ABSTRACT
Purpose: To estimate the predictive accuracy and clinical usefulness of the Chedoke–McMaster Stroke Assessment (CMSA) predictive equations.
Method: A longitudinal prognostic study using historical data obtained from 104 patients admitted post cerebrovascular accident was undertaken. Data were abstracted for all patients undergoing rehabilitation post stroke who also had documented admission and discharge CMSA scores. Published predictive equations were used to determine predicted outcomes. To determine the accuracy and clinical usefulness of the predictive model, shrinkage coefficients and predictions with 95% confidence bands were calculated.
Results: Complete data were available for 74 patients with a mean age of 65.3±12.4 years. The shrinkage values for the six Impairment Inventory (II) dimensions varied from −0.05 to 0.09; the shrinkage value for the Activity Inventory (AI) was 0.21. The error associated with predictive values was greater than ±1.5 stages for the II dimensions and greater than ±24 points for the AI.
Conclusions: This study shows that the large error associated with the predictions (as defined by the confidence band) for the CMSA II and AI limits their clinical usefulness as a predictive measure. Further research to establish predictive models using alternative statistical procedures is warranted.
Key Words: clinical application, CMSA, prediction, rehabilitation, stroke
RÉSUMÉ
Objectif : Évaluer la précision prévisionnelle et l'utilité clinique des équations prévisionnelles du Chedoke-McMaster Stroke Assessment (CMSA).
Méthode : Une étude longitudinale prévisionnelle à l'aide de données historiques obtenues auprès de 104 patients admis à la suite d'un accident vasculaire cérébral (AVC) a été réalisée. Des données ont été extraites pour tous les patients en réadaptation à la suite de leur AVC dont les scores au CMSA étaient documentés à l'admission et au congé. Des équations prévisionnelles publiées ont été utilisées pour déterminer les résultats attendus. Pour établir la précision et l'utilité clinique du modèle prévisionnel, des coefficients de retrait et des prévisions avec bande de confiance de 95 % ont été calculés.
Résultats : Des données complètes étaient disponibles pour 74 patients dont la moyenne d'âge était de 65,3 ans ±12,4 ans. Les valeurs de retrait pour les six dimensions de l'Impairment Inventory (inventaire de déficiences; II) variaient de −0,05 à 0,09. La valeur de retrait pour l'Activity Inventory (inventaire des activités; AI) était de 0,21. L'erreur associée aux valeurs prévisionnelles était supérieure de ±1,5 stade pour les dimensions II et de ±24 points pour l'AI.
Conclusions : Cette étude démontre que l'importante erreur associée aux prévisions (définie comme la « bande de confiance ») du CMSA II et AI limite leur utilité clinique en tant que mesure prévisionnelle. Des recherches ultérieures en vue de définir des modèles prévisionnels à l'aide de procédures statistiques alternatives seraient justifiées.
Mots clés : accident vasculaire cérébral, application clinique, CMSA, prévisions, réadaptation
INTRODUCTION
More than 50,000 people have strokes in Canada each year, and there are currently 300,000 Canadians living with the effects of stroke.1 Specialized interdisciplinary stroke rehabilitation units have been shown to be effective in reducing length of stay for persons recovering from stroke.2 A recent systematic review indicated that rehabilitation incorporating high-intensity, repetitive task-specific practice can optimize motor recovery after stroke.3 Nonetheless, the personal and societal costs that arise following stroke are significant.4
Measuring clinical outcomes is an integral part of evidence-based rehabilitation.5 Kirshner and Guyatt6 have noted that measures of health status have three possible purposes: (1) to discriminate among individuals, (2) to evaluate within-person change over time, and (3) to predict outcomes. The ability to predict outcomes following stroke yields several benefits: it can identify patients who would benefit from specific rehabilitation services, facilitate the provision of education on recovery to patients and their families, and offers the potential to improve planning of stroke care and rehabilitation services.7 It has been reported that validated, standardized outcome measures should be used to develop these predictive models.7
The Chedoke–McMaster Stroke Assessment (CMSA) is a highly reliable and valid outcome measure used by rehabilitation personnel to assess impairment and activity following stroke.8 The CMSA can be used to discriminate among individuals by assigning a specific motor stage of recovery using the Impairment Inventory, and the Activity Inventory (formerly called the Disability Inventory) can be used to evaluate change in physical functioning.8 Evidence for the reliability and validity of the CMSA has been reported for patients with stroke,8–11 patients with varied neurological impairments,12 patients with acquired brain injury,13 and elderly clients receiving inpatient rehabilitation.14 Furthermore, the CMSA is reported to be useful for predicting clinical outcomes in persons with stroke in both acute15 and rehabilitation settings.16–18
The use of the CMSA to predict outcomes in patients with stroke undergoing rehabilitation was first reported by Gowland in the 1980s.16,17 Subsequently, using the current version of the CMSA and a sample of 182 patients, revised predictive equations were generated using multiple regression analysis; these were reported in the CMSA manual, published in 1995.18 Gowland reported that predicted outcomes, used in conjunction with clinical judgment, could guide clinicians in selecting appropriate physiotherapeutic interventions and could also inform discharge planning.18 To date there have been no published studies further evaluating the psychometric properties of these predictive equations and their application to clinical practice.
The purpose of this investigation was to estimate the predictive accuracy and clinical usefulness of the CMSA predictive equations for patients with stroke undergoing rehabilitation. In the absence of previous cross-validation studies, determining the accuracy of the predictive equations will provide important information to health professionals working in stroke rehabilitation about the equations' value in a clinical setting, and is in keeping with the mandate of evidence-based practice.
METHODS
Design
This longitudinal prognostic study used historical data obtained from the Rehabilitation Hospital Health Sciences Centre, a tertiary-care institution in Winnipeg, Manitoba. The data were analyzed at McMaster University. The University of Manitoba Health Research Ethics Board approved access to an anonymized database for this investigation.
Participants and Procedure
The database used in this study was originally developed for a previous study that gathered data retrospectively from patient charts. That study did not address predictive accuracy.11 Data were gathered from the charts of all patients who were admitted to the stroke rehabilitation unit between July 1996 and July 1998 and were assessed at admission and discharge with the CMSA. There was no change to usual assessment and intervention care over this period. Physiotherapists and student physiotherapists assessed patients with the CMSA according to the instructions outlined in the CMSA manual,18 and all patients received conventional stroke rehabilitation during their stay in the rehabilitation unit. The original database consisted of patient demographic information, Impairment Inventory scores, and total Activity Inventory scores for 104 patients. All physiotherapists working in the stroke rehabilitation unit were involved in reviewing the patient charts and extracting the data. The purpose of the original study was to evaluate the functional outcomes of patients participating in a stroke rehabilitation program; specifically, the study reported on the extent to which change occurred during rehabilitation and the proportion of patients who met or exceeded the predicted discharge scores.11
In January 2009, following confirmation of ethical approval for the present study, additional information was extracted from the same patients' charts by staff from the hospital's medical records department and added to the database by the current investigative team. The newly retrieved information consisted of date of stroke, date of admission to and discharge from the rehabilitation unit, and scores on the 15 individual tasks of the Activity Inventory.
For the current study, patients were excluded from the data analysis if they were admitted to the rehabilitation unit more than 45 days post stroke, if their data were incomplete, or if they had experienced a stroke event other than a unilateral stroke. These criteria were chosen in an effort to achieve a more homogeneous sample.
The Chedoke–McMaster Stroke Assessment
The CMSA comprises two components: an Impairment Inventory (II), which is used to assess motor control across six dimensions, namely the arm, the hand, the leg, the foot, postural control, and shoulder pain; and an Activity Inventory (AI), which assesses functional mobility, including gross motor function and walking.8 The II quantifies the degree of motor control according to seven stages across the six dimensions. Scores are determined by the quality of movement; a score of 1 indicates severe motor impairment, and a score of 7 indicates normal movement. The AI consists of two indices: the Gross Motor Function Index (GMFI) measures functional mobility across 10 items, including moving in bed, transferring in and out of bed, and getting on and off the floor; and the Walking Index (WI) assesses the patient's ability to walk on smooth surfaces and rough terrain and to climb stairs across five items. Fourteen of the 15 AI items are scored from 1 (needs maximal assistance) to 7 (completely independent), while the fifteenth item, a 2-minute walk test,19 is scored relative to age-specific walking speed, either 0 (≤70 years of age: walking 96 m) or 2 (>70 years of age: walking 84 m). The maximum total score of the AI is 100; higher scores indicate greater functional independence. The CMSA manual describes the administration and scoring guidelines for the measure.18,20 Training for use of the CMSA includes a standardized 1-day training workshop,21 videoconferencing,22 and use of a bilingual CD-ROM for self-directed learning.23
Using the predictive equations and applying the patient's CMSA scores at admission, predicted discharge scores can be determined for each II stage and for the total AI and each AI index. Table 1 provides Gowland's predictive equations, which we applied to patient scores in the study database.18
Table 1.
Chedoke–McMaster Stroke Assessment Predictive Equations for Clients Undergoing Rehabilitation18
| Outcome Variable | R2 | Predictive Equation |
|---|---|---|
| Total AI | 0.73 | 17.45+(0.88×Gross Motor Function)+(4.30×Leg) |
| GMFI | 0.70 | 24.94+(0.76×Gross Motor Function)−(0.30×Weeks) |
| WI |
0.71 |
(0.28×Gross Motor Function)+(1.23×[Postural Control+Leg])*−4.55 |
| II | ||
| Shoulder Pain | 0.55 | 2.33+(0.44×Shoulder Pain)+(0.28×Arm) |
| Postural Control | 0.60 | 2.23+(0.35×Postural Control)+(0.30×Leg) |
| Arm | 0.80 | 0.82+(1.03×Arm)−(0.03×Weeks) |
| Hand | 0.78 | 0.53+(0.98×Hand) |
| Leg | 0.69 | 1.83+(0.77×Leg)−(0.02×Weeks) |
| Foot | 0.73 | 1.11+(0.90×Foot)−(0.03×Weeks) |
AI=Activity Inventory; GMFI=Gross Motor Function Index; WI=Walking Index; II=Impairment Inventory
Sum of scores for the stages of Postural Control and Leg
Data Analysis
We calculated means, standard deviations (SD), and quartiles for the CMSA scores (II dimensions, GMFI, WI, total AI). Next, we applied Gowland's predictive equations to patients' admission scores, as reported in the database, to obtain predicted discharge score estimates for the six II dimensions and for the AI and its two indices.18 For each item, the predicted estimate (independent variable) was regressed against the discharge score reported in the database (dependent variable) using a linear model. We also calculated the percentage of variance explained by the model ( ) and the residual or error variance ( ).
A predictive model is reliable to the extent that it generalizes to samples other than the one used to create it.24 To examine the extent to which Gowland's models predicted item scores for our sample, we calculated the shrinkage as , where is the squared correlation coefficient reported by Gowland and is the corresponding coefficient when Gowland's predictive equations were applied to our data.24 Kleinbaum et al. have stated that shrinkage values <0.10 indicate a reliable model.24 To help ascertain the clinical usefulness of the predictive equations, we calculated 95% prediction bands for each item's predicted values, as follows:
where is the predicted value for the corresponding X0 obtained by applying the Gowland predictive model, n is the number of patients contributing data, t is the critical value from the t-distribution on n−2 degrees of freedom (corresponding to a 2-tailed α value of 0.05), Sy/x is the square root of the error variance, is the mean predicted value for the item, and is the variance of the predicted values.24 The value obtained from this analysis conveys the amount of uncertainty associated with a predicted value for an individual in the units of the original measurement. The amount of uncertainty depends on the X value and is smallest when .
The final aspect of our analysis was to consider whether a linear model—as assumed by Gowland—provided the best fit for the predictive models. This was accomplished in two steps. First we examined scatter plots of the actual discharge (dependent variable) and admission (independent variable) data with the line of best fit superimposed on the graph. When a curvilinear relationship was evident, we examined higher-order polynomials and tested whether the addition of higher-order terms contributed to the predictive ability of the revised model.
Our sample size was one of convenience, determined by the number of eligible patients in our database.
RESULTS
Subjects
Of the 104 patients admitted to the rehabilitation unit between July 1996 and July 1998 who constituted the initial database, 30 were excluded from the present study owing to missing data, because they had had other than a unilateral stroke, or because they were admitted to the rehabilitation unit more than 45 days post stroke.
The other 74 patients were included in the data analysis. Patients had a mean (SD) age of 65.3 (12.4) years. Of these patients, 26 (35%) were female. The five most prevalent comorbidities were hypertension, diabetes mellitus (type I or type II), hyperlipidemia, musculoskeletal disorders, and a previous stroke or transient ischemic attack (TIA). A summary of patient characteristics is given in Table 2.
Table 2.
Patient Characteristics (n=74)
| Characteristic | Summary Value |
|---|---|
| Age | 65.3 (12.4) |
| mean (SD), years | |
| Gender (F/M) | 26/48 |
| Days post stroke | 15.6 (8.6) |
| mean (SD) | |
| LOS | 44.8 (24.4) |
| mean (SD) | |
| Type of stroke | |
| Left CVA | 41 |
| Right CVA | 33 |
LOS=length of stay; CVA=cerebro-vascular accident
The mean, standard deviation, and quartiles for CMSA admission, discharge, and prediction scores for the study population are provided in Table 3.
Table 3.
Summary Statistics for Chedoke–McMaster Stroke Assessment Values
| Outcome Variable |
Admission |
Discharge |
Predicted |
|---|---|---|---|
| Mean (SD) Quartiles |
Mean (SD) Quartiles |
Mean (SD) Quartiles |
|
| II dimensions | |||
| Hand | 3.8 (2.1) | 4.6 (2.1) | 4.2 (2.0) |
| 2, 4, 6 | 3, 6, 6 | 2.5, 4.5, 6.4 | |
| Arm | 3.7 (2.1) | 4.6 (2.1) | 4.6 (2.1) |
| 2, 4, 6 | 3, 5, 6 | 2.8, 4.8, 6.9 | |
| Leg | 4.3 (1.7) | 5.2 (1.5) | 5.1 (1.3) |
| 3, 5, 6 | 4, 6, 6 | 4.1, 5.6, 6.4 | |
| Foot | 3.6 (1.8) | 4.4 (1.9) | 4.3 (1.7) |
| 2, 4, 5 | 3, 5, 6 | 2.8, 4.6, 5.5 | |
| Shoulder pain | 5.9 (1.2) | 5.8 (1.6) | 6.0 (1.0) |
| 6, 6, 7 | 5, 7, 7 | 5.2, 6.1, 6.8 | |
| Postural control | 4.3 (1.1) | 5.1 (1.1) | 5.0 (0.8) |
| 4, 4, 5 |
5, 5, 6 |
4.5, 5.1, 5.8 |
|
| AI | 56.2 (18.1) | 76.4 (17.6) | 77.9 (18.7) |
| 43, 55, 71 | 65, 79, 90 | 63.5, 80.4, 92.9 | |
| GMFI | 47.2 (14.1) | 59.4 (10.7) | 60.2 (10.8) |
| 37, 47, 58 | 52, 62, 69 | 52.7, 60.4, 68.8 | |
| WI | 8.7 (4.9) | 17.1 (7.2) | 19.2 (6.8) |
| 4, 8, 12 | 13, 15, 22 | 13.8, 19.8, 24.7 |
II=Impairment Inventory; GMFI=Gross Motor Function Index; WI=Walking Index; AI=Activity Inventory
Predictive Ability
Table 4 summarizes the accuracy of the predictive equations. Reported in this table are the shrinkage values and three 95% prediction-band intervals (lowest, middle, and highest scale X values) for each CMSA II dimension and for the AI and its indices. Shrinkage values varied from −0.05 to 0.09 for the II dimensions; shrinkage values for the GMFI, WI, and AI were 0.19, 0.24, and 0.21 respectively.
Table 4.
Reliability and Accuracy Summary for Predictive Equations
| Outcome Variable | Reported |
Obtained |
Shrinkage ( |
Sy/x* | 95% Prediction Bands for Predicted Values of** |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 7 | ||||||||
| Hand | 0.78 | 0.76 | 0.02 | 1.06 | ±2.16 | ±2.13 | ±2.15 | |||
| Arm | 0.80 | 0.79 | 0.01 | 0.96 | ±1.96 | ±1.93 | ±1.94 | |||
| Shoulder pain | 0.55 | 0.49 | 0.06 | 1.13 | ±2.64 | ±2.33 | ±2.23 | |||
| Leg | 0.69 | 0.74 | −0.05 | 0.75 | ±1.60 | ±1.51 | ±1.53 | |||
| Foot | 0.73 | 0.78 | −0.05 | 0.91 | ±1.87 | ±1.83 | ±1.86 | |||
| Postural control |
0.60 |
0.51 |
0.09 |
0.77 |
±1.78 |
±1.56 |
±1.60 |
|||
|
1 |
35 |
70 |
||||||||
| GMFI |
0.70 |
0.51 |
0.19 |
7.49 |
±17.83 |
±15.57 |
±15.11 |
|||
|
1 |
15 |
30 |
||||||||
| WI |
0.71 |
0.47 |
0.24 |
5.30 |
±11.1 |
±10.66 |
±10.81 |
|||
|
10 |
50 |
90 |
||||||||
| Total AI | 0.73 | 0.52 | 0.21 | 11.79 | ±25.73 | ±24.04 | ±23.73 | |||
GMFI=Gross Motor Function Index; WI=Walking Index; AI=Activity Inventory
Sy/x is the square root of the residual variance.
These values represent the lowest, middle, and highest possible scores.
For the II, the error associated with predictive values amounts to approximately ±2 stages for each dimension; more error is associated with end-range scores.
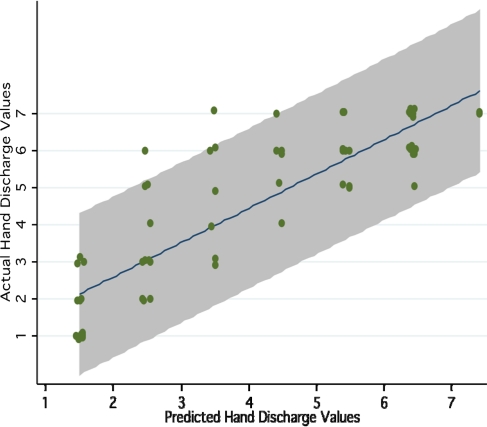
Figure 1 displays a scatter plot of the actual and predicted discharge values for the Hand Impairment item. This figure also shows the line of best fit and the 95% prediction bands.
Figure 1.
Scatter plot of actual versus predicted CMSA Hand Impairment values, including 95% prediction bands (shaded area)
Model Evaluation
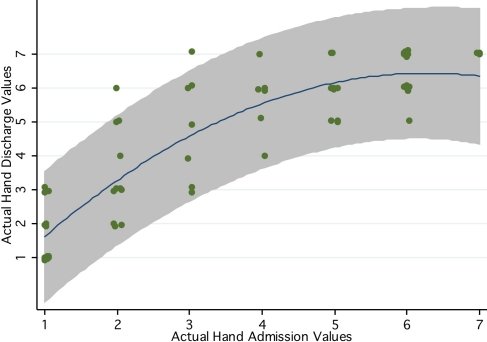
Our lack-of-fit analysis revealed that a second-degree polynomial provided better predictive models for CMSA hand and arm impairment data. The additional predictive ability of models that included a squared term were as follows: R2 hand=0.77, R2 hand+hand2=0.81 (F(1,82) hand2|hand=19.80, p<0.001); R2 arm=0.79, R2 arm+arm2=0.83 (F(1,82) arm2|arm=25.40, p<0.001). Figure 2 is a graphical representation of the curvilinear relationship between actual admission and discharge hand-impairment data.
Figure 2.
Actual CMSA hand-impairment values at admission and discharge, including 95% prediction bands (shaded area), for the second-degree polynomial model
DISCUSSION
The goal of our study was to estimate the predictive accuracy and clinical usefulness of reported CMSA equations. To meet this objective, we applied statistical procedures to examine the relationships between the actual results in our sample of patients undergoing rehabilitation following stroke and the outcomes predicted using Gowland's predictive equations. The shrinkage coefficients for the stages of the II were all less than 0.10, which suggests that the predictive models proposed by Gowland18 are supported by our data. In contrast, the shrinkage values for the total AI score and for the GMFI and WI scores greatly exceeded the critical value of 0.10, indicating that the predictive models proposed by Gowland are not supported by our data. One possible explanation for the large shrinkage value is that there were differences between the two samples of patients (e.g., the timing of the post-stroke assessments); it is also possible that differences in physiotherapy management contributed to differences in outcomes between Gowland's patients and those in the database used for the present study. Unfortunately, information on the patients in the sample used by Gowland to generate the predictive equations was not available to explore these potential explanations.
While the results of this analysis suggest that the shrinkage for the II scores is acceptable, we further considered the confidence bands of the predicted outcomes to gain additional information. The clinical usefulness of the predictive equations is directly related to the width of the prediction bands: the narrower the width, the greater the confidence in predicting an individual patient's discharge score. The width of prediction bands depends on the variability in the data, the mean score, and the distance of a particular value from the mean score: the greater the distance from the mean score, the greater the width of the prediction band. Table 3 reports the width of the 95% prediction bands for three impairment values (stages 1, 4, and 7); for most impairment values, the 95% prediction bands are approximately ±2 stages. This width is likely too large to provide a confident prediction of a patient's outcome. We offer the following clinical vignette to assist in the interpretation of our findings.
Clinical Vignette
A 64-year-old man was admitted to the rehabilitation unit after a right cerebral vascular accident (CVA) 16 days ago. The physiotherapist assesses the patient using the CMSA within the first week of admission. The CMSA stage for the hand at admission is 3. The physiotherapist calculates expected discharge scores using Gowland's predictive equations. The predicted discharge score for the hand is stage 3.5. By applying the 95% prediction bands (3.5±2.13) from the current study (see Table 3), the physiotherapist finds that the patient's hand stage at discharge could fall anywhere between 1 (i.e., 1.37) and 6 (i.e., 5.63). Stage 1 indicates that the hand will be flaccid and that there will be no evidence of any reflexive or active movement, while Stage 6 indicates that coordination and patterns of movement for the hand will be near normal.18 The range of the predicted score of the stage of the arm at discharge (5 stages in total) is too large to provide useful information to the clinician.
We also considered whether a more complex model (in this case, higher-degree polynomial) might provide a better fit than the models proposed by Gowland et al.18 We found that for the measures of hand and arm impairment, a second-degree polynomial fit our data better statistically; however, confidence in the predicted scores, as represented by the 95% prediction bands, did not improve appreciably. This finding is illustrated for the hand in Figure 2, in which the prediction band continues to be approximately ±2 stages wide.
We wish to stress that our study focused on the predictive ability of the CMSA and not on its properties in assessing patient outcomes. Proficient outcome measures must display high levels of discrimination and the ability to detect change, and previous investigations have provided support for these measurement properties as they apply to the CMSA when applied to patients with stroke.8–11,18 The role of the CMSA as a valuable discriminative and evaluative measure is not altered by the findings of the present study.
LIMITATIONS
Our study has several limitations, many of which arise from the use of a historical database. Despite efforts to achieve a large sample size, the database was missing data for a number of patients, who were therefore excluded from the analysis. Because there was no standard protocol for data collection, patients were assessed at different time points (including time post stroke and length of time between initial and discharge assessment), producing a non-homogenous sample. In addition, at the time of assessment the patients' CMSA scores were recorded on paper score sheets, which were stored in the patient files; for the purpose of the current study, these scores were then recorded on a new data-collection sheet by staff in medical records and subsequently added to the database by members of our research team. The transferring of data on several occasions by different individuals may have resulted in errors in the database used for the study.
Furthermore, we have limited information about the graduate and student physiotherapists who collected data for the original study. For example, there are no data on their experience in working with patients with stroke or on their formal training (or lack thereof) in administering and scoring the CMSA. The accuracy of the data they collected may also have influenced our results.
The results of this study suggest that statistical procedures other than linear regression can be considered to develop new and more accurate prediction models. Future research to develop predictive equations should involve a prospective study with a larger sample. Methodological considerations would include specifying assessment times related to time post stroke, rather than using admission and discharge dates, to increase accuracy of predictions.25 The collection of more comprehensive demographic information about the patients in the sample and about the persons who gather the data, as well as a description of the care received during rehabilitation, would increase the generalizability of the results.
CONCLUSION
For approximately 20 years, clinicians in rehabilitation settings have been using the CMSA predictive equations developed by Gowland to enhance clinical decision making.18 The results of this study indicate that the shrinkage values for the AI models are too large for the models to be considered reliable. Furthermore, the confidence bands associated with both II and AI scores are too large to be considered clinically useful. Future research to identify models that rehabilitation professionals can use in predicting outcomes for individual clients with stroke would facilitate evidence-based practice. The importance of the CMSA's role as a discriminative and evaluative measure for persons with stroke remains unchanged.
KEY MESSAGES
What Is Already Known on This Topic
The CMSA is a highly valid and reliable measure to discriminate and detect change in persons post stroke. There has been no research to evaluate the accuracy of the predictive equations developed for use with clients undergoing rehabilitation for stroke.
What This Study Adds
The results of this study call into question the clinical usefulness of the predictive equations developed for stroke rehabilitation. Future research using alternative predictive models is warranted.
Dang M, Ramsaran KD, Street ME, Syed SN, Barclay-Goddard R, Stratford P, Miller PA. Estimating the accuracy of the Chedoke–McMaster Stroke Assessment predictive equations for stroke rehabilitation. Physiother Can. 2011;preprint. doi:10.3138/ptc.2010-17
REFERENCES
- 1.Heart and Stroke Foundation of Canada. Statistics [Internet] Ottawa: The Foundation; n.d.. [cited 2010 Mar 12]. Available from: http://www.heartandstroke.com/site/c.ikIQLcMWJtE/b.3483991/k.34A8/Statistics.htm. [Google Scholar]
- 2.Foley N, Salter K, Teasell R. Specialized stroke services: a meta-analysis comparing three models of care. Cerebrovasc Dis. 2007;23:194–202. doi: 10.1159/000097641. doi: 10.1159/000097641. [DOI] [PubMed] [Google Scholar]
- 3.Langhorne P, Coupar F, Pollack A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8:741–54. doi: 10.1016/S1474-4422(09)70150-4. doi: 10.1016/S1474-4422(09)70150-4. [DOI] [PubMed] [Google Scholar]
- 4.Teasell RW, Foley NC, Salter KL, Jutai JW. A blueprint for transforming stroke rehabilitation care in Canada: the case for change. Arch Phys Med Rehabil. 2008;89:575–8. doi: 10.1016/j.apmr.2007.08.164. doi: 10.1016/j.apmr.2007.08.164. [DOI] [PubMed] [Google Scholar]
- 5.Law M, editor. Evidence-based rehabilitation. Thorofare, NJ: Slack; 2002. [Google Scholar]
- 6.Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chron Dis. 1985;38:27–36. doi: 10.1016/0021-9681(85)90005-0. doi: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- 7.Feigin VL, Barker-Collo S, McNaughton H, Brown P, Kerse N. Long-term neuropsychological and functional outcomes in stroke survivors: current evidence and perspectives for new research. Int J Stroke. 2008;3:33–40. doi: 10.1111/j.1747-4949.2008.00177.x. doi: 10.1111/j.1747-4949.2008.00177.x. [DOI] [PubMed] [Google Scholar]
- 8.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke–McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 9.Gowland C, Huijbregts M, McClung A, McNern A. Measuring clinically-important change with the Chedoke-McMaster Stroke Assessment. Can J Rehabil. 1993;7:14–6. [Google Scholar]
- 10.Huijbregts MP, Gowland C, Gruber R. Measuring clinically important change with the Activity Inventory of the Chedoke-McMaster Stroke Assessment. Physiother Can. 2000;52:295–304. [Google Scholar]
- 11.Barclay-Goddard R, Szturm T. Use of the Chedoke–McMaster Stroke Assessment for stroke program evaluation. Proceedings of the 2001 Canadian Physiotherapy Association national congress. Physiother Can. 2001;53(Suppl.):S14. [Google Scholar]
- 12.Barclay-Goddard R. Physical function outcome measurement in acute neurology. Physiother Can. 2000;52:138–45. [Google Scholar]
- 13.Crowe JM, Harmer D, Sharp J. Reliability of the Chedoke–McMaster Disability Inventory in acquired brain injury. Physiother Can. 1996;4:S25. [Google Scholar]
- 14.Sacks L, Yee K, Huijbregts MJ, Miller PA, Aggett T, Salbach N. Validation of the Activity Inventory of the Chedoke–McMaster Stroke Assessment and the Clinical Outcomes Variable Scale to evaluate mobility in geriatric clients. J Rehabil Med. 2010;42:90–2. doi: 10.2340/16501977-0477. doi: 10.2340/16501977-0477. [DOI] [PubMed] [Google Scholar]
- 15.Miller P, Gowland C, Crowe J, Christie H, Barclay-Goddard R, Orrett V, et al. Predicting impairment and disability in patients with acute stroke; Podium presentation at the Canadian Physiotherapy Association Congress 1997; Winnipeg, MB. [Google Scholar]
- 16.Gowland C. Recovery of motor function following stroke: profile and predictors. Physiother Can. 1982;34:77–84. [Google Scholar]
- 17.Gowland C. Predicting sensorimotor recovery following stroke rehabilitation. Physiother Can. 1984;36:313–20. [Google Scholar]
- 18.Gowland C, Van Hullenaar S, Torresin W, Moreland J, Vanspall B, Barreca S, et al. Chedoke–McMaster Stroke Assessment: development, validation, and administration manual. Hamilton (ON): McMaster University; 1995. [Google Scholar]
- 19.Miller PA, Moreland J, Stevenson TJ. Measurement properties of a standardized version of the two-minute walk test for individuals with neurological dysfunction. Physiother Can. 2002;54:241–8. 257. [Google Scholar]
- 20.Miller P, Huijbregts M, Gowland C, Barreca S, Torresin W, Moreland J, et al. Chedoke–McMaster Stroke Assessment: development, validation, and administration manual. 2nd ed. Hamilton (ON): McMaster University; 2008. [Google Scholar]
- 21.Miller P, Stratford P, Gowland C, VanHullenaar S, Torresin W. Comparing two methods to train therapists to use the Chedoke–McMaster Stroke Assessment; Proceedings of the 13th International Congress of the World Confererations of Physical Therapy; 1999 May 23–28; Yokohama, Japan. Tokyo: Japanese Physical Therapy Association; 1999. p. 127. [Google Scholar]
- 22.Miller P, Huijbregts M, French E, Taylor D, Reinikka K, Berezny L, et al. Videoconferencing a full day training workshop: effectiveness, acceptability, and cost. J Contin Educ Health Prof. 2008;28:1–14. doi: 10.1002/chp.192. [DOI] [PubMed] [Google Scholar]
- 23.Miller P, Paquet N, Huijbregts M. Chedoke–McMaster Stroke Assessment learning resource [CD-ROM] Ottawa: University of Ottawa; 2007. [Google Scholar]
- 24.Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. 2nd ed. Boston: PWS-Kent; 1988. [Google Scholar]
- 25.Gresham GE. Stroke outcome research. Stroke. 1986;12:358–60. doi: 10.1161/01.str.17.3.358. [DOI] [PubMed] [Google Scholar]