Abstract
Concurrent radiochemotherapy for medulloblastoma includes the microtubule disrupting agent vincristine; however, vincristine alone or as part of a combined treatment regimen is highly toxic. A major goal is therefore to replace vincristine with novel potent chemotherapeutic agents—in particular, with microtubule stabilizing and destabilizing compounds—with a larger therapeutic window. Here, we investigated the antiproliferative, cytotoxic and radiosensitizing effect of patupilone (epothilone B [EPO906]), a novel, non–taxane-related and nonneurotoxic microtubule-stabilizing agent in human medulloblastoma cell lines. The antiproliferative and cytotoxic effects of patupilone alone and in combination with ionizing radiation was determined in the 3 representative human medulloblastoma cell lines D341Med, D425Med, and DAOY. Patupilone alone effectively reduced the proliferative activity and clonogenicity of all medulloblastoma cell lines tested at picomolar concentrations (50–200 pM) and resulted in an at least additive anticlonogenic effect in combination with clinically relevant doses of ionizing radiation (2 or 5 Gy). Cell-cycle analysis revealed a sequential G2-M arrest and sub-G1 accumulation in a dose- and treatment-dependent manner after exposure to patupilone. In tumor xenografts derived from D425Med cells, a minimal treatment regimen with patupilone and fractionated irradiation (1 × 2 mg/kg plus 3 × 3 Gy) resulted in an extended tumor growth delay for the 2 single treatment modalities alone and a supra-additive treatment response for the combined treatment modality, with complete tumor regressions. These results demonstrate the potent efficacy of patupilone against medulloblastoma cell lines and indicate that patupilone represents a promising candidate to replace vincristine as part of a combined treatment strategy with ionizing radiation.
Keywords: apoptosis, autophagy, ionizing radiation, medulloblastoma, patupilone
Medulloblastoma is the most common malignant brain tumor of childhood.1 Standard therapy for medulloblastoma comprises neurosurgical resection, radiotherapy, and chemotherapy. However, nearly one-half of all patients die of progressive disease, and survivors experience considerable adverse effects,2–4 including radiation-dependent reduction of neurocognitive performance.5–8 Medulloblastoma is a relatively radiation sensitive tumor entity. Concurrent radiochemotherapy for medulloblastoma often includes the microtubule-disrupting agent vincristine. However, use of vincristine alone or as part of a combined treatment regimen is highly toxic;3,9 therefore, treatment modifications have been reported frequently.10–12 Of note, excellent outcomes were reported in a multi-institution phase II trial in which patients with average- and high-risk medulloblastoma were treated with risk-adapted craniospinal radiotherapy without concurrent vincristine therapy. In this trial, vincristine was only part of the dose-intensive postirradiation treatment (cyclophosphamide, cisplatin, and vincristine), challenging the value of concomitant vincristine treatment during radiotherapy.13 Therefore, a major goal is to replace vincristine, as part of multimodality treatment regimens, with novel potent chemotherapeutic agents, such as carboplatin, which is under clinical investigation, either a) together with vincristine during craniospinal radiotherapy—as in the COG 99701 trial (ClinicalTrials.gov number NCT00003203) for patients with metastatic medulloblastoma14— b) with the aim of substituting vincristine during craniospinal radiotherapy—as in the future SIOP-Europe PNET 6 medulloblastoma study for “standard-risk” patients with medulloblastoma—or c) with other promising microtubule-stabilizing and -destabilizing agents, which have been investigated thoroughly in the preclinical setting and which show promising activity against a panel of xenograft-derived embryonal tumors.15
Patupilone (epothilone B [EPO906]), which is currently being tested in phase III clinical trials,16 is a novel microtubule-targeting cytotoxic agent that is structurally unrelated to paclitaxel and docetaxel. Patupilone is 3–20 times more potent than the taxanes in vitro and has shown a safe toxicity profile in adults.17 Patupilone stabilizes preformed microtubules and leads to aberrant spindle formation during mitosis. Patupilone has been shown to induce regression of various types of human tumors in vivo, including glioma, lung, colon, breast, prostate, and ovarian carcinomas. Of note and in contrast to paclitaxel, patupilone is equally cytotoxic to paclitaxel-susceptible and paclitaxel-resistant cells that display a multidrug-resistance phenotype due to overexpression of the P-glycoprotein (P-gp) efflux pump.17 The combined treatment with patupilone and ionizing irradiation showed a supra-additive effect in vitro and in vivo in lung and colon carcinoma models.18,19 Combination of ionizing radiation and patupilone has also been tested in a phase I trial involving brain tumors.20
We investigated the efficacy of patupilone alone and in combination with ionizing radiation in different human medulloblastoma cell lines. We showed that patupilone alone is extremely potent at picomolar concentrations in medulloblastoma cell lines, and combined treatment with clinically relevant doses of ionizing radiation results in an at least additive anticlonogenic effect in vitro and induces a strong supra-additive treatment response in vivo.
Materials and Methods
Cell Cultures, Reagents, and Irradiation
Patupilone was kindly provided by Novartis Pharma. D425Med human medulloblastoma cells were purchased from the American Type Culture Collection. D341Med and D425Med human medulloblastoma cells were the kind gift of Dr Henry Friedman (Duke University). All medulloblastoma cells were cultured in Richter's zinc option medium supplemented with 10% fetal bovine serum (nonessential amino acids were added to the medium of D341Med and D425Med cells to a final concentration of 1%). All cell cultures were maintained at 37°C in a humidified atmosphere with 5% CO2.
For in vitro assays, a stock solution (1 mM) of patupilone was prepared in DMSO and further diluted with water/DMSO- and serum-containing media. Irradiation was performed at room temperature, using a Pantak Therapax 300 kV X-ray unit at 0.7 Gy/min. 3-Methyladenine (Sigma) was prepared in water at a stock solution of 100 mM and further diluted in serum-containing media. Bafilomycin-1 (Sigma) was prepared in DMSO at a stock solution of 100 μM and further diluted in serum-containing media. The broad-range caspase inhibitor z-VAD-FMK (Calbiochem; Merck Chemicals) was prepared in DMSO at a concentration of 10 μM and further diluted in serum-containing media.
Cell Proliferation, Clonogenic Cell Survival, and Cell Viability Assay
Proliferative activity was assessed in 96-well F-plates using the Alamar Blue (Biosource International) and MTS assays (Promega). Absorption was measured at 570 and 630 nm (Alamar blue) using a GenTec spectrophotometer or at 490 nm (MTS) using a microplate spectrophotometer (Molecular Devices).19,30,31 The half-maximal inhibitory concentration (IC50) values were calculated from the regression curve using GraphPad Prism software, version 4 (GraphPad Software).30 Each experiment was performed at least in triplicate. For the cell viability assay, 400 000 or 500 000 cells were seeded and treated 24 h thereafter. Cell viability was determined 72 h after treatment began using the trypan blue exclusion assay. Each experiment was performed at least in duplicate. To determine clonogenic cell survival, the number of single-seeded cells was adjusted to obtain 100 colonies per cell culture dish with a given treatment. After treatment with different regimens, cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and allowed to grow for 14 days before fixation in methanol/acetic acid (ratio, 75%:25%) and staining with crystal violet. Only colonies with >50 cells/colony were counted. For combined treatment, cells were pre-incubated with patupilone or control solution 1 h before irradiation. Clonogenic cell survival assays were repeated as independent experiments at least twice.
Cell-Cycle Analysis
For cell-cycle analysis, medulloblastoma cells were treated with patupilone for 6, 12, and 24 h, respectively. Both floating and adherent cells were collected. After washing twice in phosphate-buffered saline (PBS), cells were stained with propidium iodide (50 µg/mL) (Becton-Dickinson) in PBS containing 100 U/mL RNase A (Qiagen) for 30 min at room temperature. The percentage of cells in the different phases of the cell cycle was determined by evaluating DNA content, as described elsewhere.30,31
Caspase-3 Activity Assay
Asp-Glu-Val-Asp (DEVD)ase activity was determined in cytosolic cell extracts. Cells were treated with increasing concentrations of patupilone for 6, 12, 24, and 48 h. Cells were harvested thereafter by trypsin/EDTA, centrifuged, and washed with precooled PBS. The cell pellet was suspended in 5 volumes of precooled buffer A (20 mM HEPES-KOH [pH 7.5], 10 mM KCl, 1.5 mM MgCl2, 1 mM sodium EDTA, 1 mM sodium EGTA, 1 mM dithiothreitol [DDT], 250 mM sucrose, and 0.1 mM phenylmethylsulfonyl fluoride [PMSF] supplemented with protease inhibitors [5 mg/mL pepstatin A, 10 mg/mL leupeptin, 2 mg/mL aprotinin, 2 mg/mL DTT, and 1 mM of PMSF]). After incubation on ice for 15 min, the cells were disrupted by freezing and thawing. Cell lysates were centrifuged at 1000g for 10 min at 4°C, and the supernatant was further centrifuged at 100 000g for 30 min. The resulting supernatant (S-100 fraction) was stored at −80°C. To determine caspase 3-like activity, 75 μg of protein from the S-100 fraction was incubated at 37°C with the colorimetric caspase 3 substrate N-acetyl-Asp-Glu-Val-Asp p-nitroanilide (100 mM; Ac-DEVD-pNA; Calbiochem) and 1 mM dATP in a final volume of 120 μL. Cleavage of the caspase substrate was monitored at 405 nm using a GenTec spectrophotometer.
Detection and Quantification of Acidic Vesicular Organelles (AVOs) with Acridine Orange
To detect and quantify AVOs, cellular vital staining with acridine orange was performed. In acridine orange-stained cells, the cytoplasm and nucleolus fluoresce bright green and dim red, whereas the acidic compartments fluoresce bright red.32 The intensity of the red fluorescence isproportional to the degree of acidity and/or the volume of the cellular acidic compartment and was measured 6, 12, 24, and 48 hafter exposure to patupilone alone or 48 h after treatment with patupilone combined with ionizing radiation. After treatment with patupilone alone or in combination with irradiation, both adherent and suspended cells were stained at the indicated time points with acridine orange (1 mg/mL) for a period of 15 min, harvested by trypsin/EDTA, and collected in PBS. As a negative control, 0.5 μM bafilomycin A1 (Sigma) was added 30 min before acridine orange staining. Green (510–530 nm) and red (465 nm) fluorescence emission from 5 × 105 cells illuminated with blue (488 nm) excitation light was measured with a FACS Calibur from Becton Dickinson using CellQuest software. The ratio of red to green fluorescence was determined in control and treated cells and normalized in relation to untreated cells.
Tumor Xenograft in Nude Mice and Application of Treatment Regimes
D425Med cells (6 × 106) were injected subcutaneously on the backs of 4–6-week-old athymic nude mice. Tumor volumes were determined from caliper measurements of tumor length (L) and width (l) according to the formula (L x l2)/2. Tumors were allowed to expand to a volume of 200 mm3 (±10%) before treatment start. With the use of a customized shielding device, mice were given strictly loco regional radiotherapy of 3 × 3 Gy on 3 consecutive days using a Gulmay 200 kV X-ray unit at 100 cGy/min at room temperature. Patupilone (2 mg/kg; dissolved in 30% PEG-300/70% saline) was applied intravenously 24 h before the first treatment with ionizing radiation (at day 0 of the treatment; n= 5 per group). Tumor growth was monitored daily.
Results
Patupilone Strongly Reduces Proliferation and Viability in Human p53wt and p53mt Medulloblastoma Cell Lines
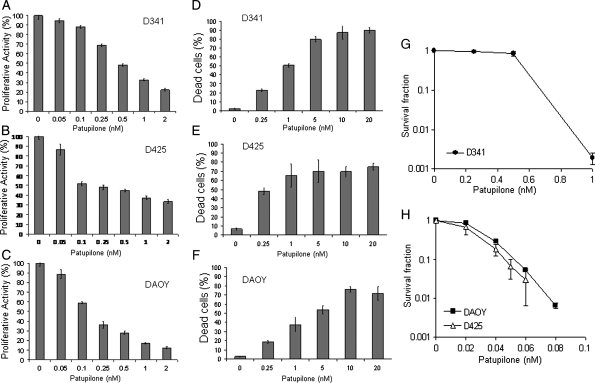
The antiproliferative effect of the microtubule-stabilizing agent patupilone was tested in the 3 representative human medulloblastoma cell lines D341Med, D425Med, and DAOY. Patupilone reduced the proliferative activity in the D341 cell line, with an IC50 of 0.53 nM (95% confidence interval [CI], 0.45–0.62) (Fig. 1A); in the D425Med cell line, with an IC50 of 0.37 nM (95% CI, 0.14–0.96) (Fig. 1B); and in the DAOY cell line, with an IC50 of 0.19 nM (95% CI, 0.12–0.29) (Fig. 1C). Likewise, cell viability, as detected by trypan blue exclusion, was reduced to 50% when treated with subnanomolar concentrations of patupilone (D341Med, 0.25 nM; D425Med and DAOY, 0.1 nM) (Fig. 1D–F). Of note, up to 10-fold higher IC50 values were obtained when the different cell lines were treated with the microtubule-destabilizing agent vincristine (Supplement 1).
Fig. 1.
Antiproliferative and cytotoxic effects of patupilone in human medulloblastoma cell lines. A–C, D341Med (A), D425Med (B), and DAOY (C) human medulloblastoma cells were treated with increasing doses of patupilone, and the antiproliferative activity was determined after 72 h of exposure. D–F, D341Med (D), D425Med (E), and DAOY (F) human medulloblastoma cells were treated with increasing doses of patupilone, and cell viability was determined by trypan blue exclusion after 72 h of exposure. (G) Clonogenicity of the D341Med cells after treatment with increasing doses of patupilone. (H) Clonogenicity of the D425Med and DAOY cells after treatment with increasing doses of patupilone. Results of a representative experiment are shown (n = 3).
Next, clonogenic cell survival was determined in the 3 cell lines after treatment with increasing concentrations of patupilone. In the D341Med cell line, the effect of patupilone on clonogenic survival was at dose range of patupilone similar to the level of proliferative activity and viability (IC50, 0.50–0.75 nM). However, the clonogenicity of D425Med and DAOY cells was already strongly reduced at a 10-fold lower concentration of patupilone (IC50, 30 pM) (Fig. 1G, H). These results overall demonstrate that patupilone is highly potent against different medulloblastoma cell lines. These medulloblastoma cell lines differ in the expression of and mutations in specific genes (eg, p53, c-myc). However, a differential treatment sensitivity so far cannot be attributed to a specific genetic background.21
Patupilone Sequentially Induces a G2-M-Phase Arrest and Apoptosis in Medulloblastoma Cell Lines
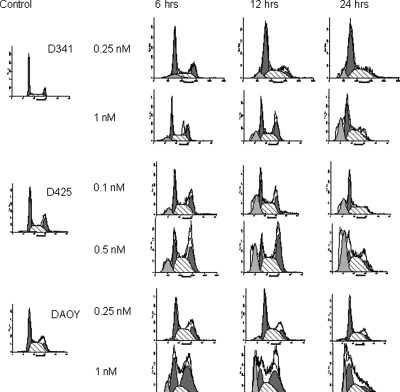
To investigate patupilone-induced alterations of cell cycle progression, we determined the cell-cycle distribution over time in the 3 medulloblastoma cell lines following treatment with low and high concentrations of patupilone (0.1–1 nM). Low-dose treatment with patupilone resulted in minor changes in cell-cycle distribution in all 3 cell lines but also in a small accumulation of cells in a sub-G1-peak in the D341Med and D425Med cell population, which is indicative of apoptosis. On the other hand, exposure to increased concentrations of patupilone resulted in extended G2-M-phase accumulation in all 3 medullobastoma cell lines (D341Med and DAOY cell lines, 1 nM; D425Med cell line, 0.5 nM patupilone) (Fig. 2). Twelve hours after patupilone exposure, 33.3% (D341Med), 40.6% (D425Med), and 46.2% (DAOY) of cells were accumulated in the G2-M phase, compared with 16.5% (D341Med), 26.3% (D425Med), and 17.5% (DAOY) of the untreated cell populations (see Table I). Accumulation of cells in the G2-M phase was most prominent in the DAOY cell line, probably because of an inactive G1 checkpoint. After the patupilone-induced G2-M-phase redistribution, extended accumulation of cells in a subG1-peak was observed in the D341Med and DAOY cells after treatment with 1 nM patupilone and in the D425Med cells after treatment with 0.5 nM of patupilone, again indicative of patupilone-induced, late apoptosis. These results demonstrate a dose-dependent sequential antiproliferative and cytotoxic effect of patupilone in the medulloblastoma cell lines.
Fig. 2.
Cell-cycle analysis after treatment with patupilone. Human medulloblastoma cell lines D341Med (top row), D425Med (middle row), and DAOY (bottom row) were treated with patupilone at low concentrations (D425Med and DAOY, 0.1 nM; D341Med, 0.25 nM) or high concentrations (D425Med, 0.5 nM; D341Med and DAOY, 1 nM) and subjected to cell-cycle determination by propidium iodide staining and FACS analysis at the indicated time points.
Table 1.
Cell-cycle analysis following treatment with high doses of patupilone.
| Cell line | 0 h (%) | 12 h (%) | 24 h (%) | |
|---|---|---|---|---|
| D341 | G0-1 | 53 | 30.22 | 42.53 |
| G2-M | 17 | 33.26 | 12.55 | |
| S | 30 | 36.52 | 44.92 | |
| sub-G1 | 0.6 | 16.85 | 26.03 | |
| D425 | G0-1 | 39.7 | 15.36 | 21.52 |
| G2-M | 26 | 40.65 | 21.74 | |
| S | 34 | 44 | 56 | |
| sub-G1 | 2 | 22.47 | 38.32 | |
| DAOY | G0-1 | 35 | 25.2 | 44.87 |
| G2-M | 17.5 | 46.21 | 25.05 | |
| S | 47 | 28.6 | 30.08 | |
| sub-G1 | 1 | 16.6 | 14.79 |
Patupilone Induces Apoptosis and Autophagy in Medulloblastoma Cell Lines
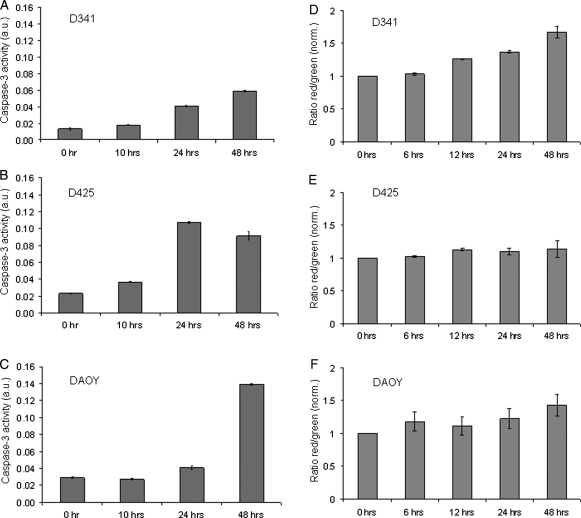
To further investigate patupilone-induced apoptosis, caspase-3 activity was assessed in the 3 medulloblastoma cell lines treated with the same concentrations as used for FACS analysis (see above). Caspase-3 activity was increased over time in the D425Med and the DAOY cell lines (Fig. 3B and 3C) and, to a smaller extent, in the D341Med cell line, reflecting the outcome of subG1-accumulation, as obtained by FACS analysis (Fig. 3A). Of note, pretreatment with the broad-range caspase inhibitor z-VAD-FMK did not rescue D425Med or DAOY cell lines from undergoing cell death, as assessed by the trypan blue viability assay (data not shown).
Fig. 3.
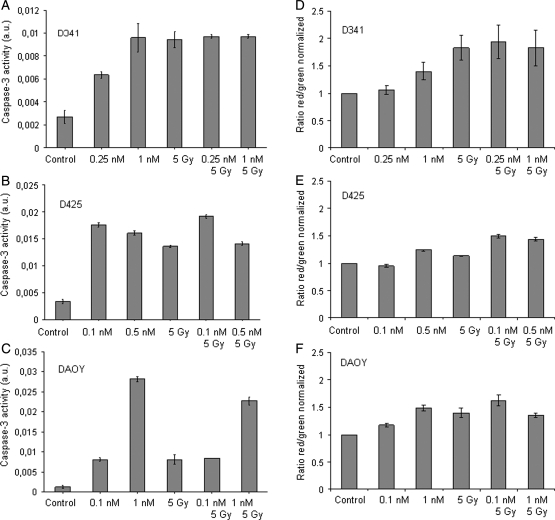
Caspase-3 activity and formation of acidic vesicular organelles (AVOs) after treatment with patupilone. Caspase-3 activity in patupilone-treated medulloblastoma cells (D341Med [A], D425Med [B], and DAOY [C]) was determined at the indicated time points. (D–F) AVO formation was determined in patupilone-treated medulloblastoma cells ([D] D341Med, 1 nM; [E] D425Med, 0.5 nM; [F] DAOY, 1 nM). Green (510–530 nm) and red (465 nm) fluorescence emission from 5 × 105 cells illuminated with blue (488 nm) excitation light was measured with a FACSCalibur at the indicated time points.
Comparable treatment sensitivity but a reduced amount of apoptosis in the D341Med cell line suggested another form of cell death induced by patupilone in this cell line. As a marker for autophagy, the fractional volume of acidic vesicular organelles (AVO) in control and patupilone-treated cells was quantified. A time-dependent increase in the amount of AVOs was exclusively observed in the D341Med (Fig. 3D) but not in the D425Med and DAOY medulloblastoma cell lines (Fig. 3E and 3F). To inhibit the autophagic process induced by patupilone, cells were pretreated with bafilomycin A1, which prevents the fusion of autophagosomes with lysosomes. High concentrations of bafilomycin A1 alone (100 nM) were not toxic to the D341Med cells, but interestingly, pretreatment with bafilomycin A1 strongly sensitized D341Med cells to patupilone (0.5 nM), resulting in nearly 100% dead cells (Supplementary Fig. S1). To determine a putative switch to apoptotic cell death after inhibition of autophagy, caspase-3 activity was assessed in cells pretreated with bafilomycin A1. No additional increase in caspase-3 activity could be detected after patupilone treatment (data not shown). These results suggest that after a G2-M-phase arrest, patupilone induces late apoptosis in the medulloblastoma cell lines D425Med and DAOY. On the other hand, D341Med cells are apoptosis resistant, and patupilone-induced autophagy might initially protect these cells from undergoing cell death not related to apoptosis.
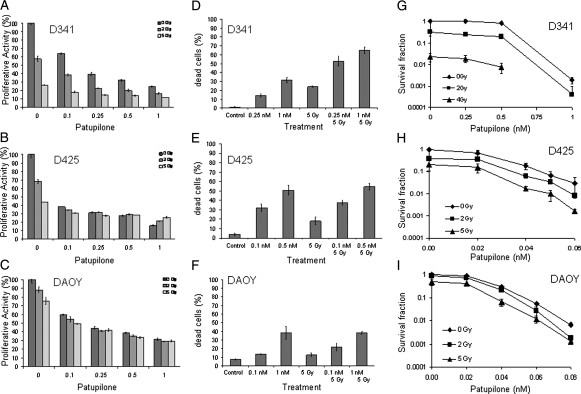
Patupilone Sensitizes Medulloblastoma Cell Lines to Ionizing Radiation
Patupilone is a promising agent for combined treatment with ionizing radiation. We therefore investigated its antitumor effect in these 3 medulloblastoma cell lines in combination with increasing doses of ionizing radiation. The D341Med cell line was clearly more radiosensitive than the D425Med and DAOY cell lines with regard to the level of proliferative activity, viability, and clonogenic survival (Fig. 4A–C). On the level of the short-term end points (ie, proliferative capacity and viability), the combined treatment modality induced an additive effect in the D341Med cell line but not in the D425Med and DAOY cell lines (Fig. 4A–F). More important, combined treatment reduced clonogenicity in all cell lines in an at least additive and comparable extent. As expected from the response to patupilone alone (see above), the D425Med and DAOY cell lines were sensitized to ionizing radiation at a 10-fold lower concentration of patupilone, compared with the D341Med cells (Fig. 4G–I). On the basis of the additive effects of combined treatment on the level of cell viability and clonogenicity, caspase-3 activity and the fractional volume of acidic vesicular organelles were determined in response to treatment. Caspase-3 activity in response to irradiation was least induced in the DAOY cells; however, no additive caspase-3 activation could be observed in all 3 cell lines in response to combined treatment with ionizing radiation (Fig. 5A–C). The autophagy-related end point was most prominently induced in the D341Med cell line in response to irradiation (Fig. 5D–F); however, a minor additive effect could only be detected in the D425Med cell line in response to the combined treatment modality. Overall, these data demonstrate that patupilone enhances the effect of ionizing radiation in human medulloblastoma cell lines with regard to viability and clonogenicity. However, combined treatment of these meduloblastoma cell lines does not result in enhanced amounts of apoptosis or autophagy in these cell lines.
Fig. 4.
Antiproliferative and cytotoxic effect of patupilone in combination with ionizing radiation. (A–C) Human medulloblastoma cell lines D341Med (A), D425Med (B), and DAOY (C) were treated with patupilone alone or in combination with clinically relevant doses of ionizing radiation (2–5 Gy). Patupilone was added 30–60 min before irradiation. The antiproliferative activity was determined after 72 h of exposure. (D–F) Human medulloblastoma cell lines D341Med (D), D425Med (E) and DAOY (F) were treated with patupilone at low (D425Med and DAOY, 0.1 nM; D341Med, 0.25 nM) or high (D425Med, 0.5 nM; D341Med and DAOY, 1 nM) concentrations, alone or in combination with ionizing radiation (5 Gy), and cell viability was determined by trypan blue exclusion after 72 h of exposure. (G–H) Clonogenicity of human medulloblastoma cells after treatment with increasing doses of patupilone or in combination with ionizing radiation.
Fig. 5.
Apoptosis and autophagy after combined treatment with patupilone and ionizing radiation. (A–C) Human medulloblastoma cell lines D341Med (A), D425Med (B), and DAOY (C) were treated with patupilone at low (D425Med and DAOY, 0.1 nM; D341Med, 0.25 nM) or high (D425, 0.5 nM; D341Med and DAOY, 1 nM) concentrations, alone or in combination with ionizing radiation. Caspase-3 activity was determined after 48 h. (D–F) Human medulloblastoma cell lines D341Med (D), D425Med (E), and DAOY (F) were treated with patupilone at low (D425Med and DAOY, 0.1 nM; D341Med, 0.25 nM) or high (D425Med, 0.5 nM; D341Med and DAOY, 1 nM) concentrations, alone or in combination with ionizing radiation. Green (510–530 nm) and red (465 nm) fluorescence emission from 5 × 105 cells illuminated with blue (488 nm) excitation light was measured with a FACSCalibur at the indicated time points or after 48 h.
Radiosensitizing Effect of Patupilone on Tumor Xenografts
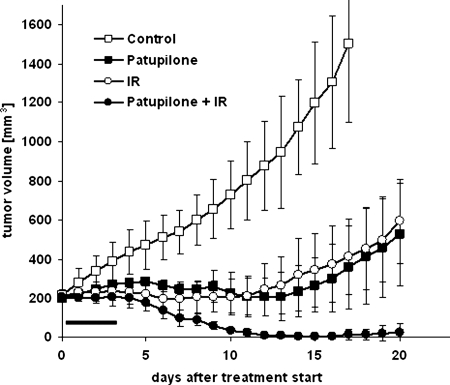
To determine an at least additive effect of the combined treatment modality in vivo, a combined treatment regimen with patupilone and ionizing radiation was tested against xenografts derived from human D425Med medulloblastoma cells, which were subcutaneously injected into the backs of nude mice. Treatment was started when tumors reached a minimal size of 200 mm3 ± 10% (on days 20–27 after cell injection). In vivo studies were performed with loco regional application of ionizing radiation using a shielding device and a minimal fractionated treatment regimen of 3 Gy on 3 consecutive days. This daily dose is applied when fractionated radiotherapy is used for the treatment of human malignancies. For practical reasons only 3 fractions were chosen as the treatment regimen, but the response to such a regimen was previously found to be useful for treatment evaluation.19 Fig. 6 summarizes the effect of tumor treatment with patupilone alone (2 mg/kg patupilone once), ionizing radiation alone (vehicle combined with 3 × 3 Gy), and patupilone and ionizing radiation in combination (2 mg/kg once combined with 3 × 3 Gy), compared with a vehicle alone–treated control group. Patupilone was applied 24 h before the first of 3 fractions of irradiation applied on 3 consecutive days. Determination of treatment-related body weight changes did not reveal a patupilone-dependent transient weight loss after patupilone application (data not shown), and no skin changes or tissue damage were observed in the co-irradiated healthy tissue area around the tumor during the follow-up period of tumor growth. Treatment with patupilone or ionizing radiation alone resulted in a partial tumor growth suppression over 10 days, whereas combined treatment exerted a strong supra-additive tumor growth control, with complete tumor regression in the follow-up period (P< .005, for ionizing radiation or patupilone alone vs combined treatment) (Fig. 6). Interestingly, tumors only slowly regressed after the end of treatment, coinciding with the in vitro results that treatment-induced apoptosis might only play a minor role for the treatment response of these medulloblastoma cells to ionizing radiation and patupilone. Complete visible tumor regression was observed in all mice treated with the combined treatment modality. In 2 of 5 mice, slow-growing tumor recurrences could be observed 25–30 days after the start of treatment. No recurrences at all occurred in the remaining cohort of mice treated with the combined treatment modality (data not shown). Overall, these results demonstrate that patupilone might be a promising alternative for a combined treatment regimen using microtubule inhibitors and ionizing radiation.
Fig. 6.
The effect of patupilone and ionizing radiation alone or in combination on the growth of D425Med-derived xenografts in nude mice. Mice were treated with patupilone (2 mg/kg once) and ionizing radiation (3 × 3 Gy on consecutive days), alone and in combination, with administration of patupilone or the vehicle 24 h prior to the first fraction of ionizing radiation. The horizontal bar indicates days of treatment. Each curve represents the mean tumor volume per group ± standard error.
Discussion
Vincristine-associated side effects in medulloblastoma have led to an intense search for novel microtubule-interfering agents with radiosensitizing potential and devoid of toxicities. Here, we investigated the effect of the novel clinically relevant microtubule inhibitor patupilone alone and in combination with ionizing radiation on 3 human medulloblastoma cell lines and determined a strong cytotoxic potency of patupilone at picomolar concentrations. Importantly, patupilone was 10-fold more potent than vincristine at inhibiting proliferation at subnanomolar concentrations (IC50 for patupilone, 0.1–0.25 nM; IC50 for vincristine, 1–1.4 nM) in all medulloblastoma cell lines tested. Cell-cycle analysis revealed that patupilone sequentially induced a strong G2-M-phase arrest in all cell lines, followed by signs of apoptosis in the 2 medulloblastoma cell lines D425Med and DAOY. In combination with ionizing radiation, an at least additive cytotoxic effect against both radiation-susceptible and radiation-resistant medulloblastoma tumor cell lines was observed. These results demonstrate a potent cytotoxic effect of patupilone alone and in combination with ionizing radiation, and they suggest that such a combined treatment modality qualifies for additional preclinical and clinical testing in medulloblastoma. Patupilone and other epothilone-derivatives are currently being tested in clinical phase II/III trials in adults, and there is an ongoing phase I/II trial of combined treatment with patupilone and radiotherapy in brain tumors.
We previously investigated the cytotoxic effect of patupilone alone or as part of a combined treatment modality with ionizing radiation against tumor cells derived from different tumor entities. Interestingly, the combination of patupilone with ionizing radiation resulted only in an additive cytotoxic effect against various cancer cell types only in vitro, but it resulted in a supra-additive tumor growth delay when tested against tumor xenografts derived from the same tumor cells as those tested in vitro.18,19 To the same extent, we could also now demonstrate an at least additive effect on combined treatment in the medulloblastoma cell lines in vitro and, more important, a strong supra-additive treatment response, including complete tumor regressions in tumor xenografts treated with a minimal combined treatment regimen in vivo. The accumulation of tumor cells in the most radiosensitive G2-M phase of the cell cycle represents the major rational for the sensitization to ionizing radiation,22–24 although other, S-phase progression-related mechanisms have also been observed.19 Additional anti-vascular and anti-angiogenic effects might contribute to the supra-additive tumor growth delay observed in vivo, and in fact, direct targeting of endothelial cells25–27 and indirect, anti-angiogenic interference with the secretion of pro-angiogenic factors from tumor cells have been proposed.
Interestingly, the semisynthetic epithilone B derivative ixabepilone has previously been investigated against several pediatric cancer models and revealed broad-spectrum activity.15 To our knowledge, this is the first report to have investigated the potency of patupilone alone and in combination with ionizing radiation in medulloblastoma cell lines and tumor xenografts, and we observed a differential cell line–dependent response with regard to the patupilone-induced mode of cell death. A strong G2-M-phase arrest was induced in all cell lines by patupilone 6 and 12 h after the commencement of treatment with low subnanomolar concentrations (0.1–1 nM). However, we also observed an initial longer-lasting accumulation of cells in the radioresistant S-phase in D425Med and D341 cell lines (data not shown), as previously manifested in other tumor cells in response to low-dose treatment with patupilone.19 The combined treatment with ionizing radiation in all cell lines resulted in an at least additive cytotoxic effect. After G2-M-phase arrest, patupilone potently induced apoptosis in the D425Med and the DAOY medulloblastoma cell lines, as indicated by caspase-3 activation and the occurrence of a subG1-peak cell population by flow cytometry. The D341Med medulloblastoma cell line was less susceptible to patupilone in terms of proliferation, clonogenic cell survival, and the apoptosis level, with an IC50 of patupilone 10-fold higher than the IC50 for the 2 other medulloblastoma cell lines. Interestingly, the fractional volume of patupilone-induced acidic vesicular organelles was increased in this cell line versus the other 2 cell lines, indicating an enhanced patupilone-dependent autophagic process. These medulloblastoma cell lines differ in their expression level and mutations of specific genes; however, a differential treatment sensitivity thus far could not be attributed to a specific genetic background.21
In addition to apoptosis, several additional cell death pathways exist, such as autophagy, necrosis, senescence, and mitotic catastrophe. Autophagy, which is also called self-cannibalism, includes the degradation and recycling of intracellular proteins and small organelles. Autophagy may protect cells under the condition of environmental stress but may also lead to cell death. Patupilone only induced autophagy-related acidic vacuolar organelles in the D341Med cells, which were also less susceptible to patupilone, compared with the 2 other medulloblastoma cell lines. Interestingly, interference with autophagy sensitized cells to patupilone-induced cell death, indicating that autophagy acts as a cell-protective rather than a cell death–related response to patupilone in these cells. However, how microtubule-stabilizing agents promote autophagy on the molecular level is far from clear. Only recently, a novel functional link has been suggested between autophagy and microtubules that is relevant for the cellular redistribution of the autophagy related LC3-protein and coordinated fusion of lysosomes and autophagosomes.28
Microtubule inhibitors and ionizing radiation also induce mitotic catastrophe as a mode of cell death,29 because they result in aberrant chromosomal segregation and failed mitosis. Treatment with patupilone led to a strong accumulation of cells in G2-M and multinucleation, as indicators for mitotic catastrophe, which has been identified in all 3 medulloblastoma cell lines (data not shown). Treatment-induced mitotic catastrophe can also trigger other late cell death end points, including apoptosis and independent of the original cytotoxic insult. Thus, we cannot exclude that patupilone-induced apoptosis in the medulloblastoma cells is a secondary end point and that the cells have already undergone mitotic catastrophe. Because pretreatment of cells with the broad-range caspase inhibitor did not reduce patupilone-induced cell viability, activation of apoptosis-related end points might indeed represent a secondary mode of cell death.
Overall, we demonstrated that patupilone is a very potent cytotoxic agent against several medulloblastoma cells lines, strongly reduces clonogenic survival alone and in combination with ionizing radiation, and induces different modes of cell death in a cell-line-dependent way. The strong treatment response also determined in vivo against tumor xenografts suggests that patupilone is a promising agent for combined treatment modality instead of vincristine and merits further preclinical investigation and eventually clinical evaluation.
Supplementary Material
Conflict of interest. The authors declare no conflict of interest.
Funding
We acknowledge the following sources of funding: Oncosuisse, the Radiumfonds, the Swiss National Foundations (to C.O. and M.P.); the Swiss National Fonds, the Swiss Research Foundation Child and Cancer (to A.O.v.B., M.A.G.); German Children‘s Cancer Foundation/Deutsche Kinderkrebsstiftung (to A.O.v.B., S.R.); and Fonds für Medizinische Forschung UZH (to A.B.-T.)
Supplementary Material
Acknowledgments
Patupilone was kindly provided by Novartis Pharma (Basel, Switzerland). We thank Paul McSheehy for his continuous support of our patupilone-related research program and Oralea Buechi for her technical support with the flow cytometry experiments.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol (Berl) 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhl J. Modern treatment strategies in medulloblastoma. Childs Nerv Syst. 1998;14(1-2):2–5. doi: 10.1007/s003810050164. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children's Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 4.Reeves CB, Palmer SL, Reddick WE, et al. Attention and memory functioning among pediatric patients with medulloblastoma. J Pediatr Psychol. 2006;31(3):272–280. doi: 10.1093/jpepsy/jsj019. [DOI] [PubMed] [Google Scholar]
- 5.Radcliffe J, Packer RJ, Atkins TE, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole-brain radiotherapy. Ann Neurol. 1992;32(4):551–554. doi: 10.1002/ana.410320411. doi:10.1002/ana.410320411. [DOI] [PubMed] [Google Scholar]
- 6.Silber JH, Radcliffe J, Peckham V, et al. Whole-brain irradiation and decline in intelligence: the influence of dose and age on IQ score. J Clin Oncol. 1992;10(9):1390–1396. doi: 10.1200/JCO.1992.10.9.1390. [DOI] [PubMed] [Google Scholar]
- 7.Chapman CA, Waber DP, Bernstein JH, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: role of age and perioperative factors. J Child Neurol. 1995;10(3):209–212. doi: 10.1177/088307389501000308. doi:10.1177/088307389501000308. [DOI] [PubMed] [Google Scholar]
- 8.Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol. 1996;29(1):91–101. doi: 10.1007/BF00165522. doi:10.1007/BF00165522. [DOI] [PubMed] [Google Scholar]
- 9.Packer RJ, Sutton LN, Goldwein JW, et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J Neurosurg. 1991;74(3):433–440. doi: 10.3171/jns.1991.74.3.0433. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg HS, Chamberlain MC, Glantz MJ, Wang S. Adult medulloblastoma: multiagent chemotherapy. Neuro Oncol. 2001;3(1):29–34. doi: 10.1215/15228517-3-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Bueren AO, von Hoff K, Benesch M, Rutkowski S. Dose reductions of vincristine in children with medulloblastoma treated in the maintenance arm of the prospective multicenter trial HIT'91. Klin Padiatr. 2009;221(6):396–397. doi: 10.1055/s-0029-1238278. [DOI] [PubMed] [Google Scholar]
- 12.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17(3):832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 13.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. doi:10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 14.Jakacki RBP, Zhou T, Holmes E, Packer RJ, Goldwein J, et al. Outcome for metastatic (M+) medulloblastoma (MB) treated with carboplatin during craniospinal radiotherapy (CSRT) followed by cyclophosphamide (CPM) and vincristine (VCR): Preliminary results of COG 99701. ASCO Annual Meeting Proceedings Part I, Journal of Clinical Oncology.2007. [Google Scholar]
- 15.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11(19 Pt 1):6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. doi:10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 16.Ten Bokkel Huinink WW, Sufliarsky J, Smit WM, et al. Safety and efficacy of patupilone in patients with advanced ovarian, primary fallopian, or primary peritoneal cancer: a phase I, open-label, dose-escalation study. J Clin Oncol. 2009;27(19):3097–3103. doi: 10.1200/JCO.2008.20.4826. doi:10.1200/JCO.2008.20.4826. [DOI] [PubMed] [Google Scholar]
- 17.Rothermel J, Wartmann M, Chen T, Hohneker J. EPO906 (epothilone B): a promising novel microtubule stabilizer. Semin Oncol. 2003;30(3 Suppl 6):51–55. doi: 10.1016/s0093-7754(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 18.Bley CR, Jochum W, Orlowski K, et al. Role of the microenvironment for radiosensitization by patupilone. Clin Cancer Res. 2009;15(4):1335–1342. doi: 10.1158/1078-0432.CCR-08-0969. doi:10.1158/1078-0432.CCR-08-0969. [DOI] [PubMed] [Google Scholar]
- 19.Hofstetter B, Vuong V, Broggini-Tenzer A, et al. Patupilone acts as radiosensitizing agent in multidrug-resistant cancer cells in vitro and in vivo. Clin Cancer Res. 2005;11(4):1588–1596. doi: 10.1158/1078-0432.CCR-04-1800. doi:10.1158/1078-0432.CCR-04-1800. [DOI] [PubMed] [Google Scholar]
- 20.Fogh S, Machtay M, Werner-Wasik M, et al. Phase I Trial Using Patupilone (Epothilone B) and Concurrent Radiotherapy for Central Nervous System Malignancies. Int J Radiat Oncol Biol Phys. 2010;77(4):1009–1016. doi: 10.1016/j.ijrobp.2009.06.050. [DOI] [PubMed] [Google Scholar]
- 21.Saylors RL, 3rd, Sidransky D, Friedman HS, et al. Infrequent p53 gene mutations in medulloblastomas. Cancer Res. 1991;51(17):4721–4723. [PubMed] [Google Scholar]
- 22.Altmann KH, Wartmann M, O'Reilly T. Epothilones and related structures–a new class of microtubule inhibitors with potent in vivo antitumor activity. Biochim Biophys Acta. 2000;1470(3):M79–91. doi: 10.1016/s0304-419x(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys. 2004;59(4):928–942. doi: 10.1016/j.ijrobp.2004.03.005. doi:10.1016/j.ijrobp.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Sinclair WK. Cyclic x-ray responses in mammalian cells in vitro. Radiat Res. 1968;33(3):620–643. doi:10.2307/3572419. [PubMed] [Google Scholar]
- 25.Bocci G, Nicolaou KC, Kerbel RS. Protracted low-dose effects on human endothelial cell proliferation and survival in vitro reveal a selective antiangiogenic window for various chemotherapeutic drugs. Cancer Res. 2002;62(23):6938–6943. [PubMed] [Google Scholar]
- 26.Ferretti S, Allegrini PR, O'Reilly T, et al. Patupilone induced vascular disruption in orthotopic rodent tumor models detected by magnetic resonance imaging and interstitial fluid pressure. Clin Cancer Res. 2005;11(21):7773–7784. doi: 10.1158/1078-0432.CCR-05-1165. doi:10.1158/1078-0432.CCR-05-1165. [DOI] [PubMed] [Google Scholar]
- 27.Thorpe PE. Vascular targeting agents as cancer therapeutics. Clin Cancer Res. 2004;10(2):415–427. doi: 10.1158/1078-0432.ccr-0642-03. doi:10.1158/1078-0432.CCR-0642-03. [DOI] [PubMed] [Google Scholar]
- 28.Shen S, Kepp O, Martins I, et al. Defective autophagy associated with LC3 puncta in epothilone-resistant cancer cells. Cell Cycle. 2010;9(2):377–383. doi: 10.4161/cc.9.2.10468. doi:10.4161/cc.9.2.10468. [DOI] [PubMed] [Google Scholar]
- 29.Demidenko ZN, Kalurupalle S, Hanko C, Lim CU, Broude E, Blagosklonny MV. Mechanism of G1-like arrest by low concentrations of paclitaxel: next cell cycle p53-dependent arrest with sub G1 DNA content mediated by prolonged mitosis. Oncogene. 2008;27(32):4402–4410. doi: 10.1038/onc.2008.82. doi:10.1038/onc.2008.82. [DOI] [PubMed] [Google Scholar]
- 30.von Bueren AO, Shalaby T, Oehler-Janne C, et al. RNA interference-mediated c-MYC inhibition prevents cell growth and decreases sensitivity to radio- and chemotherapy in childhood medulloblastoma cells. BMC Cancer. 2009;9:10. doi: 10.1186/1471-2407-9-10. doi:10.1186/1471-2407-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Bueren AO, Shalaby T, Rajtarova J, et al. Anti-proliferative activity of the quassinoid NBT-272 in childhood medulloblastoma cells. BMC Cancer. 2007;7:19. doi: 10.1186/1471-2407-7-19. doi:10.1186/1471-2407-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanzawa T, Germano IM, Komata T, Ito H, Kondo Y, Kondo S. Role of autophagy in temozolomide-induced cytotoxicity for malignant glioma cells. Cell Death Differ. 2004;11(4):448–457. doi: 10.1038/sj.cdd.4401359. doi:10.1038/sj.cdd.4401359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.