Abstract
Background:
The most appropriate inotropic agent for use in the newborn is uncertain. Dopamine and epinephrine are commonly used, but have unknown effects during hypoxia and pulmonary hypertension; the effects on the splanchnic circulation, in particular, are unclear.
Methods:
The effects on the systemic, pulmonary, hepatic, and mesenteric circulations of infusions of dopamine and epinephrine (adrenaline) were compared in 17 newborn piglets. Three groups [control (n = 5), dopamine (n = 6) and epinephrine (n = 6)] of fentanyl anesthetized newborn piglets were instrumented to measure cardiac index (CI), hepatic arterial and portal venous blood flow, mean systemic arterial pressure (SAP), mean pulmonary arterial pressure (PAP), and arterial, portal and mixed venous oxygen saturations. Systemic, pulmonary, and mesenteric vascular resistance indices [systemic vascular resistance index (SVRI), pulmonary vascular resistance index (PVRI), mesenteric vascular resistance index (MVRI)], and systemic and splanchnic oxygen extraction and consumption were calculated. Alveolar hypoxia was induced, with arterial oxygen saturation being maintained at 55-65%. After 1 h of stabilization during hypoxia, each animal received either dopamine or epinephrine; randomly administered doses of 2, 10, and 32 μg kg-1 min-1 and 0.2, 1.0, and 3.2 μg kg-1 min-1 respectively were infused for 1 h at each dose. Results were compared with the 1 h hypoxia values by two-way analysis of variance.
Results:
Epinephrine increased CI at all doses, with no significant effects on SAP and SVRI. Although epinephrine increased PAP at 3.2 μg kg-1min-1, it had no effect on PVRI. Dopamine had no effect on CI, SAP, and SVRI, but increased PAP at all doses and PVRI at 32 μg kg-1min-1. The SAP/PAP ratio was decreased with 32 μg kg-1min-1 dopamine, whereas epinephrine did not affect the ratio. In the mesenteric circulation, dopamine at 32 μg kg-1 min-1 increased portal venous flow and total hepatic blood flow and oxygen delivery, and decreased MVRI; epinephrine had no effect on these variables. Epinephrine increased hepatic arterial flow at 0.2 μg kg-1 min-1; dopamine had no effect on hepatic arterial flow at any dose. Despite these hemodynamic changes, there were no differences in systemic or splanchnic oxygen extraction or consumption at any dose of dopamine or epinephrine.
Conclusions:
Epinephrine is more effective than dopamine at increasing cardiac output during hypoxia in this model. Although epinephrine preserves the SAP/PAP ratio, dopamine shows preferential pulmonary vasoconstriction, which might be detrimental if it also occurs during the management of infants with persistent fetal circulation. Dopamine, but not epinephrine, increases portal flow and total hepatic flow during hypoxia.
Keywords: inotropes, regional flow, oxygen extraction, piglets
Introduction
Among the inotropes available for cardiovascular support in critically ill newborns, dopamine and epinephrine (adrenaline) are commonly used in neonatal intensive care units [1]. With increasing clinical and animal data showing that hemodynamic responses to inotropes in newborns differ from those in adults and older children [2, 3, 4], it is uncertain whether these agents are appropriate in the treatment of shock or hypotension in sick newborns who are at risk for the development of persistent fetal circulation and necrotizing enterocolitis. Indeed, the appropriate catecholamine in various clinical situations also remains undetermined for the critically ill adult.
The adrenoceptors in the pulmonary and mesenteric vasculature mature differently. For example, the neonatal pulmonary vasculature appears to be deficient in dopaminergic receptors [2, 5], whereas α, β and dopamin-ergic receptors are present in the mature mesenteric vas-culature [6]. The functional maturity and expression of the various adrenoceptors in the newborn vary greatly [7]. We have previously reported the responses of the pulmonary and mesenteric circulation to dopamine and epinephrine infusions in anesthetized normoxic [8] and hypoxic [9] piglets. In this acutely instrumented hypoxic model, epinephrine, at a low dose (0.2 μg kg-1 min-1), produced a pulmonary vasodilatation; in comparison, dopamine had no such effect. However, there are no data on the effects on mesenteric hemodynamics and oxygen metabolism of infusions of either dopamine or epinephrine during hypoxia.
The objectives of this study were to evaluate the effects of dopamine and epinephrine infusions in hypoxic piglets on systemic, pulmonary, and mesenteric circulations, and on systemic and splanchnic oxygen metabolism.
Materials and methods
Seventeen newborn piglets (1-3 days of age), weighing 1.4-2.4 kg (mean 1.89 kg), were obtained. Anesthesia was induced with inhaled halothane (5%, decreasing to 2%). A double lumen external jugular catheter and a common carotid arterial line were inserted. A right atrial catheter was established through the right external jugular vein. After tracheotomy and the commencement of assisted ventilation, anesthesia was maintained by a 10 μg kg-1 dose of fentanyl and the piglets were paralysed with 0.1 mg kg-1 doses of pancuronium; halothane was discontinued after a maximum of 20 min. Dextrosesaline solution was infused at a rate of 15-20 ml kg-1 h-1 while the skin incisions were open. Piglets were ventilated at pressures of 16/4 cmH2O at a rate of 12-18 breaths per minute.
A left thoracotomy was then performed in the 4th intercostal space. The pericardium was opened and a 20-gauge catheter was inserted into the root of the pulmonary artery for the measurement of pulmonary artery pressure. A 6 mm transit time ultrasound flow probe (Transonic Corporation, Ithaca, NY, USA) was placed around the main pulmonary artery to measure cardiac output. A midline laparotomy was performed. A 5-Fr Argyle catheter was inserted through the umbilical vein into the portal venous system. Two Transonic transit time ultrasound flow probes (2 mm and 1 mm) were placed around the portal vein and the common hepatic artery respectively. The neck incision, thoracotomy, and laparotomy were closed with sutures after these procedures had finished. Blood gases were drawn and 15 min of recording was done to ensure that the animal was stable. Stability, which usually occurred 20-30 min after completion of the surgical procedure, was defined as (1) heart rate and blood pressure within 10% of the post-anesthetic presurgical values, (2) right atrial pressure of 3-8 mmHg, (3) arterial PaO2 75-120 mmHg, PaCO2 37-43 mmHg and pH 7.35-7.45. The surgical procedure usually finished within 75 min. Fentanyl infusion at 5 μg kg-1 h-1 was used for analgesia and sedation for the rest of the experiment. Rectal temperature was maintained between 38.0 and 38.5°C by means of a heating blanket and an infrared heating lamp.
Five piglets were used as controls. After a baseline monitoring period of at least 15 min, simultaneous blood samples were drawn for determination of arterial, mixed venous and portal venous oxygen saturation by co-oximeter (Hemoximeter, Copenhagen, Denmark). The inspired oxygen concentration was decreased to 12% and then adjusted to achieve an arterial saturation of between 55% and 65% (PaO2 usually 40-50 mmHg); blood gas estimation was repeated at 30 min intervals. The following hemodynamic variables were monitored continuously for 4 h of hypoxia: mean systemic arterial pressure (SAP), mean pulmonary arterial pressure (PAP), right atrial pressure (RAP), heart rate, pulse oximetry oxygen saturation (Nellcor, Hayward, CA, USA), pulmonary blood flow, portal venous flow and hepatic arterial flow. Analog outputs of the pressure amplifiers and flow monitors were digitized by a DT 2801-A analog to digital converter board (Data Translation, Mississauga, Ontario, Canada) in a Dell 425E personal computer. Software was custom written using the Asyst programming environment. All signals were acquired continuously at 24 Hz and saved on hard disk. Three-minute averages of the hemodynamic variables and oxygen saturation variables [arterial (SaO2), mixed venous (SvO2), and portal venous (SpO2) saturations] were measured at 60 min intervals during the 4 h of hypoxia. Cardiac index (CI), portal venous flow index (PVFI), and hepatic arterial flow index (HAFI) were calculated by dividing the non-indexed variables by body weight.
Six piglets were prepared for each of the dopamine and epinephrine infusion groups. Hypoxia, with an arterial oxygen saturation between 55% and 65%, was induced as above. After 1 h of systemic hypoxia, baseline recordings of the above hemodynamic and oxygenation variables were made. Each piglet received either dopamine or epinephrine and was administered all three doses, which were selected in random order as determined by a Latin-Square method. Dopamine and epinephrine were infused at doses of 2, 10, and 32 μg kg-1 min-1 and 0.2, 1.0, and 3.2 μg kg-1 min-1respectively. The total intravenous fluid rate was kept constant throughout the infusions. The drug infusion was continued for 60 min. The hemodynamic (3 min averaged values) and oxygen saturation variables at 30 and 60 min of each infusion dose were collected for analysis. Blood lactate was measured after 60 min of hypoxia and after 60 min at each dose of the drug.
We calculated the following variables at individual doses:
1. Systemic vascular resistance index (SVRI) = (SAP - RAP)/CI.
2. Pulmonary vascular resistance index (PVRI) = PAP/CI.
3. Mesenteric vascular resistance index (MVRI) = SAP/PVFI.
4. Total hepatic flow index (THFI) = PVFI + HAFI.
5. Systemic oxygen extraction (systemic EO2) = [(SaO2 - SvO2)/SaO2] × 100%.
6. Splanchnic oxygen extraction (splanchnic EO2) = [(SaO2 - SpO2)/SaO2] × 100%.
7. Systemic oxygen consumption (systemic VO2) = CI × (SaO2 - SvO2) × 1.34 × [Hb].
8. Splanchnic oxygen consumption (splanchnic VO2) = PVFI × (SaO2 - SpO2) × 1.34 × [Hb].
9. Hepatic oxygen delivery (hepatic DO2) = (HAFI × SaO2 + PVFI × SpO2) × 1.34 × [Hb].
10.Ratio of hepatic arterial oxygen delivery to total hepatic DO2 (hepatic DO2 ratio) = [HAFI × SaO2/(HAFI × SaO2 + PVFI × SpO2)] × 100%.
The protocol was approved by the Laboratory Animal Care Committee of University of Alberta, and complied with the guidelines of the Canadian Council on Animal Care.
Statistical analysis
One-way repeated-measures analysis of variance (ANOVA) was used to analyze the variables at different doses within groups. Two-way ANOVA was used to identify the difference between groups at different doses. The data were analyzed with a software program (Sigma Stat version 1.01; Jandel Scientific, San Rafael, CA, USA). Dunnett's post-hoc test was used, if the overall ANOVA was significant, to compare differences with the values obtained after 1 h of hypoxia (the 'hypoxia baseline'). P < 0.05 was considered significant. All results are expressed as means ± SD.
Results
Controls (n = 5)
After 1 h of systemic hypoxia, significant increases in PAP, PVRI, and CI were found (Table 1). No significant changes in SAP, SVRI, PVFI, HAFI, THFI, or MVRI were demonstrated. Hypoxia increased systemic EO2 and splanchnic EO2 significantly. Hepatic DO2 ratio was not affected. The control animals had no significant change in any of the recorded hemodynamic and metabolic variables over the subsequent 3 h of the study in comparison with the 1 h values. At 1 h of hypoxia the control group values for the above variables were not significantly different from the hypoxia baseline values in the other two groups.
Table 1.
Effects (means ± SD) of prolonged hypoxia in five anesthetized control piglets
| Normoxia | 1 h hypoxia | 2 h hypoxia | 3 h hypoxia | 4 h hypoxia | |
| Cardiac index (ml kg-1 min-1) | 136 ± 25 | 151 ± 49* | 154 ± 50* | 151 ± 35* | 142 ± 43 |
| Arterial saturation (%) | 99 ± 0.5 | 61 ± 6* | 63 ± 3* | 59 ± 4* | 60 ± 4* |
| Systemic DO2 (ml kg-1 min-1) | 20 ± 3.4 | 14 ± 2.2* | 13 ± 1.6* | 14 ± 3.7* | 13 ± 3.7* |
| SAP (mmHg) | 83 ± 20 | 79 ± 15 | 79 ± 15 | 75 ± 15 | 73 ± 16* |
| PAP (mmHg) | 25 ± 2 | 37 ± 7* | 37 ± 7* | 38 ± 7* | 40 ± 6* |
| PVRI (mmHg ml-1 kg-1 min-1) | 0.19 ± 0.04 | 0.27 ± 0.11* | 0.25 ± 0.09* | 0.26 ± 0.07* | 0.30 ± 0.06* |
| SAP/PAP ratio | 3.3 ± 0.6 | 2.2 ± 0.5* | 2.2 ± 0.5* | 2.0 ± 0.4* | 1.8 ± 0.4* |
| Systemic EO2 (%) | 27 ± 5.5 | 46 ± 14* | 44 ± 13* | 43 ± 13* | 44 ± 11* |
EO2, oxygen extraction; DO2, oxygen delivery. *P < 0.05 compared with normoxic baseline.
Dopamine (n = 6) (Table 2)
Table 2.
Effects (means ± SD) of dopamine infusions in six anesthetized hypoxic piglets
| Normoxia | 1 h hypoxia | 2i | 2f | 10i | 10f | 32i | 32f | |
| SAP/PAP ratio | 3.4 ± 0.65§ | 2.0 ± 0.27 | 1.7 ± 0.32 | 1.6 ± 0.21 | 1.7 ± 0.33 | 1.8 ± 0.60 | 1.4 ± 0.12*† | 1.4 ± 0.20*† |
| MVRI (mmHg ml-1 kg-1 min-1) | 1.86 ± 0.49 | 2.00 ± 0.55 | 2.07 ± 0.82 | 1.98 ± 0.84 | 1.99 ± 0.86 | 2.07 ± 0.86 | 1.45 ± .74*† | 1.21 ± .46*† |
| Systemic DO2 (ml kg-1 min-1) | 33 ± 5§ | 19 ± 5 | 23 ± 8 | 24 ± 8 | 21 ± 6 | 21 ± 6 | 20 ± 4 | 19 ± 4 |
| Systemic EO2 (%) | 28 ± 11.7§ | 43 ± 12.6 | 42 ± 12.7 | 42 ± 13.8 | 39 ± 7.5 | 39 ± 10.8 | 42 ± 11.4 | 40 ± 13.1 |
| Splanchnic EO2 (%) | 20 ± 6.7§ | 39 ± 9.3 | 37 ± 13.2 | 35 ± 14.6 | 33 ± 11.3 | 30 ± 9.1 | 24 ± 11.4 | 26 ± 13.9 |
| Systemic VO2 (ml kg-1 min-1) | 7.02 ± 3.36 | 6.16 ± 2.14 | 6.78 ± 2.80 | 7.13 ± 2.57 | 6.54 ± 3.40 | 6.04 ± 2.00 | 6.27 ± 2.14 | 5.80 ± 2.33 |
| Splanchnic VO2 (ml kg-1 min-1) | 1.19 ± 0.42 | 1.07 ± 0.15 | 1.10 ± 0.32 | 1.11 ± 0.22 | 1.07 ± 0.36 | 0.97 ± 0.36 | 0.84 ± 0.49 | 1.02 ± 0.44 |
| Hepatic DO2 (ml kg-1 min-1) | 5.86 ± 0.92§ | 2.29 ± 0.87 | 2.56 ± 1.07 | 2.88 ± 1.26 | 2.73 ± 0.85 | 2.82 ± 0.90 | 3.32 ± 1.26 | 3.61 ± 1.25* |
| Hepatic DO2 ratio (%) | 17 ± 14.2 | 20 ± 13.1 | 18 ± 13.2 | 19 ± 14.2 | 18 ± 12.1 | 16 ± 11.1 | 13 ± 11.3 | 10 ± 8.6 |
| (HAFI + PVFI)/CI (%) | 28 ± 4§ | 23 ± 3 | 23 ± 5 | 23 ± 4 | 26 ± 12 | 27 ± 11 | 27 ± 5 | 32 ± 6! |
| Arterial lactate (mM) | 9.2 ± 6.6 | 9.4 ± 4.7 | 12.0 ± 7.1 | 10.2 ± 4.9 |
i, initial (3 min average at 30 min of infusion); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with variables at 1 h of hypoxia (one-way repeated measures ANOVA); †P < 0.05 compared with variables during all doses of epinephrine infusion (two-way ANOVA); §P < 0.05 for difference between normoxia baseline and 1 h of hypoxia. EO2, oxygen extraction; DO2, oxygen delivery; MVRI, mesenteric vascular resistance index; hepatic DO2 ratio, the proportion of hepatic DO2 accounted for by hepatic arterial oxygen delivery; (HAFI + PVFI)/CI, total hepatic blood flow as a proportion of the cardiac index.
There was no significant effect on SAP, CI (Fig. 1) or calculated SVRI (Fig. 2) with any dose of dopamine, PAP was elevated at all doses, and a significant increase in calculated PVRI was demonstrated only at 10 μg kg-1 min-1 dopamine. The SAP/PAP ratio was lowered significantly with 32 μg kg-1 min-1 dopamine (Table 2). The effects on PAP and the SAP/PAP ratio were sustained throughout the infusions. There were significant increases in PVFI and THFI (Fig. 3), with decreases in calculated MVRI, at a dose of 32 μg kg-1 min-1 dopamine. The SAP/PAP ratio during 32 μg kg-1 min-1 dopamine, at both the initial and final 30 min, was significantly lower than at the 1 h baseline, and was lower than all doses of epinephrine. The changes in PVFI and calculated MVRI with 32 μg kg-1 min-1 dopamine at the final 30 min were significantly different from these variables at the 1 h baseline and at all doses of the epinephrine group.
Figure 1.
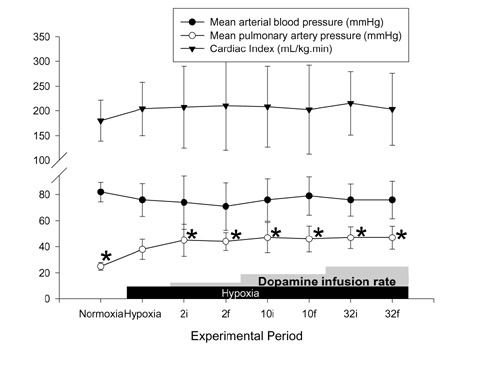
Effects of hypoxia and dopamine infusion on cardiac index, and systemic and pulmonary artery pressures. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
Figure 2.
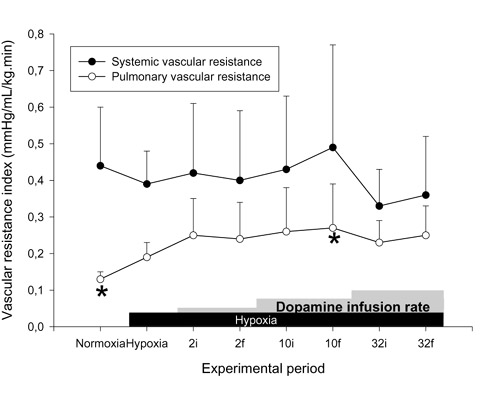
Effects of hypoxia and dopamine infusion on systemic and pulmonary vascular resistance indices. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
Figure 3.
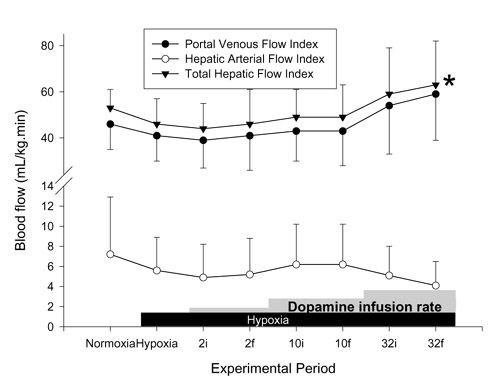
Effects of hypoxia and dopamine infusion on portal venous, hepatic arterial, and total hepatic blood flows. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
The decrease in mesenteric vascular resistance and the increase in hepatic venous flow during the highest dose of dopamine, with a stable CI, led to an increase in the total hepatic blood flow as a proportion of cardiac output.
No significant changes in systemic EO2, systemic VO2, splanchnic EO2, splanchnic VO2, and hepatic DO2 ratio were found with any dose of dopamine infusion. At 32 μg kg-1min-1 dopamine, hepatic DO2 increased significantly from the 1 h baseline. Serum lactate concentration was elevated by hypoxia but was not significantly affected by dopamine.
Epinephrine (n = 6) (Table 3)
Table 3.
Effects (means ± SD) of epinephrine infusions in six anesthetized hypoxic piglets
| Normoxia | 1 h hypoxia | 0.2i | 0.2f | 1.0i | 1.0f | 3.2i | 3.2f | |
| SAP/PAP ratio | 3.4 ± 0.52§ | 2.2 ± 0.46 | 2.0 ± 0.48 | 2.0 ± 0.40 | 2.0 ± 0.23 | 2.0 ± 0.34 | 2.1 ± 0.43 | 2.0 ± 0.44 |
| MVRI (mmHg ml-1 kg-1 min-1) | 1.67 ± 0.39 | 2.07 ± 0.80 | 2.15 ± 1.10 | 2.22 ± 1.31 | 2.43 ± 1.53 | 2.71 ± 2.20 | 2.37 ± 1.11 | 2.37 ± 1.28 |
| Systemic DO2 (ml kg-1 min-1) | 35 ± 12§ | 20 ± 7 | 23 ± 6 | 20 ± 9 | 21 ± 6 | 18 ± 5 | 22 ± 4 | 20 ± 7 |
| Systemic EO2 (%) | 29 ± 5.4§ | 43 ± 9.8 | 42 ± 5.7 | 40 ± 3.8 | 42 ± 10.7 | 38 ± 3.8 | 39 ± 6.2 | 42 ± 3.6 |
| Splanchnic EO2 (%) | 20 ± 6.2§ | 37 ± 6.8 | 36 ± 7.0 | 41 ± 9.7 | 40 ± 6.7 | 41 ± 8.4 | 35 ± 11.4 | 42 ± 10.8 |
| Systemic VO2 (ml kg-1 min-1) | 7.65 ± 1.53 | 7.36 ± 2.13 | 8.16 ± 2.65 | 7.30 ± 1.35 | 8.21 ± 2.24 | 7.23 ± 1.28 | 7.63 ± 2.47 | 7.87 ± 1.63 |
| Splanchnic VO2 (ml kg-1 min-1) | 1.25 ± 0.41 | 1.37 ± 0.36 | 1.27 ± 0.57 | 1.23 ± 0.36 | 1.29 ± 0.48 | 1.33 ± 0.27 | 1.19 ± 0.59 | 1.32 ± 0.40 |
| Hepatic DO2 (ml kg-1 min-1) | 5.97 ± 1.24§ | 2.93 ± 0.76 | 3.07 ± 0.61 | 2.79 ± 0.89 | 2.61 ± 0.52 | 2.59 ± 0.62 | 2.83 ± 0.69 | 2.62 ± 0.71 |
| Hepatic DO2 ratio (%) | 16 ± 7.6 | 19 ± 9.8 | 28 ± 15.7 | 32 ± 17.8*‡ | 29 ± 14.6 | 23 ± 12.8 | 24 ± 12.1 | 29 ± 18.8 |
| (HAFI + PVFI)/CI (%) | 33 ± 10§ | 26 ± 7 | 23 ± 3 | 22 ± 4 | 20 ± 3 | 20 ± 5 | 21 ± 5 | 21 ± 3 |
| Arterial lactate (mM) | 8.9 ± 3.4 | 12.6 ± 5.2 | 14.0 ± 7.0* | 13.5 ± 5.4* |
i, initial (3 min average at 30 min of infusion); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with variables at 1 h of hypoxia (one-way repeated measures ANOVA); ‡P < 0.05 Compared with variables during all doses of dopamine infusion (two-way ANOVA); §P < 0.05 for difference between normoxia baseline and 1 h of hypoxia. EO2, oxygen extraction; DO2, oxygen Delivery; MVRI, mesenteric vascular resistance index; hepatic DO2 ratio, the proportion of hepatic DO2 that is accounted for by hepatic arterial oxygen delivery; (HAFI + PVFI)/CI, total hepatic Blood flow as a proportion of the cardiac index.
PAP was significantly increased at the final 30 min of 3.2 μg kg-1min-1 epinephrine infusion (Fig. 4). There was no significant increase in SAP at this epinephrine dose. The SAP/PAP ratio was not changed with epinephrine infusions (Table 3). Sustained and significant increases in CI were found at all doses of epinephrine. Calculated SVRI was decreased significantly with lower doses of epinephrine (0.2 and 1.0 μg kg-1min-1), but calculated PVRI was not different from the 1 h hypoxia value at any dose (Fig. 5). No significant change was found in PVFI, THFI (Fig. 6), and calculated MVRI with any dose of epinephrine. HAFI was increased significantly with 0.2 μg kg-1 min-1 epinephrine. The CI with 3.2 μg kg-1 min-1 epinephrine was significantly higher than during dopamine infusion at any dose. The increase in HAFI, at 0.2 μg kg-1 min-1, was significantly higher than that produced by any dose of dopamine.
Figure 4.
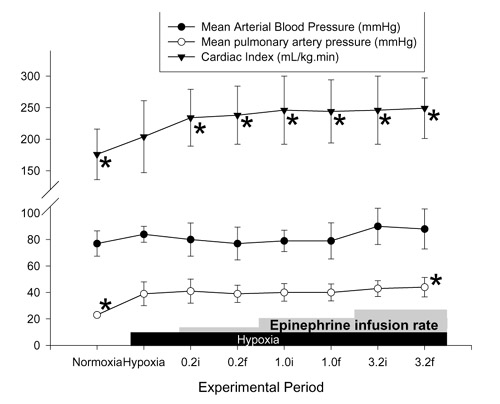
Effects of hypoxia and epinephrine infusion on cardiac index, and systemic and pulmonary artery pressures. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
Figure 5.
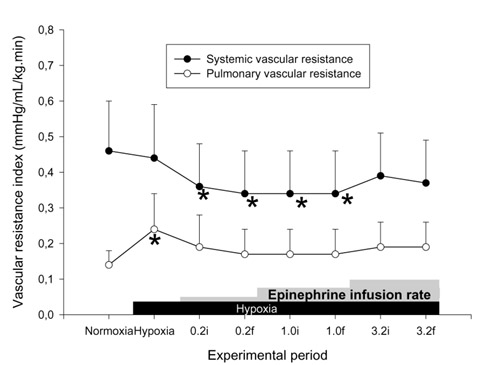
Effects of hypoxia and epinephrine infusion on systemic and pulmonary vascular resistance indices. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
Figure 6.
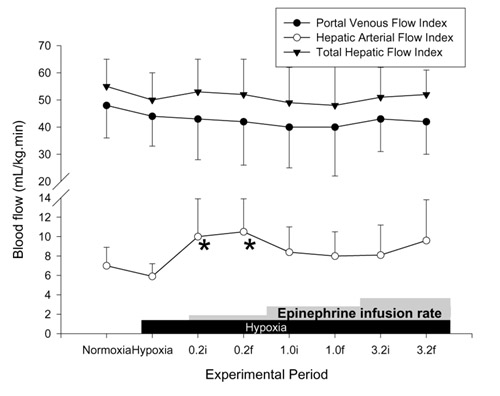
Effects of hypoxia and epinephrine infusion on portal venous, hepatic arterial, and total hepatic blood flows. i, initial (3 min average at 30 min of infusion at that dose); f, final (3 min average at 60 min of infusion). *P < 0.05 compared with effects of hypoxia.
There was no change in mesenteric vascular resistance and increase in CI with epinephrine; there was therefore a trend to a decrease in the total hepatic blood flow when expressed as a proportion of cardiac output, which was not statistically significant.
There were no significant changes in systemic EO2, systemic VO2, splanchnic EO2, splanchnic VO2, and hepatic DO2 during epinephrine infusion in comparison with the 1 h baseline. A significant elevation in hepatic DO2 was found at the final recording obtained during 0.2 μg kg-1 min-1 epinephrine (at 1 h), and this was significantly elevated compared with the baseline hypoxia and all doses of dopamine. The serum lactate was elevated by 1 h of hypoxia to a level equivalent to that in the dopamine group, and was further elevated by either 1.0 or 3.2 μg kg-1 min-1 epinephrine (but not by 0.2 μg kg-1 min-1).
Discussion
Both dopamine and epinephrine are commonly used medications in the treatment of shock and hypotension in sick newborns. Our study is the first that compares the effects of dopamine and epinephrine infusions on regional hemodynamics and oxygen metabolism in a newborn mammal. It is also important to realize that all previous studies of the effects of inotropes in the newborn have used infusions for a maximum of 15-20 min. The prolonged inotrope infusions in our experiment are unique and are somewhat more relevant to the problem of cardiovascular support for the critically ill newborn, who might receive these drugs for hours or days.
Similarly, many newborns receiving these drugs are hypoxic, receive large doses of opiates to reduce instability, are critically ill and stressed, and might have recently had major surgery. Thus, although acutely instrumented models are often criticized for being 'unphysiologic,' the stress of surgery might, in some ways, represent the clinical situation in which these drugs are actually used more accurately than healthy, chronically instrumented, models. Nevertheless, the animal model employed in the present study does not completely mirror the conditions in critically ill newborn humans. Although sick hypoxic newborns are usually hypotensive as well, it is also important to realize that the animals had no underlying disease condition; some such conditions, for example sepsis, might modify responses to infused catecholamines. Because of potential differences in drug metabolism, the number, affinity, and maturation of adrenergic receptors, and cardiovascular reflexes, the responses described to any inotropic agent in a non-human mammal should be taken as only a guide to potential effects, which must be confirmed in human newborns.
We chose the empirical doses in this comparison study on the basis of our previous paper showing that a tenfold higher dose of dopamine achieves a similar increase in CI to that of epinephrine [9]. The random order of administration of the doses was designed to eliminate the possible effects of bias related to progressive cumulative doses and the duration of systemic hypoxia. There is no commercially available co-oximetry system specifically designed for piglet blood. The only commonly used oximeter with animal coefficients, the IL282, does not include piglet blood settings. However, we have previously shown, when using these devices, that the apparent carboxyhemoglobin is erroneously elevated when using blood with very different optical characteristics [10]; the apparent carboxyhemoglobin levels in our piglets were almost always less than 2%, suggesting that the oxygen saturation values should be reliable. Furthermore, the trends shown are likely to be accurate, even if the actual values are somewhat imprecise.
This study confirms the differential responses in systemic, pulmonary, and mesenteric circulations with dopamine and epinephrine infusions that we have previously reported in anesthetized normoxic piglets [8]. Such responses differ from responses seen in adult subjects; these differences might be related to differential maturation of adrenoceptors and functional immaturity of the receptor mechanisms in newborns [11, 12], as well as to differences in the ultrastructure and metabolism of the myocardium [13, 14]. The ontogeny of the adrenoceptors seems to vary in the regional circulations and therefore the responses to inotropes are different in different vascular beds [15, 16, 17].
Our findings suggest that epinephrine, being both an α and a β adrenoceptor agonist, would be a more appropriate agent for use in inotropic support for hypoxic newborns if the same effects are present in the human infant. We demonstrated an increase in oxygen delivery consequent on the use of epinephrine during hypoxia; SAP was maintained and CI increased throughout the dose range (0.2-3.2 μg kg-1 min-1). An increase in cardiac output and oxygen delivery would be important in shocked hypoxic newborns. Dopamine did not affect the SAP and CI at any dose, although it might increase SAP and CI at a dose of 32 μg kg-1min-1 in normoxic conditions, as previously described in other studies [11, 18, 19, 20]. This is consistent with clinical reports showing that dopamine might increase blood pressure in hypotensive newborns but with no increase in cardiac output; indeed cardiac output seems to decrease [21]. In our previous experiment we did not demonstrate any further increase in CI with either dopamine or epinephrine infusions during hypoxia with arterial oxygen saturation between 45% and 50% [9]. The difference in the effects of hypoxia on the responses to inotropes of cardiac output in this and the previous study might well be related to the difference in the severity of the hypoxia [22].
O'Laughlin et al demonstrated an increase in cardiac output during dopamine infusion in hypoxic unanesthetized newborn lambs at a mean postnatal age of 6.5 days [23]. The differences in the results of the two studies might represent a species difference, a postnatal age effect, an anesthesia effect, or some other detail of the experimental maneuvers. The drug infusions in O'Laughlin's study were begun after 30 min of hypoxia; we have shown in a piglet model that 30 min is an insufficient period for the stabilization of cardiac output after initiation of this degree of hypoxia [24]. It could therefore be that the dopamine infusion in O'Laughlin's study was begun at a time when the cardiac output was still increasing. The doses also seem to have been given in sequential rather than random order, which can lead to apparent effects that are due to the order of administration rather than a true dosage effect. O'Laughlin also reported drug effects after 15 min; we did not measure hemodynamics at this time, so we might have missed transient effects of the drugs.
The relative effects of epinephrine on systemic and pulmonary pressures are potentially favourable if they can be reproduced in newborns with persistent pulmonary hypertension. The SAP/PAP ratio is crucially important for the direction of shunting across the ductus arteriosus, which determines the oxygen content of the blood distributed to various organs. In the presence of a lowered SAP/PAP ratio, owing to hypoxic pulmonary vasoconstriction, epinephrine did not alter the ratio but did increase cardiac output and therefore oxygen delivery. However, dopamine infusion at a high dose (32 μg kg-1 min-1) had a detrimental effect on the SAP/PAP ratio [25]; with no significant effect on CI this could lead to a decrease in tissue oxygen delivery if ductal shunt were reversed and systemic oxygen saturations fell as a consequence. The differences between the two drugs might well be because epinephrine is a potent β2 agonist, whereas dopamine has little effect at this receptor, and there seems to be enhanced β2-adrenoceptor responsiveness in the pulmonary vasculature during hypoxia [26].
Dopamine increased mesenteric flow at the highest dose (32 μg kg-1min-1). In our previous normoxic experiments there were no significant changes in PVFI and calculated MVRI with dopamine infusion [7, 8]. We have shown, with a selective agonist, active vasodilatation mediated by specific dopamine receptors in the mesenteric circulation of the newborn piglet [7]. Thus hypoxia seems to have enhanced the vasodilatory efficacy of dopamine in the circulation of the bowel, which might be via downregulation of α receptors [27] in the mesenteric circulation and/or increased effects of stimulating dopaminergic receptors. However, despite this apparent beneficial effect in the mesenteric blood flow, we did not investigate the mucosal blood flow in the gut, which is particularly vulnerable to hypoxic-ischemic insult. Indeed, a harmful effect of dopamine infusion on the mucosal blood flow has been reported [28]. Whereas epinephrine infusion showed a vasoconstrictive effect on the mesenteric vasculature in the previous normoxic experiment [8], this decrease in mesenteric flow was not apparent in this hypoxic model; the possible mechanisms for this difference include an effect of hypoxia on the activity of α receptors, and an enhanced responsiveness to β2 stimulation. Thus the differences in both the epinephrine and dopamine responses during hypoxia would be explained by a reduction in α-mediated vasoconstriction.
Epinephrine infusions should be used cautiously despite the lack of effects on the bowel circulation seen in this study, in view of the results of the previous study, which did show a reduction in bowel perfusion during epinephrine infusion at high dose [8]. Vasoconstriction with high doses of epinephrine could subject the hypoxic bowel in sick newborns to ischemic injury and increase the risk for the development of necrotizing enterocolitis [29, 30].
Dopamine demonstrates a potentially hepatoprotective effect at its highest dose. At 32 μg kg-1 min-1, dopamine improved hepatic DO2 as a result of mesenteric vasodilatation without a concomitant increase in splanchnic EO2 or splanchnic VO2. The increase in HAFI and hepatic DO2 ratio with 0.2 μg kg-1 min-1 epinephrine infusion is interesting. It demonstrates a probable β2-vasodilatation effect with epinephrine at low dose during hypoxia (as also reflected in the decrease in calculated SVRI); a low dose of epinephrine could also be protective and improve hepatic perfusion and oxygen delivery in hypoxic newborns. Further studies on hepatic perfusion and oxygen metabolism in systemic hypoxia are required for an evaluation of the hepatoprotective role of inotropes.
No effect on systemic or splanchnic VO2 or EO2 was demonstrated with either inotrope despite the increase in systemic oxygen delivery with epinephrine infusions. Anaerobic metabolism is the main source of ATP production during hypoxia. It is advantageous for the tissue to minimize oxygen consumption during systemic hypoxia [31]. Although we require cautious interpretation of the negative findings because of the small sample size and thus the limited statistical power, we did not show an effect on oxygen metabolism with either catecholamine. A dopamine-related increase in oxygen consumption has been shown in a study of endotoxic dogs during normoxia [32]. In the same experiment, during a 30 min hypoxic challenge, a decrease in systemic VO2 with no improvement in systemic EO2 was demonstrated. We did not confirm this in our study, which might be related to the difference in oxygen metabolism in isolated hypoxia as opposed to hypoxia and sepsis, and to the duration of hypoxia between studies.
Conclusion
During severe alveolar hypoxia in the newborn piglet, epinephrine increases cardiac output whereas dopamine has no effect. Epinephrine preserves the SAP/PAP ratio, whereas dopamine causes pulmonary vasoconstriction. Epinephrine has no effect on splanchnic blood flow, whereas dopamine increases both portal and total hepatic flow. A reconsideration of the approach to the sick newborn infant is warranted.
Abbreviations
CI = cardiac index; EO2 = oxygen extraction; HAFI = hepatic arterial flow index; hepatic DO2 = hepatic oxygen delivery; hepatic DO2 ratio = ratio of hepatic arterial oxygen delivery to total hepatic oxygen delivery; MVRI = mesenteric vascular resistance index; PAP = mean pulmonary arterial pressure; PVFI = portal venous flow index; PVRI = pulmonary vascular resistance index; SaO2 = arterial saturation; SAP = mean systemic arterial pressure; SpO2 = portal venous saturation; SvO2 = mixed venous saturation; SVRI = systemic vascular resistance index; VO2 = oxygen consumption; THFI = total hepatic flow index.
Acknowledgments
Acknowledgement
This study was supported by the Heart and Stroke Foundation of Canada and Perinatal Research Centre, University of Alberta, Edmonton, Canada.
References
- Zaritsky A, Chernow B. Use of catecholamines in pediatrics. J Pediatr. 1984;15:341–350. doi: 10.1016/s0022-3476(84)80003-7. [DOI] [PubMed] [Google Scholar]
- Driscoll DJ, Pinsky WW, Entman ML. How to use inotropic drugs in children. Drug Ther. 1979;9:124–134. [Google Scholar]
- Driscoll DJ, Gillette PC, Lewis RM, Hartley CJ, Schwartz A. Comparative hemodynamic effects of isoproterenol, dopamine and dobutamine in the newborn dog. Pediatr Res. 1979;13:1006–1009. doi: 10.1203/00006450-197909000-00011. [DOI] [PubMed] [Google Scholar]
- Roze J, Tohier C, Maingneneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child. 1993;69:59–63. doi: 10.1136/adc.69.1_spec_no.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak M, Drummond WH. Systemic and pulmonary vascular effects of selective dopamine receptor blockade and stimulation in lambs. Pediatr Res. 1993;33:181–184. doi: 10.1203/00006450-199302000-00018. [DOI] [PubMed] [Google Scholar]
- Pawlik W, Mailman D, Shanbour LL, Jacobson ED. Dopamine effects on the intestinal circulation. Am Heart J. 1976;91:325–331. doi: 10.1016/s0002-8703(76)80216-5. [DOI] [PubMed] [Google Scholar]
- Pearson RJ, Barrington KJ, Jirsch DW, Cheung PY. Dopaminergic receptor-mediated effects in the mesenteric vasculature and renal vasculature of the chronically instrumented newborn piglet. Crit Care Med. 1996;24:1706–1712. doi: 10.1097/00003246-199610000-00018. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Barrington KJ, Pearson RJ, Bigam DL, Finer NN, Van Aerde JE. Systemic, pulmonary and mesenteric perfusion and oxygenation effects of dopamine and epinephrine. Am J Respir Crit Care Med. 1997;155:32–37. doi: 10.1164/ajrccm.155.1.9001285. [DOI] [PubMed] [Google Scholar]
- Barrington KJ, Finer NN, Chan WKY. A blind, randomized comparison of the circulatory effects of dopamine and epinephrine infusions in the newborn piglet during normoxia and hypoxia. Crit Care Med. 1995;23:740–48. doi: 10.1097/00003246-199504000-00024. [DOI] [PubMed] [Google Scholar]
- Ryan CA, Barrington KJ, Vaughan D, Finer NN. Directly measured arterial oxygen saturation in the newborn infant. J Pediatr. 1986;109:526–529. doi: 10.1016/s0022-3476(86)80137-8. [DOI] [PubMed] [Google Scholar]
- Gootman PM, Buckley NM, Gootman N. Postnatal maturation of the central neural cardiovascular regulatory system. Fetal and Newborn Cardiovascular Physiology. Edited by Longo LD, Reneau DD. New York: Garland Press; 1978;vol 1:93–152. [Google Scholar]
- Vapaavouri EK, Shinebourne EA, Williams RL, Heymann MA, Rudolph AM. Development of cardiovascular responses to autonomic blockade in intact fetal and neonatal lambs. Biol Neonate. 1973;22:177–188. doi: 10.1159/000240552. [DOI] [PubMed] [Google Scholar]
- Smith RE, Page E. Ultrastructural changes in rabbit heart mitochondria during the perinatal period. Dev Biol. 1977;57:109–117. doi: 10.1016/0012-1606(77)90358-x. [DOI] [PubMed] [Google Scholar]
- Lopaschuk GD, Collins-Nakai RL, Toshiyuki I. Develomental changes in energy substrate use by the heart. Cardiovasc Res. 1992;26:1172–1180. doi: 10.1093/cvr/26.12.1172. [DOI] [PubMed] [Google Scholar]
- Gootman N, Budley BJ, Gootman PM, Nagelberg JS. Age related effects of single injections of dopamine on cardiovascular function in developing swine. Dev Pharmacol Ther. 1982;4:139–150. doi: 10.1159/000457403. [DOI] [PubMed] [Google Scholar]
- Gootman N, Budley BJ, Gootman PM, Griswold PG, Mell JD, Nudel DB. Maturation in related changes in regional circulating effects of dopamine infusion in swine. Dev Pharmacol Ther. 1983;6:9–22. doi: 10.1159/000457273. [DOI] [PubMed] [Google Scholar]
- Feltes T, Hansen TN, Martin CG, Leblanc AL, Smith S, Giesler ME. The effects of dopamine infusion on regional blood flow in newborn lambs. Pediatr Res. 1987;21:131–136. doi: 10.1203/00006450-198702000-00005. [DOI] [PubMed] [Google Scholar]
- Vane D, Weber TR, Caresky J, Grosfeld JL. Systemic and renal effects of dopamine in the infant pig. J Surg Res. 1982;32:477–483. doi: 10.1016/0022-4804(82)90129-9. [DOI] [PubMed] [Google Scholar]
- Fiser DH, Fewell JE, Hill DE, Brown AL. Cardiovascular and renal effects of dopamine and dubutamine in healthy conscious piglets. Crit Care Med. 1988;16:340–3445. doi: 10.1097/00003246-198804000-00007. [DOI] [PubMed] [Google Scholar]
- Girardin E, Berner M, Rouge JC, Rivest RW, Friedli B, Paunier L. Effect of low dose dopamine on hemodynamic and renal function in children. Pediatr Res. 1989;26:200–203. doi: 10.1203/00006450-198909000-00009. [DOI] [PubMed] [Google Scholar]
- Roze JC, Tohier C, Maingueneau C, Lefevre M, Mouzard A. Response to dobutamine and dopamine in the hypotensive very preterm infant. Arch Dis Child. 1993;69:59–63. doi: 10.1136/adc.69.1_spec_no.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng ML, Levy MN, DeGeest H, Zieske H. Effects of myocardial hypoxia on left ventricular performance. Am J Physiol. 1966;211:43–50. doi: 10.1152/ajplegacy.1966.211.1.43. [DOI] [PubMed] [Google Scholar]
- O'Laughlin MP, Fisher DJ, Dreyer WJ, O'Brian ES. Augmentation of cardiac output with intravenous catecholamines in unanesthetized hypoxemic newborn lambs. Pediatr Res. 1987;22:667–674. doi: 10.1203/00006450-198712000-00011. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Barrington KJ, Bigam DL. Temporal effects of prolonged hypoxaemia and reoxygenation on systemic, pulmonary and mesenteric perfusions in newborn piglets. Cardiovasc Res. 1998;39:451–458. doi: 10.1016/s0008-6363(98)00080-7. [DOI] [PubMed] [Google Scholar]
- Mentzer R, Alegre CA, Nolan SP. The effect of dopamine and isoproterenol on the pulmonary circulation. J Thorac Cardiovas Surg. 1976;71:807. [PubMed] [Google Scholar]
- Lock JE, Olley PM, Coceani F. Enhanced β adrenergic receptor responsiveness in hypoxic neonatal pulmonary circulation. Am J Physiol. 1981;240:H697–H703. doi: 10.1152/ajpheart.1981.240.5.H697. [DOI] [PubMed] [Google Scholar]
- Tateishi J, Faber JE. ATP-sensitive K+ channels mediate α2D-adrenergic receptor contraction of arteriolar smooth muscle and reversal of contraction by hypoxia. Circ Res. 1995;76:53–63. doi: 10.1161/01.res.76.1.53. [DOI] [PubMed] [Google Scholar]
- Neviere R, Mathieu D, Chagnon JL, Lebleu N, Wattel F. The contrasting effects of dobutamine and dopamine on gastric mucosal perfusion in septic patients. Am J Respir Crit Care Med. 1996;154:1684–1688. doi: 10.1164/ajrccm.154.6.8970355. [DOI] [PubMed] [Google Scholar]
- Ballance WA, Dahms BB, Shenker N, Kliegman RM. Pathology of neonatal necrotizing enterocolitis: a ten year experience. J Pediatr. 1990;117:S6–S13. doi: 10.1016/s0022-3476(05)81124-2. [DOI] [PubMed] [Google Scholar]
- Konto WP, Jr, Wilson R. Epidemiology of necrotizing enterocolitis with etiologic implications. Perinatol Neonatol. 1983;7:63–68. [Google Scholar]
- Suguihara C, Bancalari E, Hehre D, Duara S, Gerhardt T. Changes in ventilation and oxygen consumption during acute hypoxia in sedated newborn piglets. Pediatr Res. 1994;35:536–540. [PubMed] [Google Scholar]
- Cain SM, Curtis SE. Systemic and regional oxygen uptake and delivery and lactate flux in endotoxic dogs infused with dopexamine. Crit Care Med. 1991;19:1552–1560. doi: 10.1097/00003246-199112000-00019. [DOI] [PubMed] [Google Scholar]


