Abstract
Glioblastoma multiforme (GBM) is the most common and aggressive brain cancer, and despite treatment advances, patient prognosis remains poor. During routine animal studies, we serendipitously observed that fenbendazole, a benzimidazole antihelminthic used to treat pinworm infection, inhibited brain tumor engraftment. Subsequent in vitro and in vivo experiments with benzimidazoles identified mebendazole as the more promising drug for GBM therapy. In GBM cell lines, mebendazole displayed cytotoxicity, with half-maximal inhibitory concentrations ranging from 0.1 to 0.3 µM. Mebendazole disrupted microtubule formation in GBM cells, and in vitro activity was correlated with reduced tubulin polymerization. Subsequently, we showed that mebendazole significantly extended mean survival up to 63% in syngeneic and xenograft orthotopic mouse glioma models. Mebendazole has been approved by the US Food and Drug Administration for parasitic infections, has a long track-record of safe human use, and was effective in our animal models with doses documented as safe in humans. Our findings indicate that mebendazole is a possible novel anti-brain tumor therapeutic that could be further tested in clinical trials.
Keywords: albendazole, animal models, antiparasitic, benzimidazole, drug discovery, glioblastoma, mebendazole, preclinical trial, tubulin
Glioblastoma multiforme (GBM), which has been classified as a grade IV astrocytoma, is a highly aggressive tumor that invades early into surrounding brain tissues, making cure via surgical resection almost impossible. Standard of care currently consists of radiotherapy combined with chemotherapy of DNA-alkylating/methylating temozolomide (TMZ), which has increased the mean duration of patient survival to 15 months.1 Currently, only 10% of patients with GBM, including all post-treatment living conditions, survive 5 years after diagnosis, despite continuous work and improvement of GBM therapy.2,3
There has been no shortage of clinical trials for GBM. More than 600 clinical trials related to GBM have been run or are actively recruiting, according to the US National Institutes of Health's website (http://www.ClinicalTrials.gov). Unfortunately, few if any recent clinical trials show a clear survival benefit for patients with GBM. The present clinical trial system often does not address the difficulties of GBM therapy. Many therapies tried in the clinic were developed on the basis of results for other cancers, do not account for insufficient drug delivery to the brain, and do not address treatment-resistant migrating glioblastoma cells.4
There is a need to broaden the available treatments for GBM by introducing new therapeutic agents. One possible means to expedite initiation of GBM clinical trials is to examine previously established drugs with known track records of safety in humans, regardless of their intended use. However, searching for anticancer activity in compounds that have been “generally regarded as safe” may or may not turn up a promising drug suitable for GBM.
Mebendazole (MBZ), methyl N-[6-(benzoyl)-1H-benzimidazol-2-yl] carbamate, is a drug developed to treat human helminthic infections. It has been approved by the US Food and Drug Administration (FDA) and is available as generic drug for treating roundworm, common hookworm, American hookworm, pinworm, and whipworm. Clinical application of MBZ has been well documented at various doses for the rarer echinococcosis (hydatid disease).5–8 The closely related albendazole (ABZ) has been approved for treating an even wider range of parasites, including neurocysticercosis in the central nervous system (CNS). Both drugs are used to treat CNS parasitic infections and, therefore, have sufficient brain penetration for these indications. Sufficient drug delivery to the tumor remains a major challenge for most glioblastoma therapy.
The mechanism of action for MBZ and other benzimidazoles is to bind to the tubulin subunits in the gut epithelium of the parasite, preventing polymerization of the tubulin, causing ultrastructural changes, and eventually preventing parasite growth.9,10 Tubulin is vital to cell division and is also a cancer target for several chemotherapy drugs, including paclitaxol, cholchicine, and vincristine.
In addition to antiparasitic activity, MBZ and ABZ have shown preclinical anticancer activity in adrenocortical carcinoma, lung cancer, ovarian cancer, and melanoma cells,11–15 but thus far, the benzimidazole family has not been tested against glioblastoma or any other brain tumors.
Fenbendazole, a benzimidazole antihelminthic used routinely in veterinary medicine, was applied as feed supplement in our mouse colony to fight a pinworm infection. We noted problems with brain tumor intake in the xenograft model after the mice were given fenbendazole, and we further investigated this finding. This eventually led us to test the efficacy of the approved human drugs MBZ and ABZ against GBM in vitro and in vivo. We also tested whether MBZ's predicted interaction with tubulin was evident in glioblastoma cells. We found that MBZ worked best to extend survival in 2 different animal models and to explain our findings.
Materials and Methods
Cell Lines and Tissue Culture
Human GBM U87-MG (U87), D54, H80, H247, H392, H397, H502, H566, and the mouse GL261 glioma cell line were maintained in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and antibiotics. The human GBM neurosphere line 060919 was grown in NeuroCult NS-A basal medium containing NeuroCult NS-A proliferation supplements (Stem Cell Technologies), 20 ng/mL epidermal growth factor (PeproTech), 10 ng/mL basic fibroblast growth factor (PeproTech), and 4 μg/mL heparin (Stem Cell Technologies). Normal mouse nontumor astrocytes were obtained by mincing a healthy C57BL6 mouse brain and culturing it in DMEM media supplemented with 10% FBS for 2 weeks. Astrocyte populations emerged as adherent cells and were further cultured and used within 1 month. All cells were maintained at 37°C in humidified air containing 5% CO2.
Cells Growth Assay
ABZ, MBZ, thiabendazole, and fenbendazole were purchased from Sigma-Aldrich. TMZ was kindly provided by the Developmental Therapeutics Program of the National Cancer Institute (NCI)/National Institutes of Health. The viable cells were measured with a Cell Counting Kit-8 (Dojindo Laboratories) containing WST-8 tetrazolium salt at 450 nm on a PerkinElmer VICTOR3 plate reader. Half-maximal inhibitory concentrations (IC50s) were determined by incubating cells at a range of concentrations for 72 h and calculated by GraphPad Prism, version 5.0, using the log(inhibitor) versus response function and nonlinear fit.
Luciferase Expression by Lentivirus
The firefly luciferase cDNA from pGL3-basic (Promega) was subcloned in pFUGW and transfected along with CMVΔR8.91 and pMD.G in 293T cells using Lipofectamine 2000 (Invitrogen). Virus was harvested after 48 h and used to infect GL261 and 060919 cells by incubating with 8 μg/mL polybrene (Sigma).
Animal Experiments
Female C57BL/6 mice (age, 5–6 weeks) were purchased from the NCI. The syngeneic cell line GL261 was transfected with firefly luciferase with lentivirus and used for brain tumor implantation. For the implantation procedure, female C57BL/6 mice were anesthetized via intraperitoneal injection of 60 µL of a stock solution containing ketamine hydrochloride (75 mg/kg; 100 mg/mL; ketamine HCl; Abbot Laboratories), xylazine (7.5 mg/kg; 100 mg/mL; Xyla-ject; Phoenix Pharmaceutical), and ethanol (14.25%) in a sterile 0.9% NaCl solution. Using a stereotactic frame, 40, 000 GL261 cells were injected through a burr hole drilled 2 mm lateral to the sagittal suture and 1 mm anterior to the coronal suture at a depth of 3 mm below the dura at a rate of 1 µL/min.
The human GBM 060919 stem-like neurosphere cells were transfected with firefly luciferase and lentivirus, and 150,000 cells were implanted in female athymic nude mice, as described above.
At day 5 after implantation of the tumor cells, drugs were administered by oral gavage. MBZ tablets (TEVA) and ABZ tablets (GlaxoSmithKline) were resuspended in phosphate-buffered saline (PBS) and mixed with 50% of sesame oil (Sigma) to achieve better gastrointestinal absorption of the drug.5,16 Control animals were fed with PBS mixed with 50% sesame oil. TMZ was dissolved fresh for each use in PBS.
Animals were observed daily for any signs of deterioration, neurotoxicity, or movement disorders. They were inspected for signs of pain and distress, as in accordance with the Johns Hopkins Animal Care and Use Guidelines. If the symptoms persisted and resulted in debilitation, the moribund animals were euthanized according to protocol. The brain and other organs were dissected and placed in formalin for additional pathological studies.
Intracranial luciferase activity was determined by a Xenogen instrument with intraperitoneal injection of 2 mg/mouse D-luciferin potassium salt (Gold Biotechnology). Fifteen minutes after the injection, the animals were scanned for 1 min at a distance of 20 cm.
Tubulin Polymerization Assay
The tubulin polymerization assay was performed as described elsewhere.17 In brief, cells were lysed by resuspension in hypertonic buffer (2 mM of ethylene glycol tetraacetic acid, 1 mM of MgCl2, 2 mM of phenylmethylsulfonyl fluoride, 0.5% NP40, 20 mM of Tis-HCl [pH 6.8], and protease inhibitors). After brief vortexing, the samples were centrifuged at 13, 000 g for 10 min at room temperature. The supernatant containing the depolymerized tubulin was transferred to a new tube, and the pellet containing the polymerized tubulin was resuspended in an equal volume of hypertonic buffer. Equal amounts of protein were analyzed by Western blotting. The anti-α-tubulin Western blots were scanned, and the signals were qualified by the program ImageJ 1.42q (National Institutes of Health).
Western Blots
Cells were lysed in buffer as previously described elsewhere.18 Cell lysates were heated for 5 min with LDS Sample Buffer (Invitrogen) supplemented with 100 mM of dithiothreitol before loading onto a 4%–12% NuPAGE Bis-Tris Gel (Invitrogen). After transfer to a polyvinylidene fluoride membrane (Bio-Rad), immunostaining was performed in accordance with standard procedure. The following antibodies were used in this study: mouse anti-α-tubulin (Calbiochem) and anti-actin-HRP (Santa Cruz, C-11). Signals were visualized by the SuperSignal chemiluminescent system (Pierce).
Immunofluorescence Staining
The staining procedure followed the procedure described above.19 The neurosphere glioblastoma cell line 060919 was transferred from neurosphere medium and grown in DMEM medium containing 10% FBS in adherent single cell condition on chamber slides (Nunc). They were treated with 1 μM of MBZ or 10 nM of cholchicine for 24 h and then fixed for 10 min with 4% paraformaldehyde solution and permeated with methanol for 2 min with 3 washes in PBS in between and after. The slides were first blocked by 10% goat serum in PBS for 1 h at room temperature and incubated with mouse anti-α-tubulin antibody and subsequently with Alexa Fluor 594 (Texas Red) goat anti-mouse IgG (Invitrogen) in 10% goat serum in PBS at room temperature. They were then washed 3 times in PBS in between and after. After staining, the slides were covered with mounting medium containing DAPI (Vector Laboratories) and examined on a fluorescence microscope.
Statistical Analysis
The results are presented as a mean value ± standard deviation. Data were analyzed by GraphPad Prism, version 5.0. The P values were determined by a Mantel-Cox test. P values <.05 were accepted as statistically significant.
Results
We first observed the anti–brain tumor activity of benzimidazole family anthelminthics serendipitously when the animal facility started providing fenbendazole-containing food to the mice colony to eliminate pinworm infections. One of our orthotopic brain tumor models stopped forming tumors, whereas before the fenbendazole therapy, we had consistent, reproducible tumor engraftment. This prompted us to investigate commercially available benzimidazoles: fenbendazole, thiabendazole, MBZ, and ABZ, by first using GBM cell lines in vitro. On the basis of superior IC50s levels (Fig. 1A and data not shown) and FDA approval of human use, we decided to focus on MBZ and ABZ for in vivo preclinical evaluation.
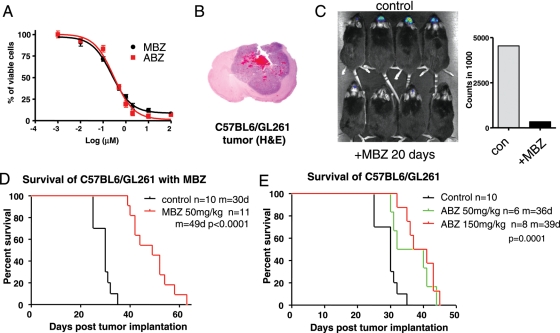
Fig. 1.
Mebendazole (MBZ) inhibited intracranial tumor growth in the syngeneic GL261 mouse model. (A) Inhibition of GL261 mouse glioma cells by MBZ and albendazole (ABZ) showing the half-maximal inhibitory concentration (IC50) of MBZ at 0.24 μM and the IC50 of ABZ at 0.30 μM. (B) Hematoxylin and eosin staining of a coronal section of a C57BL/6 mouse brain implanted with GL261 glioma cells in the frontal lobe. (C) Mice implanted with GL261 cells expressing firefly luciferase were injected with 100 mg/kg luciferin and measured by Xenogen after 20 days of MBZ treatment. Four animals per group were randomly selected and shown. The total photon counts are displayed in the bar graph to the right. (D) Kaplan-Meier survival curve of C57BL/6 mice implanted with GL261 glioma cells and treated with MBZ. MBZ was given orally beginning 5 days after tumor implantation at a daily dose of 50 mg/kg for the first 20 days of treatment then changed to 50 mg/kg for 5 days, with 2 days off, each week. MBZ treatment increased the mean survival to 49 days compared with the 30 days of control (P < .0001). m, Mean survival days; n, number of animals. (E) Kaplan-Meier survival curve of C57BL/6 mice implanted with GL261 glioma cells treated with ABZ. MBZ was given orally beginning 5 days after tumor implantation at either 50 mg/kg or 150 mg/kg, as indicated, every day for the first 20 days of treatment and subsequently changed to 5 days a week. P < .001 for ABZ, 150 mg/kg, versus control; P = .015 for ABZ, 50 mg/kg, versus control; P = .38 (not significant) for ABZ, 150 mg/kg, versus 50 mg/kg
We used a GL261 syngeneic mouse glioma model to test the in vivo efficacy of MBZ and ABZ. The syngeneic GL261 mouse glioma was induced originally by intracranial injection of 3-methylcholantrene into C57BL/6 mice.20 Intracranial GL261 tumors showed rapid growth, diverse cell populations, necrotic regions, and invasive growth pattern, along with hemorrhages, closely resembling human GBM pathology (Fig. 1B). GL261 cells in vitro were susceptible to MBZ and ABZ, with IC50 levels measured at 0.24 and 0.3 μM, respectively (Fig. 1A). In a dose escalation in C57BL6 mice, daily administration of MBZ at 100 mg/kg led to toxicities, such as weight loss, whereas administration at 50 mg/kg did not show adverse effects.
Oral administration of MBZ at 50 mg/kg from day 5 after tumor implantation slowed tumor growth. The GL261 tumors expressing luciferase were visualized via Xenogen scanning using luciferin, showing smaller tumors after 20 days of MBZ treatment, as reflected by the significantly lower luciferase signals versus those of the control group (Fig. 1C). Mean survival was increased from 30 days in the control group to 49 days in the intervention group, a 63.3% increase (Fig. 1D).
ABZ was tested with the GL261 model at 50 mg/kg and 150 mg/kg, also without showing any significant adverse effects. Compared with the same control group in Fig. 1D, 2 doses of ABZ were able to moderately extend the survival to 36 days (20%) and 39 days (30%), respectively (Fig. 1E). This survival increase was less than that with MBZ.
Next, we tested MBZ and ABZ with the 060919 human GBM stem-like neurosphere cell line. The IC50s of both drugs in vitro were determined to be similar (∼0.1 μM). Of note, this cell line showed resistance to TMZ compared to the GL261 line, with an IC50 at 148 μM (Fig. 2A). The 060919 line grown as an intracranial xenograft showed a highly invasive and neo-vascularized growth pattern similar to the morphology of human GBMs, with features such as brain tissue infiltrations, heterogeneic population, hemorrhages, neoplastic giant cells, necrotic/hypoxic tissues, and pseudopalisading cells21 (Fig. 2B). Treating the mice with MBZ prolonged the mean duration of survival to 65 days, compared with 48 days for the control group, whereas ABZ at 150 mg/kg and TMZ at 15 mg/kg failed to extend the duration of survival (Fig. 2C). The measurement of luciferase activity in 060919 tumors confirmed that the tumor growth was inhibited by MBZ treatment (Fig. 2D and E).
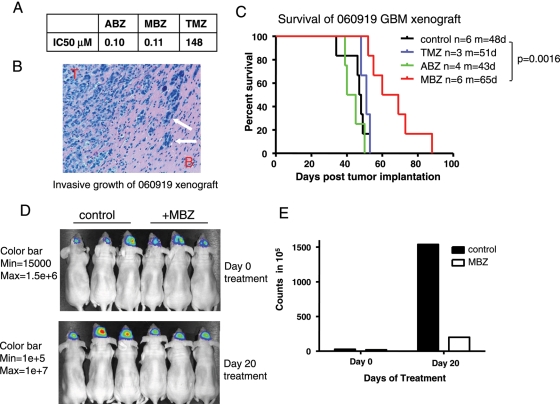
Fig. 2.
Mebendazole (MBZ) improved the survival in the 060919 human glioblastoma multiforme (GBM) xenograft mouse model. (A) The half-maximal inhibitory concentration (IC50) levels of albendazole (ABZ), MBZ, and temozolomide (TMZ) in the 060919 human GBM neurosphere line are shown. (B) Hematoxylin and eosin staining of a coronal section of a nude mouse brain implanted with 060919 cells in the frontal lobe (10x). White arrows point to the invasive tumor cells. (C) Kaplan-Meier survival curve of 060919 xenografts treated with oral MBZ (50 mg/kg), ABZ (150 mg/kg), or TMZ (15 mg/kg) started 5 days after tumor implantation. Mice were treated daily for 20 days, followed by the same daily dose for 5 days per week. MBZ treatment increased the mean survival to 65 days compared with the 48 days of control. M, mean survival in days; n, number of animals. Significance values were as follows: MBZ versus control, P = .0016; ABZ versus control, P = .45; TMZ versus control, P = .30. (D) Mice implanted with 060919 cells expressing firefly luciferase were injected with 100 mg/kg luciferin and measured by Xenogen before and after 20 days of MBZ treatment. Three animals in each group were randomly selected and shown in the picture on the left side. Different color bars were applied on day 0 and day 20 of treatment. (E) The total photon counts of animals displayed in D were shown in the graph.
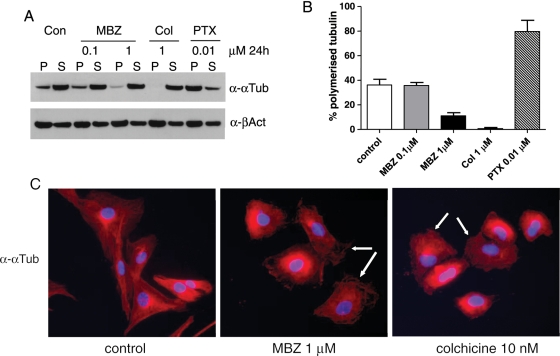
Benzimidazole family antihelminthics can exert cellular toxicity by binding to tubulin molecules and thereby disrupting their polymerization and microtubule formation in a similar fashion to colchicines.22 To test whether MBZ interferes with the microtubule formation in GBM cells, we performed a tubulin polymerization assay with the 060919 GBM cell line. Cells were incubated with 0.1 or 1 μM of MBZ, 1 μM of colchicines, or 10 nM of paclitaxel (PTX) for 24 h. PTX is known for hyperstabilizing the microtubule structure, thus causing mitotic arrest. After the lysis by hypotonic buffer and centrifugation, polymerized and depolymerized tubulin resided in the pellet and supernatant fractions, respectively. OneμM of MBZ clearly reduced the polymerized tubulin in the pellet as well as the percentage of polymerized tubulin in the total tubulin amount down to 11%, compared with 38% for the control group (Fig. 3A and B). Accordingly, colchicine severely decreased polymerized tubulin down to 1%, and PTX accumulated it up to 80%. Supplementary Material, Figure S1 shows the antitubulin polymerization of MBZ at 0.1 or 0.2 μM after 72 h, and the concentrations and time were comparable to the results of the condition used to determine the IC50 value of MBZ with 060919 cells. After 72 h, MBZ significantly inhibited the polymerization of tubulin at 0.1 μM. The depolymerization of tubulin by MBZ caused serious disruption in microtubule structure, as demonstrated by the immunofluorescent staining of α-tubulin in Fig. 3C, in which 060919 GBM stem cells were cultured adherently on the chamber slides and incubated with 1 μM of MBZ for 24 h. As a positive control, colchicine showed a similar effect.
Fig. 3.
Mebendazole (MBZ) disrupted microtubule polymerization in 060919 glioblastoma multiforme (GBM) cells. (A) 060919 GBM neurosphere cells were incubated with MBZ, colchicine (Col), or paclitaxol (PTX) at indicated concentrations for 24 h. After lysing cells with hypotonic buffer, the lysates were separated by centrifugation. The pellets (P) containing polymerized tubulin were resuspended in equal amount of lysis buffer and loaded along with the supernatant (S) containing depolymerized tubulin on SDS-PAGE for anti-α-tubulin (αTub) Western blot. The anti-β-Actin (βAct) blot served as a loading control. (B) The signals on the anti-α-tubulin blot were quantified and the percentage of polymerized tubulin (% P) induced by individual treatments was calculated with the formula: % P = P/(P + S) × 100. Mean and standard deviation (SD) are indicated. (C) MBZ disrupted the microtubule structure of 060919 cells. 060919 cells were cultured in adherent fashion with serum-containing media, treated for 24 h with 1 μM of MBZ or 10 nM cholchicine, and stained with anti-αTub antibody, Texas Red secondary antibody, and DAPI.
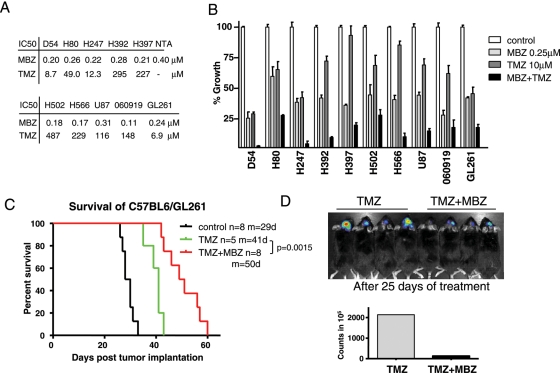
To evaluate MBZ therapy in combination with TMZ, we first tested the in vitro efficacies of MBZ and TMZ using 10 GBM cell lines. Although the IC50s of MBZ appeared to be in a close range, between 0.11 and 0.31 μM, these cell lines varied widely in response to TMZ with IC50s ranging from 8.7 to 547 μM (Fig. 4A). When used together in vitro, the combination of MBZ and TMZ slowed growth in GBM cells more than either drug alone (Fig. 4B). However, synergy was not observed in vivo. In the GL261 mouse model, TMZ at 15 mg/kg prolonged the mean survival to 41 days, compared with 29 days in the control group, in this set of experiments. With the addition of MBZ to the treatment regimen, the mean survival was extended to 50 days (Fig. 4C). This was a 72.4% improvement over the untreated control group. However, it is not significantly longer than the survival benefit achieved by MBZ alone observed in the previous set of experiments in Fig. 1D. Because the experiments were done under the same conditions, the various studies are shown superimposed in Supplementary Material, Fig. S2 for direct visual comparison.
Fig. 4.
Mebendazole (MBZ) plus temozolomide (TMZ) extends survival further than TMZ alone in the GL261 mouse model. (A) The half-maximal inhibitory concentration (IC50) levels of MBZ and TMZ with various glioblastoma multiforme (GBM) cell lines and normal mouse astrocytes. (B) Inhibition of GBM cell growth by MBZ, TMZ, or MBZ and TMZ combined. (C) Kaplan–Meier survival curve of C57BL/6 mice implanted with GL261 glioma cells and treated with TMZ (15 mg/kg) or TMZ plus MBZ (50 mg/kg). MBZ and TMZ were administered daily starting 5 days post tumor implantation for 20 days, followed by dosing 5 days per week. The TMZ + MBZ treatment increased the mean survival to 50 days, compared to the 30 days for controls (P < .0001) and 41 days for TMZ alone. The P = .0015 for TMZ plus MBZ versus TMZ. M, mean survival in days; n, number of animals. (D) Mice implanted with GL261 cells expressing firefly luciferase were injected with 100 mg/kg luciferin and measured by Xenogen after 25 days of MBZ treatment. Four animals in the TMZ and TMZ plus MBZ groups were randomly selected and are shown in the top picture, whereas the total photon counts are displayed on the lower graph. The color bars were set as follows: min = 2e + 5; max = 2e + 7.
Discussion
We accidentally found that fenbendazole, a benzimidazole, reduced brain tumor engraftment in nude mice after the mouse colony was treated for pinworms. Fenbendazole was previously reported to interfere with one lymphoma model in 2008,23 after we had already noted problems with fenbendazole disrupting brain tumor engraftment. We pursued this finding by evaluating whether the 2 most widely used human approved benzimidazoles showed efficacy against glioblastoma models.
The benzimidazoles are widely used for veterinary and human applications, with MBZ and ABZ approved for human parasitic treatment. Benzimidazole drugs, such as MBZ and ABZ, have been used to treat CNS infections of human cystic and alveolar echinococcosis since the introduction in the 1970s and have proven to be well tolerated and safe.24 Although MBZ and other benzimidazoles have reported preclinical antitumor activity, this is, to our knowledge, the first study to have related benzimidazole drugs to the treatment of a brain cancer. MBZ showed efficacy in 2 mouse models, with both having histological and phenotypic features similar to human GBM.
Although ABZ and MBZ revealed similar IC50s with both GL261 mouse glioma cells and 060919 human GBM stem-like neurosphere cells, MBZ extended survival most effectively. One explanation may be the different bioavailability of MBZ versus ABZ in our models. The limited solubility of ABZ and MBZ in water and organic solvents greatly affects absorption and behavior in the body.25 Given orally, ABZ and MBZ suffer from poor gastrointestinal absorption in human and rodents, and fatty meals are usually included to increase the absorption.5,16 The absorption of MBZ has been reported to be as low as 5%–10%, and similar low levels have been measured with ABZ in humans.25 We decided to focus on MBZ because of its superior efficacy.
MBZ and ABZ bind to the (+) end of the microtubule, inhibit tubulin polymerization, and prevent the addition of tubulin subunits in parasites.10,26 Microtubules are composed of α- and β-tubulin and are cytoskeleton components that are required for cellular transport, cell division, and maintenance of structural integrity. In addition, disruption of microtubule formation could also impair the migration of GBM cells. It was shown that the migration of neuronal cells is impaired by an α–tubulin mutation and that microtubule inhibitors can potentially block the mobility of glioma cells.27,28 It is also very likely that the migration/invasion feature of GBM cells renders them more resistant to apoptosis and cytotoxic insults.4,29
Figure 3 shows significant reduction of polymerized tubulin in 060919 cells treated with 1 μM of MBZ, as well as the disruption of microtubule structure. Other mechanisms have been proposed for benzimidazole, such as inhibition of VEGF and HIF-1α expression and inactivation of Bcl-2.15,30,31 Despite clear evidence of an anti-tubulin effect from MBZ, other mechanisms contributing to its efficacy in our preclinical models cannot be excluded. In parasites, a specific resistance to benzimidazoles was found to be conferred by changing the β-tubulin sequence at codon 200 as the result of a single-nucleotide polymorphism from TTC200 to TAC200.32,33 The possibility of tubulin mutation-based acquired resistance should be noted if MBZ therapy proceeds to clinical trials.
Antitubulin drugs other than benzimidazoles have been studied for cancer treatments, but many, such as cholchicine, exhibit severe toxicity. MBZ, flubendazole, and a number of benzimidazoles have been shown to interact with tubulin on the similar binding site–like colchicines, but distinct from the binding site of vinca alkaloids.22,34,35 Vinca alkaloids, such as vinblastine and vincrisine, have been approved for treating lymphomas, acute lymphoblastic leukemia, and nephroblastoma but are associated with considerable adverse effects, including bone marrow suppression and nervous system toxicity.
MBZ and other benzimidazoles seem to target cancer cells preferentially over normal cells, and this may suggest a favorable therapeutic index for in vivo applications. In vitro, MBZ displayed a slightly higher IC50 of 0.4 μM in mouse astrocytes, compared with those in various GBM cell lines, ranging 0.11–0.31 μM. In addition, our 2 different GBM animal models showed survival benefit for MBZ with minimal toxicity. Several other studies have shown in vivo success with benzimidazoles against cancers, including non–small cell lung cancer, adrenocortical carcinoma, leukemia, and colorectal cancer.11,12,23,35,36 There is evidence that MBZ's mode of action in lung cancer cells involves prevention of polymerization of tubulin.12 An additional anti-cancer mechanism associated with MBZ is that it induces apoptosis by BCL-2 inactivation in melanoma cells.15
MBZ has not yet been tried in human cancer therapy. A previous pilot clinical study of ABZ in patients with advanced colorectal cancer and hepatocellular carcinoma at 10 mg/kg/day for 28 days demonstrated anti-tumor efficacy, but there were concerns about severe neutropenia in 3 of 10 patients.37 On the basis of our preclinical results in GBM and the problems faced by ABZ, we would favor a clinical investigation with MBZ for GBM.
We used a daily dosing for 2 months of 50 mg/kg of MBZ, which translates to 4.1 mg/kg/day based on body surface area.38 MBZ has been extensively used for human for treating echinococcosis, with multiple reports of minimal adverse effects at 50–70 mg/kg/day for 6–24 months of continuous use.7,8,39 MBZ was also reported safe in children at 100–200 mg/kg doses for 12 weeks.6 These reported safe doses suggest we could escalate to higher doses than those required for efficacy in animals.
In another study of patients with echinococcosis, dosages as high as 200 mg/kg per day for up to 48 weeks were well tolerated, with 8.6 ng/mL (0.029 μM) of MBZ measured in cerebrospinal fluid, compared to 93 ng/mL maximally attained in serum.40 Other studies have shown safe doses up to 200 mg/kg with daily use.6,41,42 With a molecular weight at 295 Da and lipophilic properties, MBZ is capable of passing through the blood-brain barrier.43 In fact, MBZ has effectively treated CNS echinococcosis in numerous clinical settings before, indicating significant CNS penetration of MBZ.44–46
To determine whether combining MBZ with TMZ was beneficial, we chose to use a modest dose of TMZ that had a survival benefit, but not so large as to mask any potential synergy. At 15 mg/kg, TMZ extended the survival rate by 41.4%. The combination of MBZ and TMZ showed a survival benefit of 72.4%, compared with 63.3% for MBZ alone in a separate set of experiments. This difference was not significant for MBZ versus MBZ plus TMZ when comparing these 2 sets of experiments. Also of interest is that TMZ showed efficacy in only 1 of our 2 mouse models, whereas MBZ showed a significant survival difference in both TMZ-susceptible GL261 (IC50 = 6.9 μM) and TMZ-resistant 060919 (IC50 = 149 μM). Of note, patients with TMZ-resistant GBMs make up more than one-half of the patient population.47 A possible next step is to determine whether MBZ has single-agent efficacy in patients for whom initial therapy has failed.
In summary, MBZ offers a highly promising opportunity for clinical application on GBM. This is because it has a long track record of safety, there is evidence of preclinical efficacy, an anti-cancer mechanism has been revealed, cost is relatively low, the drug widely available as a generic drug, there is good CNS penetration, and there is a great need for better GBM therapy.
Supplementary Material
Acknowledgments
Support provided by National Institutes of Health Grants R01 NS052507, the Virginia and D.K. Ludwig Fund for Cancer Research, the Johns Hopkins Department of Neurosurgery, Irving J. Sherman, MD, and the Irving J. Sherman Neurosurgery Research Professorship to G.J.R.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. doi:10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Nobusawa S, Lachuer J, Wierinckx A, et al. Intratumoral patterns of genomic imbalance in glioblastomas. Brain Pathol. 2010;20:936–944. doi: 10.1111/j.1750-3639.2010.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khasraw M, Lassman AB. Advances in the treatment of malignant gliomas. Curr Oncol Rep. 2010;12:26–33. doi: 10.1007/s11912-009-0077-4. doi:10.1007/s11912-009-0077-4. [DOI] [PubMed] [Google Scholar]
- 4.Lefranc F, Brotchi J, Kiss R. Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol. 2005;23:2411–2422. doi: 10.1200/JCO.2005.03.089. doi:10.1200/JCO.2005.03.089. [DOI] [PubMed] [Google Scholar]
- 5.Munst GJ, Karlaganis G, Bircher J. Plasma concentrations of mebendazole during treatment of echinococcosis: preliminary results. Eur J Clin Pharmacol. 1980;17:375–378. doi: 10.1007/BF00558451. doi:10.1007/BF00558451. [DOI] [PubMed] [Google Scholar]
- 6.Messaritakis J, Psychou P, Nicolaidou P, et al. High mebendazole doses in pulmonary and hepatic hydatid disease. Arch Dis Child. 1991;66:532–533. doi: 10.1136/adc.66.4.532. doi:10.1136/adc.66.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vutova K, Mechkov G, Vachkov P, et al. Effect of mebendazole on human cystic echinococcosis: the role of dosage and treatment duration. Ann Trop Med Parasitol. 1999;93:357–365. doi: 10.1080/00034989958357. doi:10.1080/00034989958357. [DOI] [PubMed] [Google Scholar]
- 8.El-On J. Benzimidazole treatment of cystic echinococcosis. Acta Trop. 2003;85:243–252. doi: 10.1016/s0001-706x(02)00217-6. doi:10.1016/S0001-706X(02)00217-6. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald LM, Armson A, Thompson AR, Reynoldson JA. Characterisation of benzimidazole binding with recombinant tubulin from Giardia duodenalis, Encephalitozoon intestinalis, and Cryptosporidium parvum. Mol Biochem Parasitol. 2004;138:89–96. doi: 10.1016/j.molbiopara.2004.08.001. doi:10.1016/j.molbiopara.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Kohler P. The biochemical basis of anthelmintic action and resistance. Int J Parasitol. 2001;31:336–345. doi: 10.1016/s0020-7519(01)00131-x. doi:10.1016/S0020-7519(01)00131-X. [DOI] [PubMed] [Google Scholar]
- 11.Martarelli D, Pompei P, Baldi C, Mazzoni G. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother Pharmacol. 2008;61:809–817. doi: 10.1007/s00280-007-0538-0. doi:10.1007/s00280-007-0538-0. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki J, Ramesh R, Chada S, et al. The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells. Mol Cancer Ther. 2002;1:1201–1209. [PubMed] [Google Scholar]
- 13.Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA. Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo. Clin Cancer Res. 2002;8:2963–2969. [PubMed] [Google Scholar]
- 14.Pourgholami MH, Cai ZY, Chu SW, Galettis P, Morris DL. The influence of ovarian cancer induced peritoneal carcinomatosis on the pharmacokinetics of albendazole in nude mice. Anticancer Res. 2010;30:423–428. [PubMed] [Google Scholar]
- 15.Doudican N, Rodriguez A, Osman I, Orlow SJ. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol Cancer Res. 2008;6:1308–1315. doi: 10.1158/1541-7786.MCR-07-2159. doi:10.1158/1541-7786.MCR-07-2159. [DOI] [PubMed] [Google Scholar]
- 16.Fraga Fuentes MD, Garcia Diaz B, de Juana Velasco P, Bermejo Vicedo MT. [Influence of foods on the absorption of antimicrobial agents] Nutr Hosp. 1997;12:277–288. [PubMed] [Google Scholar]
- 17.Chu SW, Badar S, Morris DL, Pourgholami MH. Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res. 2009;29:3791–3796. [PubMed] [Google Scholar]
- 18.Bai RY, Dieter P, Peschel C, Morris SW, Duyster J. Nucleophosmin-anaplastic lymphoma kinase of large-cell anaplastic lymphoma is a constitutively active tyrosine kinase that utilizes phospholipase C-gamma to mediate its mitogenicity. Mol Cell Biol. 1998;18:6951–6961. doi: 10.1128/mcb.18.12.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai R, Siu IM, Tyler BM, et al. Evaluation of retinoic acid therapy for OTX2-positive medulloblastomas. Neuro Oncol. 2010;12:655–663. doi: 10.1093/neuonc/nop062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–2400. [PubMed] [Google Scholar]
- 21.Siu IM, Tyler BM, Chen JX, et al. Establishment of a human glioblastoma stemlike brainstem rodent tumor model. J Neurosurg Pediatr. 2010;6:92–97. doi: 10.3171/2010.3.PEDS09366. doi:10.3171/2010.3.PEDS09366. [DOI] [PubMed] [Google Scholar]
- 22.Lacey E. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int J Parasitol. 1988;18:885–936. doi: 10.1016/0020-7519(88)90175-0. doi:10.1016/0020-7519(88)90175-0. [DOI] [PubMed] [Google Scholar]
- 23.Gao P, Dang CV, Watson J. Unexpected antitumorigenic effect of fenbendazole when combined with supplementary vitamins. J Am Assoc Lab Anim Sci. 2008;47:37–40. [PMC free article] [PubMed] [Google Scholar]
- 24.Vuitton DA. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev Anti Infect Ther. 2009;7:145–149. doi: 10.1586/14787210.7.2.145. doi:10.1586/14787210.7.2.145. [DOI] [PubMed] [Google Scholar]
- 25.Dayan AD. Albendazole, mebendazole and praziquantel: review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–159. doi: 10.1016/s0001-706x(03)00031-7. doi:10.1016/S0001-706X(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 26.Lacey E, Watson TR. Structure-activity relationships of benzimidazole carbamates as inhibitors of mammalian tubulin, in vitro. Biochem Pharmacol. 1985;34:1073–1077. doi: 10.1016/0006-2952(85)90611-2. doi:10.1016/0006-2952(85)90611-2. [DOI] [PubMed] [Google Scholar]
- 27.Keays DA, Tian G, Poirier K, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. doi:10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henry WI, Dubois J, Quick QA. The microtubule inhibiting agent epothilone B antagonizes glioma cell motility associated with reorganization of the actin-binding protein alpha-actinin 4. Oncol Rep. 2011;25:887–893. doi: 10.3892/or.2011.1145. [DOI] [PubMed] [Google Scholar]
- 29.Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–1403. doi: 10.1634/theoncologist.12-12-1395. doi:10.1634/theoncologist.12-12-1395. [DOI] [PubMed] [Google Scholar]
- 30.Pourgholami MH, Yan Cai Z, Lu Y, Wang L, Morris DL. Albendazole: a potent inhibitor of vascular endothelial growth factor and malignant ascites formation in OVCAR-3 tumor-bearing nude mice. Clin Cancer Res. 2006;12:1928–1935. doi: 10.1158/1078-0432.CCR-05-1181. doi:10.1158/1078-0432.CCR-05-1181. [DOI] [PubMed] [Google Scholar]
- 31.Pourgholami MH, Cai ZY, Badar S, et al. Potent inhibition of tumoral hypoxia-inducible factor 1alpha by albendazole. BMC Cancer. 2010;10:143. doi: 10.1186/1471-2407-10-143. doi:10.1186/1471-2407-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kwa MS, Veenstra JG, Roos MH. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol Biochem Parasitol. 1994;63:299–303. doi: 10.1016/0166-6851(94)90066-3. doi:10.1016/0166-6851(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 33.Kwa MS, Veenstra JG, Van Dijk M, Roos MH. Beta-tubulin genes from the parasitic nematode Haemonchus contortus modulate drug resistance in Caenorhabditis elegans. J Mol Biol. 1995;246:500–510. doi: 10.1006/jmbi.1994.0102. doi:10.1006/jmbi.1994.0102. [DOI] [PubMed] [Google Scholar]
- 34.Ireland CM, Gull K, Gutteridge WE, Pogson CI. The interaction of benzimidazole carbamates with mammalian microtobule protein. Biochem Pharmacol. 1979;28:2680–2682. doi: 10.1016/0006-2952(79)90049-2. doi:10.1016/0006-2952(79)90049-2. [DOI] [PubMed] [Google Scholar]
- 35.Spagnuolo PA, Hu J, Hurren R, et al. The antihelmintic flubendazole inhibits microtubule function through a mechanism distinct from Vinca alkaloids and displays preclinical activity in leukemia and myeloma. Blood. 2010;115:4824–4833. doi: 10.1182/blood-2009-09-243055. doi:10.1182/blood-2009-09-243055. [DOI] [PubMed] [Google Scholar]
- 36.Pourgholami MH, Akhter J, Wang L, Lu Y, Morris DL. Antitumor activity of albendazole against the human colorectal cancer cell line HT-29: in vitro and in a xenograft model of peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2005;55:425–432. doi: 10.1007/s00280-004-0927-6. doi:10.1007/s00280-004-0927-6. [DOI] [PubMed] [Google Scholar]
- 37.Morris DL, Jourdan JL, Pourgholami MH. Pilot study of albendazole in patients with advanced malignancy. Effect on serum tumor markers/high incidence of neutropenia. Oncology. 2001;61:42–46. doi: 10.1159/000055351. doi:10.1159/000055351. [DOI] [PubMed] [Google Scholar]
- 38.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. Faseb J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. doi:10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 39.Todorov T, Vutova K, Donev S, et al. The types and timing of the degenerative changes seen in the cysts during and after benzimidazole treatment of cystic echinococcosis. Ann Trop Med Parasitol. 2005;99:649–659. doi: 10.1179/136485905X65125. doi:10.1179/136485905X65125. [DOI] [PubMed] [Google Scholar]
- 40.Bryceson AD, Woestenborghs R, Michiels M, van den Bossche H. Bioavailability and tolerability of mebendazole in patients with inoperable hydatid disease. Trans R Soc Trop Med Hyg. 1982;76:563–564. doi: 10.1016/0035-9203(82)90163-8. doi:10.1016/0035-9203(82)90163-8. [DOI] [PubMed] [Google Scholar]
- 41.Bryceson AD, Cowie AG, Macleod C, et al. Experience with mebendazole in the treatment of inoperable hydatid disease in England. Trans R Soc Trop Med Hyg. 1982;76:510–518. doi: 10.1016/0035-9203(82)90151-1. doi:10.1016/0035-9203(82)90151-1. [DOI] [PubMed] [Google Scholar]
- 42.Kammerer WS, Schantz PM. Long term follow-up of human hydatid disease (Echinococcus granulosus) treated with a high-dose mebendazole regimen. Am J Trop Med Hyg. 1984;33:132–137. doi: 10.4269/ajtmh.1984.33.132. [DOI] [PubMed] [Google Scholar]
- 43.Chico LK, Van Eldik LJ, Watterson DM. Targeting protein kinases in central nervous system disorders. Nat Rev Drug Discov. 2009;8:892–909. doi: 10.1038/nrd2999. doi:10.1038/nrd2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Charles RW, Govender S, Naidoo KS. Echinococcal infection of the spine with neural involvement. Spine (Phila Pa 1976) 1988;13:47–49. doi: 10.1097/00007632-198801000-00011. doi:10.1097/00007632-198801000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Ersahin Y, Mutluer S, Guzelbag E. Intracranial hydatid cysts in children. Neurosurgery. 1993;33:219–224. discussion 224–225 doi:10.1227/00006123-199308000-00006. [PubMed] [Google Scholar]
- 46.Erdincler P, Kaynar MY, Babuna O, Canbaz B. The role of mebendazole in the surgical treatment of central nervous system hydatid disease. Br J Neurosurg. 1997;11:116–120. doi: 10.1080/02688699746456. doi:10.1080/02688699746456. [DOI] [PubMed] [Google Scholar]
- 47.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. doi:10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.