Abstract
Patients with von Hippel–Lindau (VHL) syndrome with diffuse CNS hemangioblastomas have morbidity related to their disease and require a lifetime of surgical resections. Ninety-seven percent of tumors progress, and 5-year surgery rates are 20%–60%. Stereotactic radiosurgery and fractionated radiotherapy have had limited success. For the first time, we have used infratentorial craniospinal radiation therapy (ICSRT) for VHL patients with CNS hemangioblastomas. Consecutive VHL patients treated at the National Institutes of Health with radiographic evidence of hemangioblastomas were included if they received ICSRT. Patients underwent neurologic examinations and imaging at 3- to 12-month intervals. Seven patients with 84 hemangioblastomas met eligibility criteria. ICSRT was commonly administered to 43.2 Gy in 24 fractions. Mean pre-ICSRT tumor volume was 5.48 cm3. At a mean follow-up of 73.8 months, mean post-ICSRT tumor volume was 6.87 cm3, and 91 tumors were identified. Complete radiographic resolution was achieved in 17.9% of lesions. Although many patients were no longer optimal surgical candidates, only 4 surgeries were needed for symptomatic lesions after ICSRT, compared with 33 prior. Acute toxicity was mild and no patient developed grade ≥1 late spinal cord toxicity according to the criteria of the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer, despite the high dose that the entire spinal cord received. Clinical and radiographic stability or resolution was demonstrated in the majority of tumors. Tumor growth rate in this study was less than reported in natural history studies, and the rate of surgical intervention was reduced. ICSRT was well tolerated, can decrease hemangioblastoma growth rate, and is a potential therapeutic option for VHL patients that warrants further investigation.
Keywords: central nervous system, craniospinal irradiation, hemangioblastoma, tumor volume, von Hippel-Lindau (VHL)
Von Hippel–Lindau (VHL) is an autosomal dominant syndrome characterized by a predisposition to CNS hemangioblastomas, retinal angiomas, renal cell carcinomas, pheochromocytomas, endolymphatic sac neoplasms, pancreatic islet cell tumors, and renal, pancreatic, and hepatic cysts. CNS hemangioblastomas are the most common manifestation of VHL. They are considered to be benign, highly vascular neoplasms that are typically well circumscribed and may be solid or cystic. CNS hemangioblastomas account for 2% of all intracranial tumors in adults between 25 and 40 years of age and are often associated with a defective VHL gene. Some patients with VHL present predominantly with diffuse, insidious CNS infratentorial hemangioblastomas. VHL patients often develop multiple lesions that can cause significantly debilitating neurologic morbidities, including loss of motor or sensory function, impaired gait, or even paralysis.1–3
Resection of CNS hemangioblastomas can improve patient symptomotology. Unfortunately, patients often have multiple tumors that grow and expand at different times and rates, resulting in renewed neurologic dysfunction and subjecting these patients to lifelong disability, suffering, and a need for multiple surgical interventions.
External beam radiation therapy has become an alternative treatment course for patients who have CNS hemangioblastomas that are not amenable to surgical resection. Better local control of hemangioblastomas has been demonstrated with higher total doses of conventionally fractionated external beam radiation therapy. One study that evaluated patients with CNS hemangioblastomas between 1963 and 1983 revealed local control was achieved in 57% (4/7) of patients receiving 50 Gy, compared with 33% (4/12) in patients receiving less than 50 Gy and 45% (9/20) in patients with gross residual disease. However, radiation-induced hydromyelia occurred in 2 patients in the 50-Gy cohort and required surgical intervention.4 A survival advantage with higher radiation doses was demonstrated in another study in which 24 patients were treated for CNS hemangioblastomas from 1950 to 1976. Patients receiving greater than 36 Gy had a 10-year overall survival (OS) rate of 57%, compared with 27% for those treated to less than 36 Gy.5 Drawing on this dose-response data, a more modern series treating 18 patients with 31 CNS hemangioblastomas from 1980 to 2004 achieved a 5-year OS rate of 69%, which decreased to 30% at 10 years following 50–55.8 Gy of fractionated external beam radiation therapy.6 Stereotactic radiosurgery has also been used to treat CNS hemangioblastomas, particularly for patients with cerebellar tumors. However, since radiosurgery has been found to have diminishing local control with time, this modality is often limited to the treatment of unresectable and symptomatic lesions.7
Natural history studies have demonstrated that 97% of CNS hemangioblastomas progress and that 5-year surgical intervention rates are 20% for spinal cord lesions, 38% for cerebellar tumors, and 60% for brainstem tumors. Furthermore, these tumors have biphasic growth patterns, with an active growth phase for a mean of 13 months and quiescent phase for a mean of 25 months, during which time little to no increase in size of lesions is noted.8–10 Over a 10-year period, Ammerman et al. found that 45% of hemangioblastomas that become symptomatic are not evident at initial presentation. Furthermore, of the 143 hemangioblastomas that they identified at initial presentation and followed, nearly all of those lesions had interval growth and 41% became symptomatic.9,10
It has, therefore, been hypothesized that early intervention against small or asymptomatic lesions would be clinically beneficial instead of allowing them to become larger and produce symptoms during the course of patients’ disease. Surgical resection of asymptomatic lesions is not typically done and is reserved for lesions causing symptoms or signs in order to avoid the potential morbidity associated with resection.11–13 Many symptom-producing tumors develop rapidly from lesions that initially cannot be identified by conventional radiologic neuroimaging or directly visualized during surgery and are, therefore, not amenable to surgical resection. We describe for the first time infratentorial craniospinal radiation therapy (ICSRT), which could be an alternative to current therapies for patients with diffuse hemangioblastomas due to VHL, who are typically faced with a lifetime of morbidity due to their disease and decreased quality of life from innumerable surgical procedures and other interventions. We hypothesize that ICSRT to a dose of radiation close to spinal cord tolerance could sterilize microscopic CNS hemangioblastomas, allow for stabilization or regression of existing lesions, and stabilize or improve patient symptomatology.
Methods
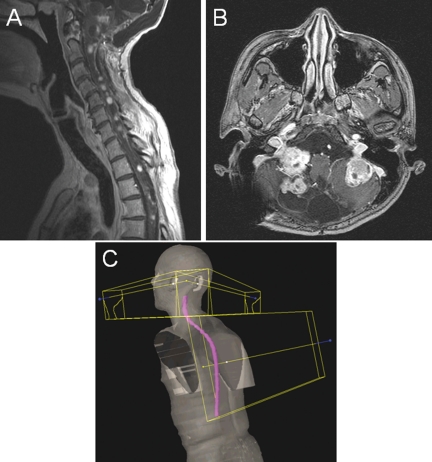
This retrospective analysis reviewed treatment courses for all patients with a diagnosis of VHL treated at the National Institutes of Health Clinical Research Center from 1998 to 2008. All patients with radiographic evidence of hemangioblastomas in both the cerebellum and the spine and brainstem who received ICSRT were included in this analysis, with a representative spine and cerebellum MRI slice noted in Figure 1A and 1B. Seven patients met these inclusion criteria and were included in this analysis.
Fig. 1.
Diffuse hemangioblastoma lesions and treatment fields. Hemangioblastomas associated with VHL disease are often diffuse and lead to significant morbidity. Depicted are representatives of the sagittal (A) and axial (B) views that were seen in the patients in this study. Also, because 98% of tumors occur in the infratentorial area, the field setup (C) included lateral beams shaped just to treat the cerebellum and a matched spine field that delivered ICSRT. Most patients were treated with ICSRT fields to 43.2 Gy, and all patients were treated without field modification or cone downs.
Patients were treated in the supine or prone position with customized thermoplastic immobilization devices. Patients were treated to the entire craniospinal axis with megavoltage photons, with the most common dose being 43.2 Gy to the entire craniospinal region without the hemispheres in 1.8-Gy fractions over 4.8 weeks. The posterior fossa was treated with an opposed lateral beam arrangement. The cranial volume extended caudally to 0.5 cm below the base of the skull and anteriorly to the posterior clinoid processes (Fig. 1C).
The spine was treated with a single posteroanterior beam (4 patients), an anteroposterior/posteroanterior technique (2 patients), or intensity-modulated radiation therapy (1 patient). The spinal volume extended cranially to the caudal border of the cranial field, caudally to the conus medullaris, and laterally to allow for a 1-cm margin on the lateral edges of the vertebral bodies. The caudal border of the spinal fields for patients with disease below the level of the termination of the spinal cord was caudal to the conus medullaris at the discretion of the treating radiation oncologist. The collimation of the cranial field was rotated to match the divergence of the spinal field. The spinal fields were typically feathered at least 0.5 cm, and the match line was moved at least twice (mean 2.4 match shifts) during the course of therapy.
Patients underwent weekly examinations during treatment. They underwent interval histories, performance status assessments, and complete neurologic examinations 1 month following the completion of ICSRT, typically followed by similar assessments at 3- to 6-month intervals for 2 to 3 years, then 6- to 12-month intervals thereafter. Patients also underwent craniospinal neuroimaging with serial MRIs of the brain and entire spinal axis at 6- to 12-month intervals following ICSRT.
Hemangioblastoma total tumor volume and number of lesions were determined from craniospinal neuroimaging. To avoid misinterpretation of a blood vessel, all contrast-enhanced lesions smaller than 3 mm in maximum diameter were excluded when determining both tumor volume and number of lesions. All lesions with any cystic component were classified as cystic lesions for analysis. Serial diagnostic MRIs were imported into a commercial treatment planning system (Eclipse; Varian Medical Systems) and fused with the CT simulation images used for craniospinal treatment planning purposes. All hemangioblastoma solid tumors, cystic lesions, and associated intracranial peritumoral edema at least 3 mm were contoured, with such lesions arising in the cerebellum distinguished from those originating in the spine or brainstem. All contours were performed by a single radiation oncologist (C.B.S.) and approved by an additional radiation oncologist (N.L.S.). Based on this hemangioblastoma delineation, separate tumor volumes for the cerebellum and the spine and brainstem were computed three-dimensionally by the treatment planning software.
Statistical analysis was performed using Microsoft Windows Office Excel 2003. Chi-squared or Fisher's exact test for independence was used to compare proportions, and Student's t-test was used for continuous measures. Statistical significance was defined as P ≤ .05.
Results
Among the study population, 4 patients were male and 3 were female. The cohort mean age at the time of ICSRT was 40.8 years (range, 32.3–52.5 y). The mean fractionated radiation dose to the posterior fossa was 44.2 Gy (range, 43.2–45.0 Gy). One patient received a stereotactic radiosurgery treatment of 22 Gy to a single dominant cerebellar lesion 12.4 months prior to treatment with ICSRT, whereas no other study patients received any additional radiation therapy prior to or after undergoing ICSRT. The mean fractionated dose to the spine was 44.1 Gy (range, 37.5–51.6 Gy). The initial target volumes for both the posterior fossa and the spine were treated without field modifications or tumor volume reductions, and therefore most patients had radiation administered to 43.2 Gy in 24 fractions over 4.8 weeks.
Number of Lesions and Tumor Volumes
Among all patients, there were a total of 84 hemangioblastomas prior to ICSRT (Table 1). Lesions were solid (n = 72) or cystic (n = 12). Patients had an average of 4.1 tumors in the cerebellum (range, 1–10) and 7.9 tumors in the spine and brainstem (range, 4–16). The mean pre-ICSRT patient tumor volume was 3.59 cm3 (range, 0.02–22.15 cm3) for cerebellar tumors and 1.90 cm3 (range, 0.13–4.16 cm3) for spine and brainstem tumors, for a mean total tumor volume of 5.48 cm3 (range, 0.19–26.31 cm3) among all study patients.
Table 1.
Number and volume of hemangioblastomas before and after ICSRT
| Pre-ICSRT | Post-ICSRT | |
|---|---|---|
| Mean number of cerebellar lesions | 4.1 | 4.1 |
| Mean number of spine/brainstem lesions | 7.9 | 8.9 |
| Cohort total number of lesions | 84 | 91 |
| Mean cerebellar lesion tumor volume | 3.59 cm3 | 4.90 cm3 |
| Mean spine/brainstem lesion tumor volume | 1.90 cm3 | 1.97 cm3 |
| Cohort mean total tumor volume | 5.48 cm3 | 6.87 cm3 |
When assessing the treatment response for the cohort within the first year following ICSRT, overall tumor volume stability of spine and brainstem lesions were noted. Surveillance imaging performed at a mean of 6.4 months following ICSRT showed that the mean tumor volume had increased 1.7% to 1.93 cm3 (range 0.09–4.31 cm3). This mean tumor volume decreased to 1.3% below the pre-ICSRT mean tumor volume at 12 months. Surveillance imaging was performed at a mean of 12.6 months following radiotherapy (1.87 cm3, range 0.09–4.39 cm3). The mean tumor volume for cerebellar tumors had increased 25.1% at 6 months surveillance (4.49 cm3, range 0.02–28.48 cm3) and 28.5% above the pre-ICSRT baseline at 12 months surveillance (4.61 cm3, range 0.01–29.11 cm3). This increase in tumor volume, however, was attributable largely to a single patient with very rapid cerebellar tumor volume growth just prior to beginning radiotherapy who experienced continued rapid growth in the first year following ICSRT.
At a mean follow-up of 73.8 months (range 40.3–155.6 months) after the completion of ICSRT, 91 total hemangioblastomas were identified among the study population, for an 8.3% increase in the number of lesions. The mean number of tumors following ICSRT remained constant in the cerebellum (4.1, range 0–9) and increased in the spine and brainstem to 8.9 (range 3–16). The mean post-ICSRT tumor volume increased 36.6% to 4.90 cm3 (range 0.00–30.53 cm3) for all cerebellar lesions but remained largely stable for spine and brainstem lesions (1.97 cm3, range 0.11–4.58 cm3, +3.8%). The mean total tumor volume for the cohort increased 25.3% to 6.87 cm3 (range 0.11–35.11 cm3).
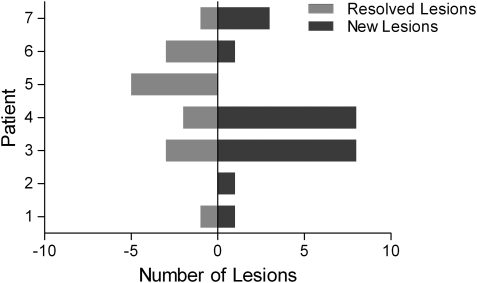
Complete radiographic resolution was achieved in 17.9% of hemangioblastomas identified on MR imaging immediately prior to ICSRT (Fig. 2). Among previously identified lesions, 9 spine and brainstem tumors and 6 cerebellar tumors resolved, with 7 (46.7%) of these lesions resolving within the first 6 months following ICSRT and the majority (66.7%) resolving within 13 months of radiation. Only 2 tumors (13.3%) resolved more than 2.5 years after ICSRT. On surveillance imaging, 22 new lesions were identified among the cohort, with 16 new spine and brainstem lesions and 6 new cerebellar tumors.
Fig. 2.
New and resolved hemangioblastomas following ICSRT. The number of lesions that have resolved are shown by bars to the left of the middle and the number of lesions that are new are to the right of the middle. Although some patients had more new than resolved lesions, the rate of new lesions is lower than historical controls.
In total, 23.9% (20/84) of hemangioblastomas identified on neuroimaging immediately prior to ICSRT were shown to have regressed following radiotherapy and remained at <50% of their original tumor volume on the most recent surveillance neuroimaging. Lesions present at initial presentation that completely resolved or remained at <50% of their original tumor volume were somewhat more likely to be solid than cystic (26.4% vs. 8.3%, P = .18), although this difference was not statistically significant. All but one lesion that showed regression were initially noted to be less than 1 cm in maximum diameter, which may be a potential threshold for treatment. Conversely, 25.0% (21/84) of lesions progressed or had an increase in volume by >50% following the completion of ICSRT. Lesions that progressed were significantly more likely to be cystic (50.0% vs. 20.8%, P = .03). The remaining 51.2% (43/84) exhibited prolonged size and volume stability following ICSRT.
Surgical Interventions
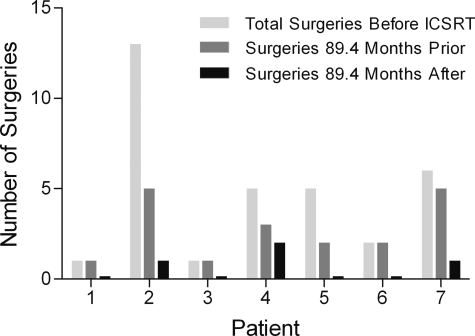
Prior to ICSRT, study patients required a total of 33 separate surgical procedures to remove 47 CNS hemangioblastomas (Fig. 3). In the 73.8 months prior to ICSRT, 17 surgical procedures to remove 28 lesions were performed, and during the mean follow-up of 73.8 months after radiation, only 4 surgical procedures among the cohort were performed to resect 8 symptomatic or progressive lesions.
Fig. 3.
Surgical interventions before and after ICSRT. The number of surgeries is depicted by a set of 3 bars for each patient: the first being the total number of surgeries the patient had before ICSRT, the second the number of surgeries in the mean of 89.2 months before ICSRT, and the third the number of surgeries in the mean of 89.2 months after ICSRT. Noted is a significant decrease of the number of surgeries the patients had after their radiation therapy.
Neurologic Signs and Symptoms
Three patients were maintained on steroid therapy prior to beginning ICSRT and all patients reported neurologic symptoms and had positive neurologic signs on physical examination prior to receiving radiotherapy. On examination, motor findings included upper extremity motor weakness (N = 3), lower extremity motor weakness (N = 3), and difficulty with ambulation (N = 4). Sensory signs and symptoms included upper extremity paresthesias or decreased sensation (N = 4), lower extremity paresthesias, decreased proprioception or decreased sensation to touch (N = 5), sustained upper extremity tremors, decreased fine motor coordination, or decreased control of range of motion (N = 3), and sustained lower extremity clonus, tremors, spasms, or decreased control of range of motion (N = 4). Patients reported pain in their necks (N = 1) and backs with or without lower extremity radiation (N = 4). Visual and hearing examinations revealed decreased visual acuity (N = 2), nystagmus or strabismus (N = 2), and hearing loss (N = 2). Patients also experienced dizziness or lightheadedness (N = 1) and nausea and emesis (N = 1).
Following ICSRT, 2 patients were successfully weaned off of steroids within 2 week and 3 months, respectively, of radiotherapy completion. Steroid administration was not started during ICSRT for any patient. One patient reported a significant improvement in lower back pain and lower extremity paresthesias following ICSRT. He was also found to have resolution of previously identified proximal lower extremity motor weakness. Signs and symptoms directly attributable to CNS hemangioblastomas exhibited prolonged stabilization in 4 patients, with 1 of these patients also having decreased back pain and decreased analgesic dependence. One patient, however, has become largely wheelchair dependent and has had increased back pain and difficulty with balance since receiving radiation therapy. His post-ICSRT course has been complicated by the diagnosis of bilateral renal cell carcinomas requiring multiple surgical procedures. One patient reported a mild increase in intermittent lower back pain with radiation into the right lower extremity that became clinically significant nearly 10 years after completing ICSRT.
Overall Survival
Three patients in this cohort, who suffered from VHL-associated pancreatic and renal neoplasms, died from progressive VHL disease that was not directly attributable to CNS hemangioblastomas. Patients died at a mean of 3.0 years following ICSRT (range, 0.3–5.4 years). For the entire cohort, the estimated OS was 85.7% at 2 years, 71.4% at 5 years, and 57.1% at 7 years.
Treatment Toxicity
ICSRT was well tolerated among the study population. Three patients developed grade ≥2 acute toxicity following the criteria of the Radiation Therapy Oncology Group/European Organisation for Research and Treatment of Cancer (RTOG/EORTC), and no patient developed acute hematologic toxicity. Acute toxicities included skin erythema (grade 1 in 2 patients, grade 2 in 1), esophagitis (grade 1 in 2, grade 3 in 1), and gastrointestinal (grade 1 abdominal discomfort in one, grade 2 nausea/emesis in two). Furthermore, no patient developed subacute radiation-induced toxicity or grade ≥1 RTOG/EORTC late toxicity of the spinal cord or brain.
Discussion
This study demonstrates that ICSRT can be administered safely and may be a novel therapeutic option that can be considered for patients with VHL predisposed to CNS hemangioblastomas.
Although complete microsurgical resection of symptomatic lesions provides excellent outcomes with tumor control in over 99% of lesions and symptom stabilization or improvement in over 90% of patients,14–17 these patients are subjected to a lifetime of surgical interventions, and a possible alternative or preventive measure would be a welcome option. To date, neither conventionally fractionated external beam radiation therapy nor stereotactic radiosurgery have proven to be successful alternatives and have been reserved for CNS hemangioblastomas not amenable to resection or recurrent lesions following resection. Most studies have shown that fractionated radiotherapy has limited local control in this patient population.4,5,18 Stereotactic radiosurgery has also been used for treatment because CNS hemangioblastomas are highly vascular tumors that are considered to be late-responding to radiotherapy and, therefore, should respond to higher biologically equivalent radiation doses in a single fraction.19 Despite high local control rates achieved in some published stereotactic radiosurgical series with limited follow-up, the use of stereotactic radiosurgery has diminishing local control at longer follow-up.7 Although stereotactic radiosurgery is similar to surgery because it is able to address only the lesion targeted, it has significantly lower local control and is, therefore, often reserved for unresectable and symptomatic lesions.
No registered clinical trials are currently assessing radiation therapy to treat VHL-associated hemangioblastomas, and ICSRT for patients with VHL has not previously been described.20 The use of ICSRT to treat small or asymptomatic diffuse craniospinal disease may prevent these tumors from progressing or becoming symptomatic during the disease course. It may also serve to prevent the development of new CNS hemangioblastomas or prevent or delay the progression of existing microscopic tumors21 that cannot be visualized by MRI but are known to progress to symptomatic lesions as per Ammerman et al.9,10 ICSRT is a treatment option that has the opportunity to improve disease outcomes, as opposed to the other local therapy options. Such treatment of asymptomatic lesions with surgery or with ablative radiosurgery is not feasible because of the risks and treatment-related morbidities associated with those interventions.
In our study ICSRT was administered, was found to be safe, and may have assisted in stabilizing VHL syndrome and decreasing intervention rates as compared with natural history studies. Although most patients were treated to a relatively high dose of 43.2 Gy to the entire craniospinal axis, no late spinal cord or brain toxicity was appreciated, showing that this regimen is reasonable for this population of patients. Five of the 7 patients in this cohort achieved stability or improvement of their preradiotherapy neurologic signs and symptoms, which compares favorably with 41% of lesions becoming symptomatic in the natural history study by Ammerman et al.9,10
In this study, the rate of surgical intervention for symptomatic or progressing lesions was reduced following ICSRT, and radiation therapy achieved prolonged stability or improvement of neurologic signs and symptoms in most patients in this cohort. This decreased surgical intervention rate is, however, confounded by the facts that (1) almost all of these patients had had so many surgical procedures in the past that they were not ideal candidates for further surgical intervention, and (2) lesions radiographically found to change or enlarge following ICSRT may have been more likely to be observed due to the belief that such changes were treatment related. Complete radiographic resolution was achieved in over one sixth (17.9%) of all tumors identified in this study prior to ICSRT, with most of these tumors resolving within the first year after radiotherapy. This compares very favorably with all previously published series that utilized external beam radiation therapy to treat CNS hemangioblastomas.4–6 It is possible that the increased utilization of MR imaging in this study compared with older fractionated radiation therapy series allowed for the identification of smaller lesions that would not have been evident using CT imaging. As this study demonstrated improved response to ICSRT in smaller lesions, it is possible that modern imaging has artificially improved the reported efficacy of radiation therapy in this series.
Although the mean total tumor volume of this cohort increased by 25.3% during the follow-up period, the rate of tumor progression in this study was significantly lower than what has been reported in natural history studies of VHL-associated hemangioblastomas, which have rates of progression up to 97%. Further evaluation would need to be done to ensure that these tumors were not just in a long quiescent phase and that the ICSRT was indeed slowing the progression and stabilizing disease. To avoid such bias, with a mean follow-up of 73.8 months in this study, we elected to report on our cohort after the mean time for tumors to complete approximately 2 complete growth and quiescent phase patterns (76 months, with a mean of 38 months per complete cycle).
Natural history studies of VHL patients being conducted at the National Institutes of Health20 may yield a better understanding of which hemangioblastomas are likely to grow and become symptomatic. This knowledge could allow for earlier interventions, when tumors may be more safely and effectively managed. As such, these studies will guide physicians in treatment decisions and avoid unnecessary surgical or radiosurgical procedures against individual tumors in these patients.
Longitudinal studies assessing ICSRT are needed to determine whether there is a decreased need for surgical intervention and decreased rates of tumor progression and symptomatology. From this study, it seems that patients with lesions less than 1 cm may benefit the most from ICSRT because all but one lesion under this threshold either regressed or remained stable after radiation was administered and because 19 of the 20 lesions that regressed were less than 1 cm in maximum diameter. It should also be determined whether ICSRT is best suited as a treatment or as a preventive measure to stop microscopic lesions from progressing to symptomatic lesions. At the present time, however, ICSRT should be considered as an alternative to such localized interventions to confer disease stability throughout the craniospinal axis for patients with VHL with diffuse CNS hemangioblastomas.
Conclusions
We first describe ICSRT as a new treatment modality for VHL patients with diffuse hemangioblastomas. ICSRT can achieve clinical and radiographic stability, reduce hemangioblastoma growth rate, and decrease the rate of surgical interventions for symptomatic lesions. ICSRT is well tolerated and may be a potential alternative to surgical resection in patients with disease not amenable to surgical resection. These findings serve as a basis for pursuing a phase II study anticipated to open at the Clinical Research Center at the National Institutes of Health in 2011 that will assess external beam infratentorial craniospinal radiation therapy in VHL patients with CNS hemangioblastomas.
Conflict of interest statement. None declared.
Funding
This work was supported by the intramural research program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Authors’ Contributions
CBS contributed to study concept and design, performed data collection and data analysis, computed tumor volumes, and drafted the manuscript. RRL contributed to study concept and design and data analysis. JO performed image registration. EHO contributed to study concept and design. KC contributed to study concept and design. NLS contributed to study concept and design, performed data collection and data analysis, computed tumor volumes, and drafted the manuscript. All authors read and approved the final manuscript.
References
- 1.Atlas SW, Lavi E, Fisher PG. Intraxial brain tumors. In: Atlas SW, editor. MRI of the Brain and Spine. 3rd ed. London: Lippincott Williams & Wilkins; 2001. pp. 389–394. [Google Scholar]
- 2.Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995;194:629–642. doi: 10.1148/radiology.194.3.7862955. [DOI] [PubMed] [Google Scholar]
- 3.Slater A, Moore NR, Huson SM. The natural history of cerebellar hemangioblastomas in von Hippel-Lindau disease. AJNR Am J Neuroradiol. 2003;24:1570–1574. [PMC free article] [PubMed] [Google Scholar]
- 4.Smalley SR, Schomberg PJ, Earle JD, Laws ER, Jr, Scheithauer BW, O'Fallon JR. Radiotherapeutic considerations in the treatment of hemangioblastomas of the central nervous system. Int J Radiat Oncol Biol Phys. 1990;18:1165–1171. doi: 10.1016/0360-3016(90)90454-r. doi:10.1016/0360-3016(90)90454-R. [DOI] [PubMed] [Google Scholar]
- 5.Sung DI, Chang CH, Harisiadis L. Cerebellar hemangioblastomas. Cancer. 1982;49:553–555. doi: 10.1002/1097-0142(19820201)49:3<553::aid-cncr2820490326>3.0.co;2-a. doi:10.1002/1097-0142(19820201)49:3<553::AID-CNCR2820490326>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Koh ES, Nichol A, Millar BA, Ménard C, Pond G, Laperriere NJ. Role of fractionated external beam radiotherapy in hemangioblastoma of the central nervous system. Int J Radiat Oncol Biol Phys. 2007;69(5):1521–1526. doi: 10.1016/j.ijrobp.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Asthagiri AR, Mehta GU, Zach L, et al. Prospective evaluation of radiosurgery for hemangioblastomas in von Hippel-Lindau disease. Neuro Oncol. 2010;12:80–86. doi: 10.1093/neuonc/nop018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:82–94. doi: 10.3171/jns.2003.98.1.0082. doi:10.3171/jns.2003.98.1.0082. [DOI] [PubMed] [Google Scholar]
- 9.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel-Lindau disease: implications for treatment. J Neurosurg. 2006;105:248–255. doi: 10.3171/jns.2006.105.2.248. doi:10.3171/jns.2006.105.2.248. [DOI] [PubMed] [Google Scholar]
- 10.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Integra Foundation Award: Long-term natural history of hemangioblastomas in von Hippel-Lindau disease: implications for treatment. Clin Neurosurg. 2006;53:324–331. [PubMed] [Google Scholar]
- 11.Constans JP, Meder F, Maiuri F, Donzelli R, Spaziante R, de Divitiis E. Posterior fossa hemangioblastomas. Surg Neurol. 1986;25:269–275. doi: 10.1016/0090-3019(86)90238-7. doi:10.1016/0090-3019(86)90238-7. [DOI] [PubMed] [Google Scholar]
- 12.Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989;70:24–30. doi: 10.3171/jns.1989.70.1.0024. doi:10.3171/jns.1989.70.1.0024. [DOI] [PubMed] [Google Scholar]
- 13.Lonser RR, Weil RJ, Wanebo JE, DeVroom HL, Oldfield EH. Surgical management of spinal cord hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:106–116. doi: 10.3171/jns.2003.98.1.0106. doi:10.3171/jns.2003.98.1.0106. [DOI] [PubMed] [Google Scholar]
- 14.Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D. Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery. 2001;48:55–62. doi: 10.1097/00006123-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Roonprapunt C, Silvera VM, Setton A, Freed D, Epstein FJ, Jallo GI. Surgical management of isolated hemangioblastomas of the spinal cord. Neurosurgery. 2001;49:321–327. doi: 10.1097/00006123-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH. Surgical management of brainstem hemangioblastomas in patients with von Hippel-Lindau disease. J Neurosurg. 2003;98:95–105. doi: 10.3171/jns.2003.98.1.0095. doi:10.3171/jns.2003.98.1.0095. [DOI] [PubMed] [Google Scholar]
- 17.Van Velthoven V, Reinacher PC, Klisch J, Neumann HP, Gläsker S. Treatment of intramedullary hemangioblastomas, with special attention to von Hippel-Lindau disease. Neurosurgery. 2003;53:1306–1313. doi: 10.1227/01.neu.0000093497.81390.29. doi:10.1227/01.NEU.0000093497.81390.29. [DOI] [PubMed] [Google Scholar]
- 18.Aksu G, Ulutin C, Fayda M, Saynak M. Cerebellar and multiple spinal hemangioblastomas and intraventricular meningioma managed with subtotal resection and external beam radiotherapy. report of a case with literature review. J BUON. 2005;10:405–409. [PubMed] [Google Scholar]
- 19.Hall EJ, Brenner DJ. The radiobiology of radiosurgery: rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381–385. doi: 10.1016/0360-3016(93)90367-5. doi:10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
- 20.ClinicalTrials.gov. Search for Clinical Trials. Available at http://clinicaltrials.gov . (Accessed March 4, 2011)
- 21.Vortmeyer AO, Frank S, Jeong SY, et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel-Lindau disease. Cancer Res. 2003;63:7051–7055. [PubMed] [Google Scholar]