51.5 | 439 | 51.2 | 444 | 51.9 |
| Tumor characteristics at baseline | ||||||
| Tumor location* | ||||||
| Proximal | 1,033 | 60.3 | 506 | 59.0 | 527 | 61.6 |
| Distal | 668 | 39.0 | 348 | 40.6 | 320 | 37.4 |
| Unknown | 12 | 0.7 | 3 | 0.4 | 9 | 1.1 |
| Depth of tumor invasion | ||||||
| T1 | 2 | 0.1 | 2 | 0.2 | 0 | 0 |
| T2 | 15 | 0.8 | 9 | 1.0 | 6 | 0.7 |
| T3 | 1,610 | 93.9 | 808 | 94.2 | 802 | 93.6 |
| T4a | 66 | 3.9 | 31 | 3.6 | 35 | 4.1 |
| T4b | 9 | 0.5 | 4 | 0.4 | 5 | 0.6 |
| Unknown | 11 | 0.6 | 3 | 0.3 | 8 | 0.9 |
| No. of nodes examined | 1,702 | 854 | 848 | |||
| Median | 12 | 12.5 | 12 | |||
| Range | 0-99 | 0-59 | 0-99 | |||
| No. of involved lymph nodes | ||||||
| 0 | 1,692 | 98.8 | 849 | 99.1 | 843 | 98.5 |
| 1 | 9 | 0.5 | 4 | 0.5 | 5 | 0.6 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | 1 | 0.1 | 1 | 0.1 | 0 | 0 |
| Unknown | 11 | 0.6 | 3 | 0.4 | 8 | 0.9 |
| Perineural invasion | ||||||
| No | 1,651 | 96.4 | 822 | 96.0 | 829 | 96.8 |
| Yes | 41 | 2.4 | 25 | 2.9 | 16 | 1.8 |
| Unknown | 21 | 1.2 | 10 | 1.1 | 11 | 1.4 |
| Peritumoral host lymphoid reaction | ||||||
| No | 1,467 | 85.6 | 751 | 87.6 | 716 | 83.6 |
| Yes | 208 | 12.1 | 90 | 10.5 | 118 | 13.8 |
| Unknown | 38 | 2.2 | 16 | 1.9 | 22 | 2.6 |
Abbreviation: CEA, carcinoembryonic antigen.
Proximal indicates cecum, ascending colon, hepatic flexure, and transverse colon; distal indicates splenic flexure, descending colon, and sigmoid colon.
The following Cancer and Leukemia Group B institutions participated in this study: Cancer Centers of the Carolinas, Greenville, SC–Jeffrey K. Giguere, MD, supported by CA29165; Christiana Care Health Services, Community Clinical Oncology Program (CCOP), Wilmington, DE–Stephen Grubbs, MD, supported by CA45418; Dana-Farber Cancer Institute, Boston, MA–Harold J. Burstein, MD, PhD, supported by CA32291; Dartmouth Medical School–Norris Cotton Cancer Center, Lebanon, NH–Konstantin Dragnev, MD, supported by CA04326; Duke University Medical Center, Durham, NC–Jeffrey Crawford, MD, supported by CA47577; Georgetown University Medical Center, Washington, DC–Minetta C. Liu, MD, supported by CA77597; Long Island Jewish Medical Center, Lake Success, NY–Kanti R. Rai, MD, supported by CA35279; Massachusetts General Hospital, Boston, MA–Jeffrey W. Clark, MD, supported by CA32291; Memorial Sloan-Kettering Cancer Center, New York, NY–Clifford A. Hudis, MD, supported by CA77651; Mount Sinai School of Medicine, New York, NY–Lewis R. Silverman, MD, supported by CA04457; Nevada Cancer Research Foundation CCOP, Las Vegas, NV–John A. Ellerton, MD, supported by CA35421; Northern Indiana Cancer Research Consortium CCOP, South Bend, IN–Rafat Ansari, MD, supported by CA86726; Rhode Island Hospital, Providence, RI–William Sikov, MD, supported by CA08025; Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, MD, supported by CA59518; Southeast Cancer Control Consortium CCOP, Goldsboro, NC–James N. Atkins, MD, supported by CA45808; State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, MD, supported by CA21060; The Ohio State University Medical Center, Columbus, OH–Clara D. Bloomfield, MD, supported by CA77658; University of California at San Diego, San Diego, CA–Barbara A. Parker, MD, supported by CA1178; University of California at San Francisco, San Francisco, CA–Charles J. Ryan, MD, supported by CA60138; University of Chicago, Chicago, IL–Hedy L. Kindler, MD, supported by CA41287; University of Illinois Minority-Based CCOP, Chicago, IL–David J. Peace, MD, supported by CA74811; University of Iowa, Iowa City, IA–Daniel A. Vaena, MD, supported by CA47642; University of Maryland Greenebaum Cancer Center, Baltimore, MD–Martin Edelman, MD, supported by CA31983; University of Massachusetts Medical School, Worcester, MA–William V. Walsh, MD, supported by CA37135; University of Minnesota, Minneapolis, MN–Bruce A. Peterson, MD, supported by CA16450; University of Missouri/Ellis Fischel Cancer Center, Columbia, MO–Michael C. Perry, MD, supported by CA12046; University of Nebraska Medical Center, Omaha, NE–Anne Kessinger, MD, supported by CA77298; University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, MD, supported by CA47559; University of Tennessee Memphis, Memphis, TN–Harvey B. Niell, MD, supported by CA47555; University of Vermont, Burlington, VT–Steven M. Grunberg, MD, supported by CA77406; Wake Forest University School of Medicine, Winston-Salem, NC–David D. Hurd, MD, supported by CA03927; Walter Reed Army Medical Center, Washington, DC–Brendan M. Weiss, MD, supported by CA26806; Washington University School of Medicine, St Louis, MO–Nancy Bartlett, MD, supported by CA77440; Weill Medical College of Cornell University, New York, NY–John Leonard, MD, supported by CA07968.
Table A2.
Statistical Significance for OS and DFS by Treatment and Prognostic Factors for Death From All Causes With Associated Kaplan-Meier Estimates of 5-Year OS and DFS
| Factor | No. of Patients | OS |
DFS |
||||
|---|---|---|---|---|---|---|---|
| 5-Year OS | 95% CI | P* (n = 366 events) | 5-Year DFS | 95% CI | P* (n = 457 events) | ||
| Treatment† | 1,713 | .71 | .64 | ||||
| Edrecolomab | 857 | 0.87 | 0.85 to 0.90 | 0.80 | 0.77 to 0.83 | ||
| Observation | 856 | 0.85 | 0.82 to 0.88 | 0.80 | 0.77 to 0.82 | ||
| Sex | 1,713 | < .001 | < .001 | ||||
| Male | 887 | 0.84 | 0.81 to 0.86 | 0.78 | 0.75 to 0.80 | ||
| Female | 826 | 0.89 | 0.86 to 0.91 | 0.82 | 0.79 to 0.85 | ||
| Race | 1,707 | .24 | .12 | ||||
| White | 1,558 | 0.88 | 0.85 to 0.90 | 0.81 | 0.78 to 0.82 | ||
| Nonwhite | 149 | 0.84 | 0.69 to 0.92 | 0.72 | 0.63 to 0.79 | ||
| Age (actual value)‡ | 1,713 | < .001 | < .001 | ||||
| Age, years | 1,713 | < .001 | < .001 | ||||
| < 70 | 1,110 | 0.89 | 0.87 to 0.91 | 0.82 | 0.80 to 0.84 | ||
| ≥ 70 | 603 | 0.81 | 0.77 to 0.84 | 0.76 | 0.72 to 0.79 | ||
| Differentiation (stratification factor) | 1,713 | .48 | .71 | ||||
| Well | 158 | 0.89 | 0.83 to 0.93 | 0.84 | 0.76 to 0.89 | ||
| Moderately well | 1,324 | 0.86 | 0.84 to 0.88 | 0.80 | 0.77 to 0.82 | ||
| Poor | 231 | 0.83 | 0.78 to 0.88 | 0.80 | 0.74 to 0.84 | ||
| Differentiation | 1,694 | .04 | .25 | ||||
| Well/moderate | 1,431 | 0.87 | 0.85 to 0.89 | 0.80 | 0.78 to 0.82 | ||
| Poor/undifferentiated | 263 | 0.83 | 0.77 to 0.87 | 0.79 | 0.73 to 0.83 | ||
| Vascular/lymphatic invasion (stratification factor) | 1,713 | .0049 | .005 | ||||
| No | 1,522 | 0.87 | 0.85 to 0.89 | 0.81 | 0.79 to 0.83 | ||
| Yes | 191 | 0.80 | 0.73 to 0.85 | 0.71 | 0.64 to 0.77 | ||
| Performance status | 1,697 | < .001 | < .001 | ||||
| 0 | 1,216 | 0.89 | 0.87 to 0.90 | 0.82 | 0.80 to 0.84 | ||
| 1-2 | 481 | 0.80 | 0.76 to 0.83 | 0.74 | 0.70 to 0.78 | ||
| Depth of tumor invasion | 1,702 | .006 | .013 | ||||
| T1-3 | 1,627 | 0.87 | 0.85 to 0.88 | 0.80 | 0.78 to 0.82 | ||
| T4 | 75 | 0.69 | 0.55 to 0.79 | 0.66 | 0.53 to 0.76 | ||
| Preoperative CEA (stratification factor), ng | 830 | .06 | .019 | ||||
| < 5 | 631 | 0.88 | 0.85 to 0.90 | 0.82 | 0.79 to 0.85 | ||
| ≥ 5 | 199 | 0.82 | 0.75 to 0.87 | 0.73 | 0.66 to 0.79 | ||
| Tumor location | 1,701 | .77 | .13 | ||||
| Proximal | 1,033 | 0.87 | 0.84 to 0.89 | 0.81 | 0.79 to 0.84 | ||
| Distal | 668 | 0.85 | 0.82 to 0.88 | 0.78 | 0.74 to 0.81 | ||
| No. of nodes examined (actual value)‡ | 1,702 | .012 | < .001 | ||||
| No. of nodes examined (median) | 1,702 | < .001 | < .001 | ||||
| < 12 | 767 | 0.83 | 0.80 to 0.85 | 0.76 | 0.72 to 0.79 | ||
| ≥ 12 | 935 | 0.89 | 0.87 to 0.91 | 0.83 | 0.81 to 0.86 | ||
| Perineural invasion | 1,692 | .025 | .06 | ||||
| No | 1,651 | 0.87 | 0.85 to 0.88 | 0.80 | 0.78 to 0.82 | ||
| Yes | 41 | 0.76 | 0.59 to 0.86 | 0.66 | 0.48 to 0.78 | ||
| Peritumoral host lymphoid reaction | 1,675 | .048 | .06 | ||||
| No | 1,467 | 0.86 | 0.84 to 0.88 | 0.80 | 0.77 to 0.82 | ||
| Yes | 208 | 0.90 | 0.84 to 0.93 | 0.84 | 0.78 to 0.89 | ||
Abbreviations: OS, overall survival; DFS, disease-free survival; CEA, carcinoembryonic antigen.
P values are associated with the log-rank test unless otherwise noted.
Stratified proportional hazards model.
Proportional hazards model.
Table A3.
Statistical Significance for Disease-Specific OS and DFS and Non–Cancer-Related OS by Treatment and Prognostic Factors With Associated Kaplan-Meier Estimates of 5-Year Survival
| Factor | No. of Patients | Disease-Specific Survival |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OS |
DFS |
Non–Cancer-Related Survival |
||||||||
| 5-Year OS | 95% CI | P* (n = 146 events) | 5-Year DFS | 95% CI | P* (n = 265 events) | 5-Year OS | 95% CI | P* (n = 162 events) | ||
| Treatment† | 1,655 | .91 | .44 | .72 | ||||||
| Edrecolomab | 829 | 0.92 | 0.90 to 0.94 | 0.83 | 0.80 to 0.86 | 0.95 | 0.94 to 0.97 | |||
| Observation | 826 | 0.93 | 0.91 to 0.94 | 0.85 | 0.83 to 0.88 | 0.93 | 0.91 to 0.95 | |||
| Sex | 1,655 | .05 | .09 | .026 | ||||||
| Male | 845 | 0.90 | 0.90 to 0.94 | 0.83 | 0.81 to 0.86 | 0.93 | 0.91 to 0.95 | |||
| Female | 810 | 0.93 | 0.92 to 0.95 | 0.85 | 0.83 to 0.88 | 0.96 | 0.94 to 0.97 | |||
| Race | 1,649 | .004 | .002 | .12 | ||||||
| White | 1,508 | 0.93 | 0.92 to 0.94 | 0.85 | 0.83 to 0.87 | 0.94 | 0.93 to 0.95 | |||
| Nonwhite | 141 | 0.86 | 0.78 to 0.92 | 0.76 | 0.67 to 0.82 | 0.95 | 0.89 to 0.98 | |||
| Age (actual value)‡ | 1,655 | .031 | .23 | < .001 | ||||||
| Age, years | 1,655 | .06 | .22 | < .001 | ||||||
| < 70 | 1,088 | 0.93 | 0.91 to 0.94 | 0.85 | 0.83 to 0.87 | 0.97 | 0.96 to 0.98 | |||
| ≥ 70 | 567 | 0.92 | 0.89 to 0.94 | 0.84 | 0.81 to 0.87 | 0.89 | 0.86 to 0.92 | |||
| Differentiation (stratification factor) | 1,655 | .46 | .71 | .57 | ||||||
| Well | 153 | 0.95 | 0.89 to 0.93 | 0.87 | 0.80 to 0.92 | 0.95 | 0.90 to 0.98 | |||
| Moderately well | 1,277 | 0.93 | 0.91 to 0.94 | 0.84 | 0.82 to 0.86 | 0.95 | 0.93 to 0.96 | |||
| Poor | 225 | 0.90 | 0.85 to 0.93 | 0.85 | 0.80 to 0.90 | 0.93 | 0.89 to 0.96 | |||
| Differentiation (well/moderate v poor/undifferentiated) | 1,636 | .004 | .24 | .55 | ||||||
| Well/moderate | 1,381 | 0.94 | 0.92 to 0.95 | 0.85 | 0.83 to 0.87 | 0.94 | 0.93 to 0.95 | |||
| Poor/undifferentiated | 255 | 0.88 | 0.83 to 0.91 | 0.83 | 0.78 to 0.87 | 0.94 | 0.90 to 0.96 | |||
| Vascular/lymphatic invasion (stratification factor) | 1,655 | .013 | .09 | .01 | ||||||
| No | 1,467 | 0.93 | 0.92 to 0.94 | 0.85 | 0.83 to 0.87 | 0.95 | 0.94 to 0.96 | |||
| Yes | 188 | 0.89 | 0.83 to 0.93 | 0.80 | 0.73 to 0.85 | 0.90 | 0.84 to 0.93 | |||
| Performance status | 1,639 | .35 | .24 | < .001 | ||||||
| 0 | 1,182 | 0.93 | 0.91 to 0.94 | 0.85 | 0.83 to 0.87 | 0.96 | 0.95 to 0.97 | |||
| 1-2 | 457 | 0.90 | 0.87 to 0.93 | 0.83 | 0.79 to 0.86 | 0.90 | 0.87 to 0.92 | |||
| Depth of tumor invasion (T1-3 v T4) | 1,642 | < .001 | .02 | .21 | ||||||
| T1-3 | 1,569 | 0.93 | 0.92 to 0.94 | 0.85 | 0.83 to 0.87 | 0.95 | 0.93 to 0.96 | |||
| T4 | 75 | 0.80 | 0.67 to 0.88 | 0.77 | 0.65 to 0.85 | 0.86 | 0.73 to 0.93 | |||
| Preoperative CEA (stratification factor), ng | 807 | .46 | .04 | .08 | ||||||
| < 5 | 614 | 0.95 | 0.92 to 0.96 | 0.87 | 0.84 to 0.90 | 0.94 | 0.92 to 0.96 | |||
| ≥ 5 | 193 | 0.91 | 0.86 to 0.95 | 0.80 | 0.73 to 0.85 | 0.92 | 0.86 to 0.95 | |||
| Tumor location | 1,643 | .08 | .002 | .14 | ||||||
| Proximal | 998 | 0.93 | 0.92 to 0.95 | 0.87 | 0.85 to 0.89 | 0.94 | 0.92 to 0.95 | |||
| Distal | 643 | 0.91 | 0.89 to 0.93 | 0.81 | 0.78 to 0.84 | 0.95 | 0.93 to 0.96 | |||
| No. of nodes examined (actual value)‡ | 1,644 | .17 | .009 | .007 | ||||||
| No. of nodes examined (median) | 1,644 | .06 | .003 | < .001 | ||||||
| < 12 | 737 | 0.91 | 0.88 to 0.93 | 0.82 | 0.79 to 0.84 | 0.93 | 0.90 to 0.94 | |||
| ≥ 12 | 907 | 0.94 | 0.92 to 0.95 | 0.87 | 0.85 to 0.89 | 0.96 | 0.94 to 0.97 | |||
| Perineural invasion | 1,634 | < .001 | .02 | .80 | ||||||
| No | 1,593 | 0.93 | 0.92 to 0.94 | 0.85 | 0.83 to 0.87 | 0.94 | 0.93 to 0.96 | |||
| Yes | 41 | 0.83 | 0.66 to 0.92 | 0.72 | 0.55 to 0.84 | 0.92 | 0.76 to 0.97 | |||
| Peritumoral host lymphoid reaction | 1,617 | .26 | .26 | .24 | ||||||
| No | 1,412 | 0.93 | 0.91 to 0.94 | 0.84 | 0.82 to 0.86 | 0.94 | 0.93 to 0.95 | |||
| Yes | 205 | 0.94 | 0.89 to 0.97 | 0.88 | 0.82 to 0.91 | 0.96 | 0.92 to 0.98 | |||
NOTE. Analysis of the DFS endpoint includes 12 additional patients who died after recurrence of disease with unknown cause of death.
Abbreviations: OS, overall survival; DFS, disease-free survival; CEA, carcinoembryonic antigen.
P values are associated with the log-rank test unless otherwise noted.
Stratified proportional hazards model.
Proportional hazards model.
Table A4.
Results Based on the Cumulative Incidence Model for Disease-Related OS Versus Other Causes of Death
| Factor | No. of Patients | P for Disease-Related OS (n = 146 events) | P for Other Cause–Related OS (n = 162 events) |
|---|---|---|---|
| Treatment | 1,655 | .94 | .72 |
| Sex | 1,655 | .07 | .03 |
| Race | 1,649 | .01 | .09 |
| Age (actual value) | 1,655 | .23 | < .001 |
| Age (< v ≥ 70 years) | 1,655 | .15 | < .001 |
| Differentiation (well/moderate v poor/undifferentiated) | 1,636 | .005 | .72 |
| Vascular/lymphatic invasion (stratification factor) | 1,655 | .02 | .02 |
| Performance status | 1,639 | .76 | < .001 |
| Depth of tumor invasion (T1-3 v T4) | 1,644 | .001 | .36 |
| Preoperative CEA (stratification factor) | 807 | .17 | .64 |
| Tumor location | 1,643 | .07 | .10 |
| No. of nodes examined (actual value) | 1,644 | < .001 | < .001 |
| No. of nodes examined (< v ≥ 12) | 1,644 | .09 | < .001 |
| Perineural invasion | 1,634 | < .001 | .95 |
| Peritumoral host lymphoid reaction | 1,617 | .29 | .26 |
Abbreviations: OS, overall survival; CEA, carcinoembryonic antigen.
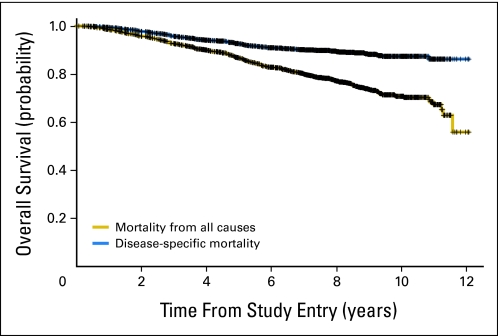
Fig A1.
Kaplan-Meier estimates of all-cause and disease-specific overall survival for all randomly assigned patients (N = 1,713).
Fig A2.
Cumulative probability of experiencing the cause-specific event before time t by years from study activation (disease-related and other causes of death) for (A) sex, (B) race, and (C) age.
Footnotes
See accompanying article on page 3153
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NCI.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00002968.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: David J. Kerr, Centocor; Anthony Fields, GlaxoSmithKline Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Donna Niedzwiecki, Monica M. Bertagnolli, Robert S. Warren, Carolyn C. Compton, Nancy E. Kemeny, Richard M. Goldberg, Robert J. Mayer, Thomas A. Colacchio
Administrative support: Monica M. Bertagnolli, Richard M. Goldberg, Thomas A. Colacchio
Provision of study materials or patients: Steven Alberts, J. Marc Pipas, Joel H. Schwartz, James Atkins, Mark O'Rourke, Michael C. Perry, Richard M. Goldberg, Robert J. Mayer, Thomas A. Colacchio
Collection and assembly of data: Donna Niedzwiecki, Monica M. Bertagnolli, Carolyn C. Compton, Al Bowen Benson III, S. Gail Eckhardt, Steven Alberts, Gity N. Porjosh, David J. Kerr, Anthony Fields, Philippe Rougier, J. Marc Pipas, Joel H. Schwartz, James Atkins, Mark O'Rourke, Michael C. Perry, Richard M. Goldberg, Thomas A. Colacchio
Data analysis and interpretation: Donna Niedzwiecki, Monica M. Bertagnolli, Robert S. Warren, Richard M. Goldberg, Robert J. Mayer, Thomas A. Colacchio
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: Results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 3.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer: International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) Investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 4.Haller DG, Catalano PJ, Macdonald JS, et al. Fluorouracil (FU), leucovorin (LV) and levamisole (LEV) adjuvant therapy for colon cancer: Five-year final report of INT-0089. Proc Am Soc Clin Oncol. 1998;17(suppl):256a. abstr 982. [Google Scholar]
- 5.Zaniboni A. Adjuvant chemotherapy in colorectal cancer with high-dose leucovorin and fluorouracil: Impact on disease-free survival and overall survival. J Clin Oncol. 1997;15:2432–2441. doi: 10.1200/JCO.1997.15.6.2432. [DOI] [PubMed] [Google Scholar]
- 6.O'Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 7.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer: International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 8.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 9.QUASAR Collaborative Group. Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 10.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: Final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 11.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 12.Wolmark N, Wieand S, Kuebler PJ, et al. A phase III trial comparing FU/LV to FU/LV + oxaliplatin in stage II or III carcinoma of the colon: Survival results of NSABP Protocol C-07. J Clin Oncol. 2008;26(suppl):1008s. abstr LBA4005. [Google Scholar]
- 13.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 14.Adjuvant therapy for patients with colon and rectum cancer. Consens Statement. 1990;8:1–25. [PubMed] [Google Scholar]
- 15.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology, v3. 2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.
- 17.Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476–487. doi: 10.1056/NEJMra040958. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB., 3rd The hope for today–the promise for tomorrow: Will oncologists meet the challenge? J Clin Oncol. 2007;25:2156–2158. doi: 10.1200/JCO.2006.09.9838. [DOI] [PubMed] [Google Scholar]
- 19.Göttinger HG, Funke I, Johnson JP, et al. The epithelial cell surface antigen 17-1A, a target for antibody-mediated tumor therapy: Its biochemical nature, tissue distribution and recognition by different monoclonal antibodies. Int J Cancer. 1986;38:47–53. doi: 10.1002/ijc.2910380109. [DOI] [PubMed] [Google Scholar]
- 20.Adams DO, Hall T, Steplewski Z, et al. Tumors undergoing rejection induced by monoclonal antibodies of the IgG2a isotype contain increased numbers of macrophages activated for a distinctive form of antibody-dependent cytolysis. Proc Natl Acad Sci U S A. 1984;81:3506–3510. doi: 10.1073/pnas.81.11.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herlyn D, Wettendorff M, Schmoll E, et al. Anti-idiotype immunization of cancer patients: Modulation of the immune response. Proc Natl Acad Sci U S A. 1987;84:8055–8059. doi: 10.1073/pnas.84.22.8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellstedt H, Frödin JE, Masucci G, et al. Monoclonal antibodies (MAb 17-1A) for the treatment of patients with metastatic colorectal carcinomas. Acta Chir Scand Suppl. 1989;549:63–70. [PubMed] [Google Scholar]
- 23.Wadler S. The role of immunotherapy in colorectal cancer. Semin Oncol. 1991;18(suppl):S27–S38. [PubMed] [Google Scholar]
- 24.Ragnhammar P, Magnusson I, Masucci G, et al. The therapeutic use of the unconjugated monoclonal antibodies (Mab) 17-1A in combination with GM-CSF in the treatment of colorectal carcinoma. Med Oncol Tumor Pharmacother. 1993;10:61–70. doi: 10.1007/BF02987770. [DOI] [PubMed] [Google Scholar]
- 25.Maeda M, Shoji M, Kawagoshi T, et al. Distribution of 111In- and 125I-labeled monoclonal antibody 17-1A in mice bearing xenografts of human pancreatic carcinoma HuP-T4. Cancer. 1994;73(suppl 3):S800–S807. doi: 10.1002/1097-0142(19940201)73:3+<800::aid-cncr2820731309>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Riethmüller G, Schneider-Gädicke E, Schlimok G, et al. Randomized trial of monoclonal antibody for adjuvant therapy of resected Dukes C colorectal carcinoma: German Cancer Aid 17-1A Study Group. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 27.Fleming ID, Cooper JS, Henson DE, et al., editors. ed 5. Philadelphia, PA: Lippincott-Raven; 1997. AJCC Manual for Staging of Cancer. [Google Scholar]
- 28.Greene FL, Page DL, Fleming ID, et al., editors. ed 6. New York, NY: Springer; 2002. AJCC Cancer Staging Manual. [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 31.Harrell FE, Jr, Lee KL, Califf RM, et al. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE. Design: S-plus function for biostatistical/epidemiological modeling, testing, estimation, validation, graphics, prediction, and typesetting by storing enhanced model design attributes in the fit, 1994. http://lib.stat.cmu.edu/DOS/S/Harrell/
- 33.Kalbfleisch JD, Prentice RL. New York, NY: John Wiley & Sons; 1980. The Statistical Analysis of Failure Time Data; pp. 163–188. [Google Scholar]
- 34.Wu Y, Takkenberg JJ, Grunkemeier GL. Measuring follow-up completeness. Ann Thorac Surg. 2008;85:1155–1157. doi: 10.1016/j.athoracsur.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a completing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 36.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 37.Therneau TM, Grambsch PM. New York, NY: Springer-Verlag; 2000. Modeling Survival Data, Extending the Cox Model; pp. 107–111. [Google Scholar]
- 38.R Development Core Team: R: A language and environment for statistical computing. http://www.R-project.org.
- 39.Fields AL, Keller A, Schwartzberg L, et al. Adjuvant therapy with the monoclonal antibody edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. J Clin Oncol. 2009;27:1941–1947. doi: 10.1200/JCO.2008.18.5710. [DOI] [PubMed] [Google Scholar]
- 40.Punt CJ, Nagy A, Douillard JY, et al. Edrecolomab alone or in combination with fluorouracil and folinic acid in the adjuvant treatment of stage III colon cancer: A randomized study. Lancet. 2002;360:671–677. doi: 10.1016/S0140-6736(02)09836-7. [DOI] [PubMed] [Google Scholar]
- 41.Niedzwiecki D, Hollis D. Interpretation of results: Data analysis and reporting of results. In: Kelly WK, Halabi S, editors. Oncology Clinical Trials: Successful Design, Conduct, and Analysis. New York, NY: Demos Medical; 2010. p. 184. [Google Scholar]
- 42.Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264–271. doi: 10.1200/JCO.2009.24.0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Voyer TE, Sigurdson ER, Hanlon AL, et al. Colon cancer survival is associated with increasing number of lymph nodes analyzed: A secondary survey of intergroup trial INT-0089. J Clin Oncol. 2003;21:2912–2919. doi: 10.1200/JCO.2003.05.062. [DOI] [PubMed] [Google Scholar]
- 44.Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65–71. doi: 10.1245/aso.2003.03.058. [DOI] [PubMed] [Google Scholar]