Abstract
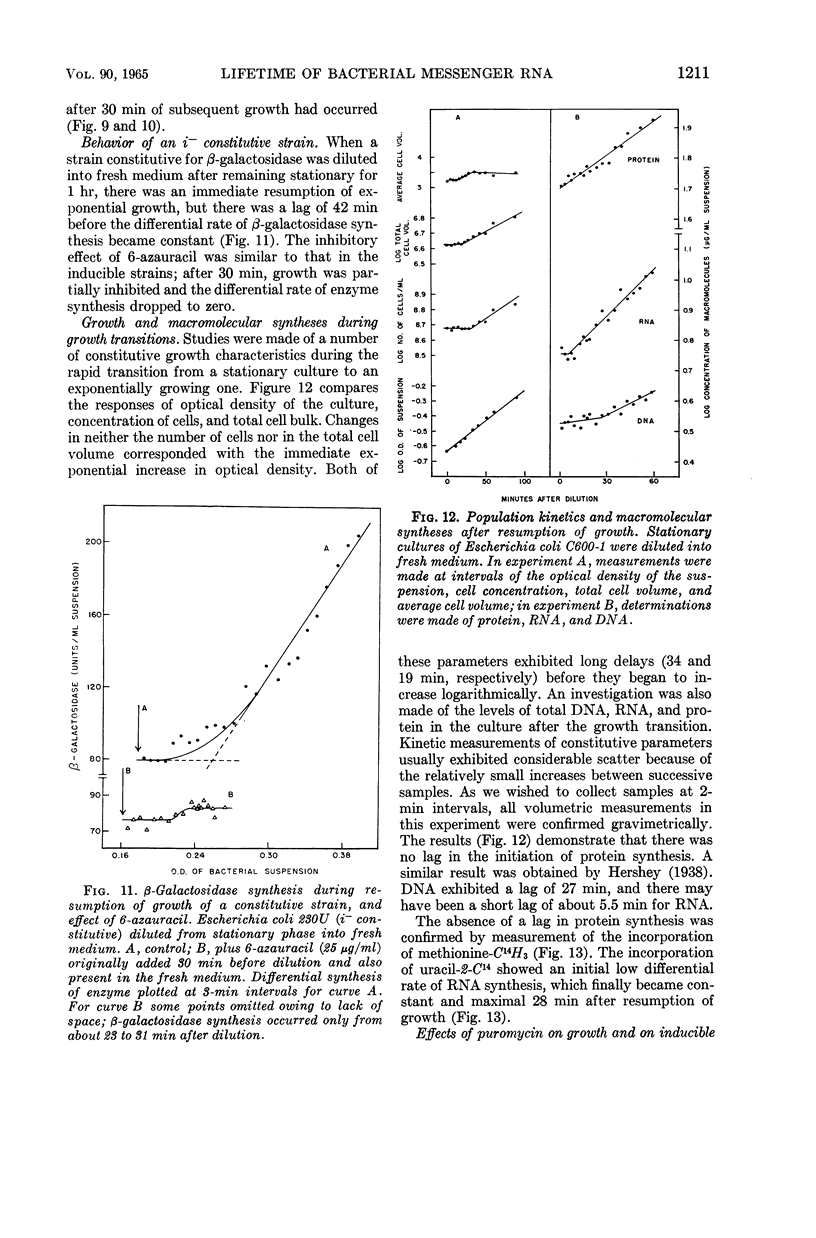
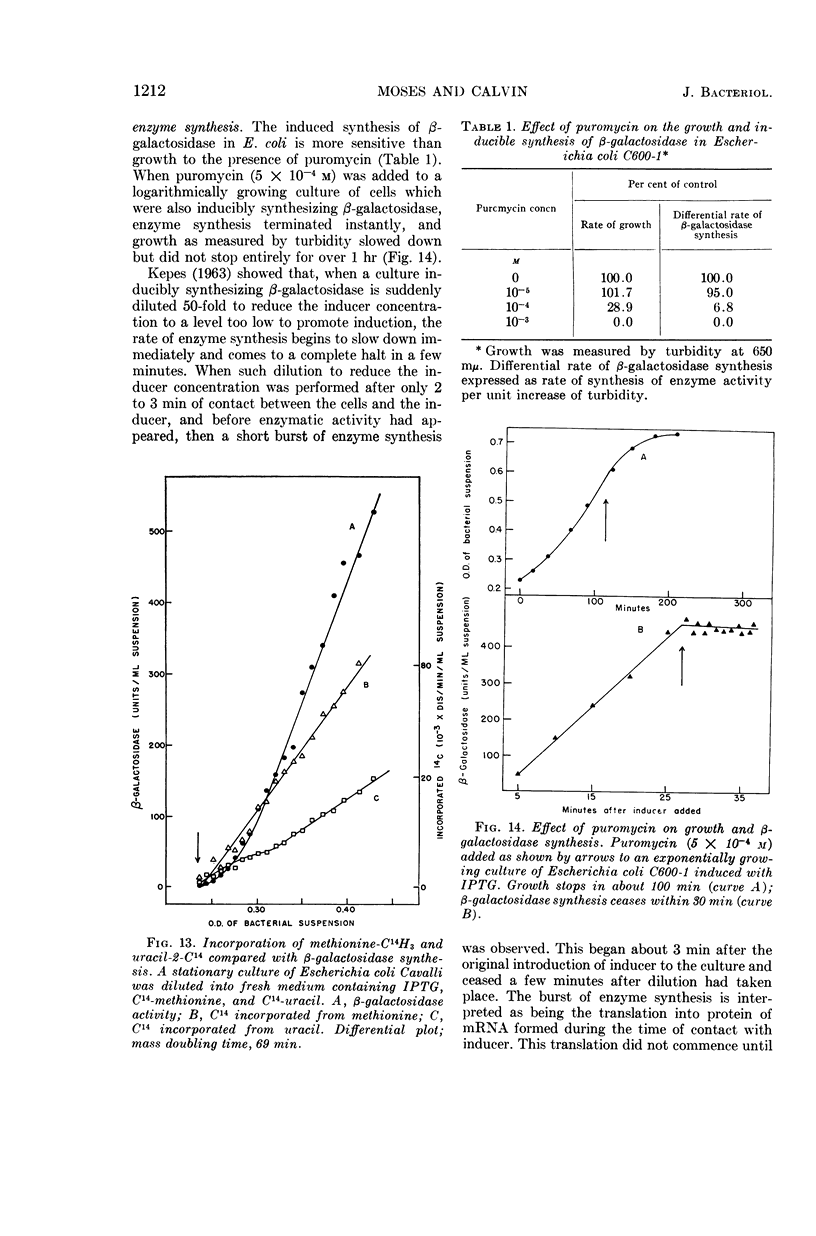
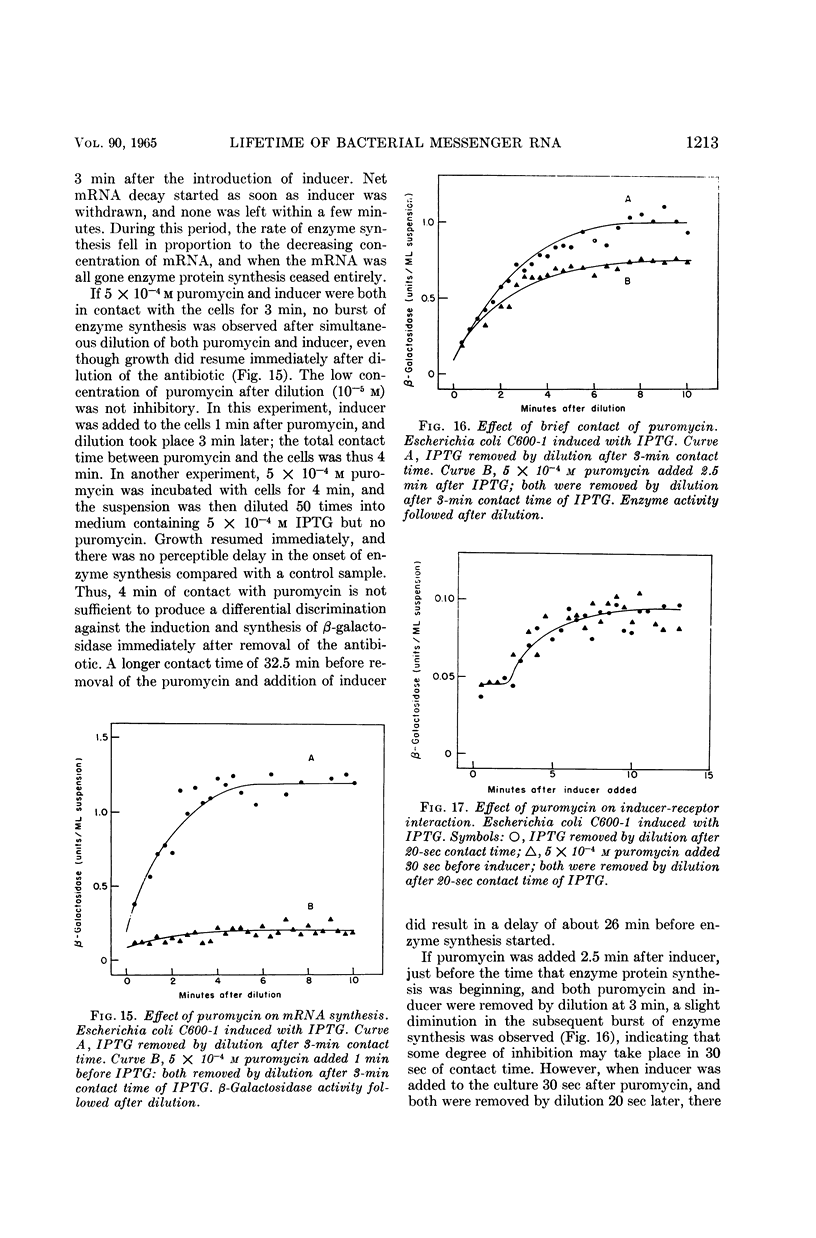
Moses, V. (University of California, Berkeley), and M. Calvin. Lifetime of bacterial messenger ribonucleic acid. J. Bacteriol. 90:1205–1217. 1965.—When cells from a stationary culture of Escherichia coli were placed in fresh medium containing inducer for β-galactosidase, growth, as represented by increase in turbidity and by total protein synthesis, started within 30 sec. By contrast, β-galactosidase synthesis was greatly delayed compared with induction during exponential growth. Two other inducible enzymes (d-serine deaminase and l-tryptophanase) and one repressible enzyme (alkaline phosphatase) showed similar lags. The lags were not due to catabolite repression. They could not be reduced by pretreatment of the culture with inducer, or by supplementing the fresh medium with amino acids or nucleotides. The lag was also demonstrated by an i− mutant constitutive for β-galactosidase synthesis. An inhibitor of ribonucleic acid (RNA) synthesis, 6-azauracil, preferentially inhibited β-galactosidase synthesis compared with growth in both inducible and constitutive strains. Puromycin, an inhibitor of protein synthesis, acted as an inhibitor at additional sites during the induction of β-galactosidase synthesis. No inhibition of the reactions proceeding during the first 20 sec of induction was observed, but puromycin seemed to prevent the accumulation of messenger RNA during the period between 20 sec and the first appearance of enzyme activity after 3 min. It is suggested that these observations, together with many reports in the literature that inducible enzyme synthesis is more sensitive than total growth to some inhibitors and adverse growth conditions, can be explained by supposing that messenger RNA for normally inducible enzymes is biologically more labile than that for some normally constitutive proteins. The possible implications of this hypothesis for the achievement of cell differentiation by genetic regulation of enzyme synthesis are briefly discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERRAH G., KONETZKA W. A. Selective and reversible inhibition of the synthesis of bacterial deoxyribonucleic acid by phenethyl alcohol. J Bacteriol. 1962 Apr;83:738–744. doi: 10.1128/jb.83.4.738-744.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEZI J. A., COWIE D. B. Kinetic studies of beta-galactosidase induction. Biophys J. 1961 Nov;1:639–647. doi: 10.1016/s0006-3495(61)86913-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES E. A., RIBBONS D. W. STUDIES ON THE ENDOGENOUS METABOLISM OF ESCHERICHIA COLI. Biochem J. 1965 May;95:332–343. doi: 10.1042/bj0950332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGELBERG H., ARTMAN M. STUDIES ON STREPTOMYCIN-DEPENDENT BACTERIA: EFFECT OF STREPTOMYCIN ON PROTEIN SYNTHESIS BY STREPTOMYCIN-SENSITIVE, STREPTOMYCIN-RESISTANT AND STREPTOMYCIN-DEPENDENT, MUTANTS OF ESCHERIHIA COLI. Biochim Biophys Acta. 1964 Feb 17;80:256–268. doi: 10.1016/0926-6550(64)90098-2. [DOI] [PubMed] [Google Scholar]
- FRETER R., OZAWA A. EXPLANATION FOR LIMITATION OF POPULATIONS OF ESCHERICHIA COLI IN BROTH CULTURES. J Bacteriol. 1963 Nov;86:904–910. doi: 10.1128/jb.86.5.904-910.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., SIDDIQI O. Suppression of mutations in the alkaline phosphatase structural cistron of E. coli. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1121–1127. doi: 10.1073/pnas.48.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN L., MICO U. J., CROCKER T. T. Nuclear-cytoplasmic relationships in human cells in tissue culture. IV. A study of some aspects of nucleic acid and protein metabolism in enucleate cells. Biochim Biophys Acta. 1960 Dec 4;45:82–86. doi: 10.1016/0006-3002(60)91428-1. [DOI] [PubMed] [Google Scholar]
- HABERMANN V. The effect of 6-azauracil on microorganisms inhibited by chloramphenicol. Biochim Biophys Acta. 1961 Apr 29;49:204–211. doi: 10.1016/0006-3002(61)90884-8. [DOI] [PubMed] [Google Scholar]
- HARTWELL L. H., MAGASANIK B. THE MOLECULAR BASIS OF HISTIDASE INDUCTION IN BACILLUS SUBTILIS. J Mol Biol. 1963 Oct;7:401–420. doi: 10.1016/s0022-2836(63)80033-9. [DOI] [PubMed] [Google Scholar]
- HENDERSON T. R. Differential inhibition of beta-galactosidase induction and synthesis by deuterium oxide. Biochem Biophys Res Commun. 1962 Oct 17;9:240–245. doi: 10.1016/0006-291x(62)90066-9. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., FURTH J. J., MALAMY M., ALEXANDER M. The role of deoxyribonucleic acid in ribonucleic acid synthesis. III. The inhibition of the enzymatic synthesis of ribonucleic acid and deoxyribonucleic acid by actinomycin D and proflavin. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1222–1230. doi: 10.1073/pnas.48.7.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- LARK K. G., LARK C. Changes during the division cycle in bacterial cell wall synthesis, volume, and ability to concentrate free amino acids. Biochim Biophys Acta. 1960 Oct 7;43:520–530. doi: 10.1016/0006-3002(60)90474-1. [DOI] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Induction and repression of beta-galactosidase in non-growing Escherichia coli. Biochem J. 1961 Jun;79:489–496. doi: 10.1042/bj0790489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTERN C. F., BRACKETT F. S., OLSON B. J. Determination of number and size of particles by electrical gating: blood cells. J Appl Physiol. 1957 Jan;10(1):56–70. doi: 10.1152/jappl.1957.10.1.56. [DOI] [PubMed] [Google Scholar]
- MCFALL E., MAGASANIK B. Thymine starvation and enzyme synthesis. Biochim Biophys Acta. 1960 Dec 18;45:610–612. doi: 10.1016/0006-3002(60)91505-5. [DOI] [PubMed] [Google Scholar]
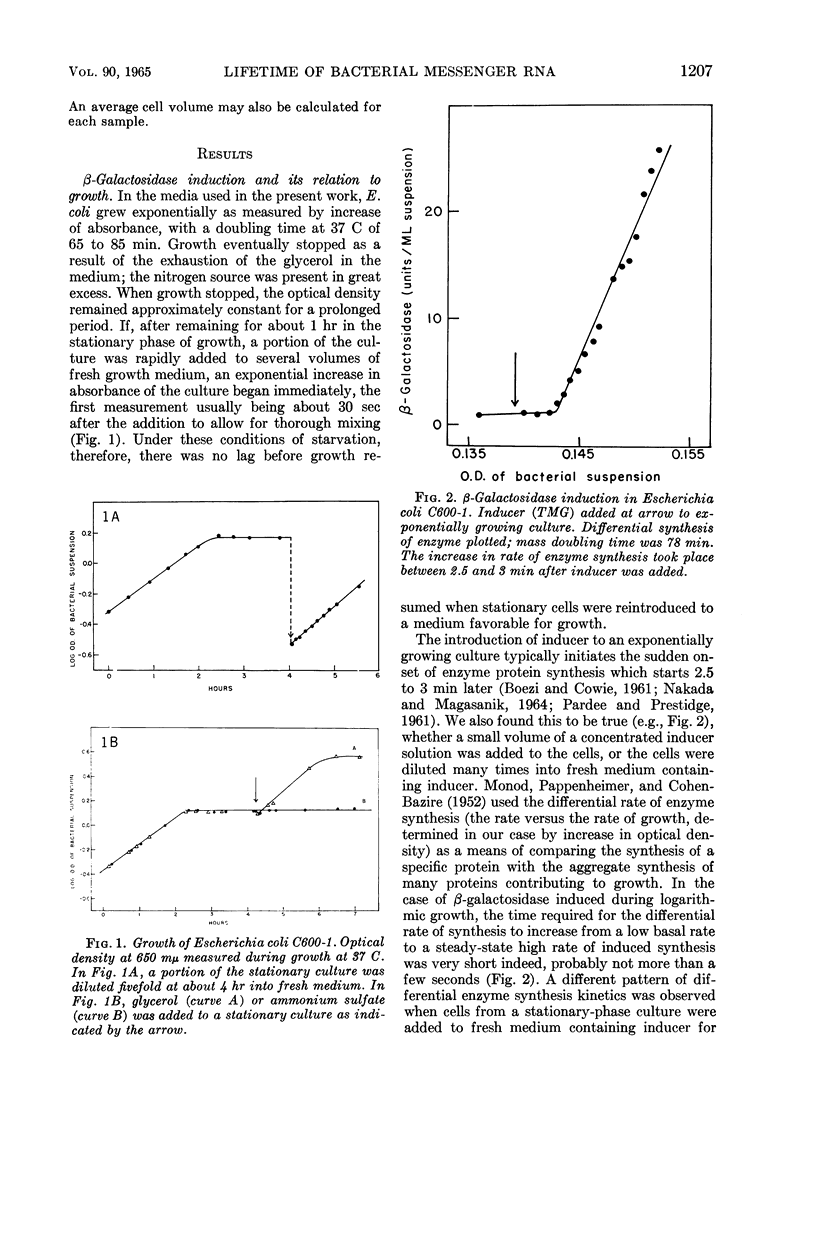
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
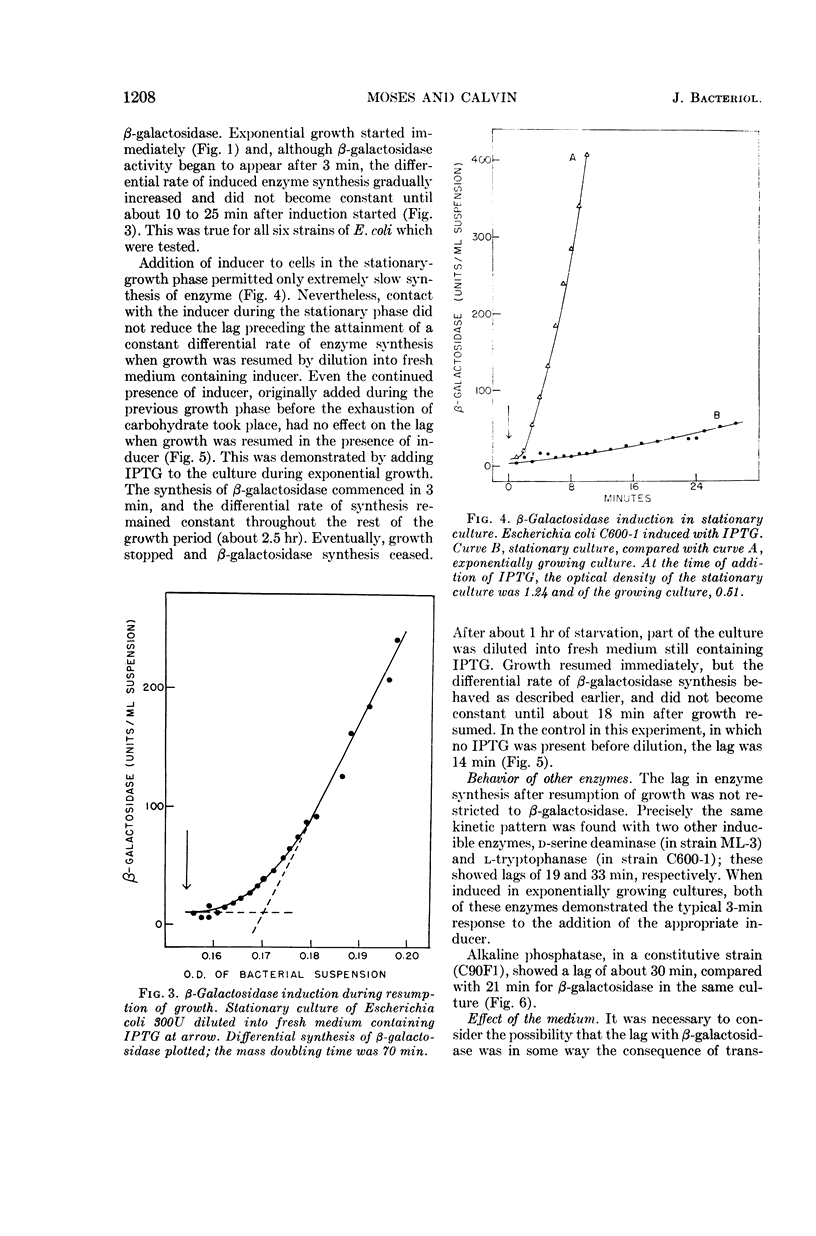
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K. CHANGES IN THE INDUCIBILITY OF GALACTOKINASE AND BETA-GALACTOSIDASE DURING INHIBITION OF GROWTH IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Oct 1;77:318–328. doi: 10.1016/0006-3002(63)90502-x. [DOI] [PubMed] [Google Scholar]
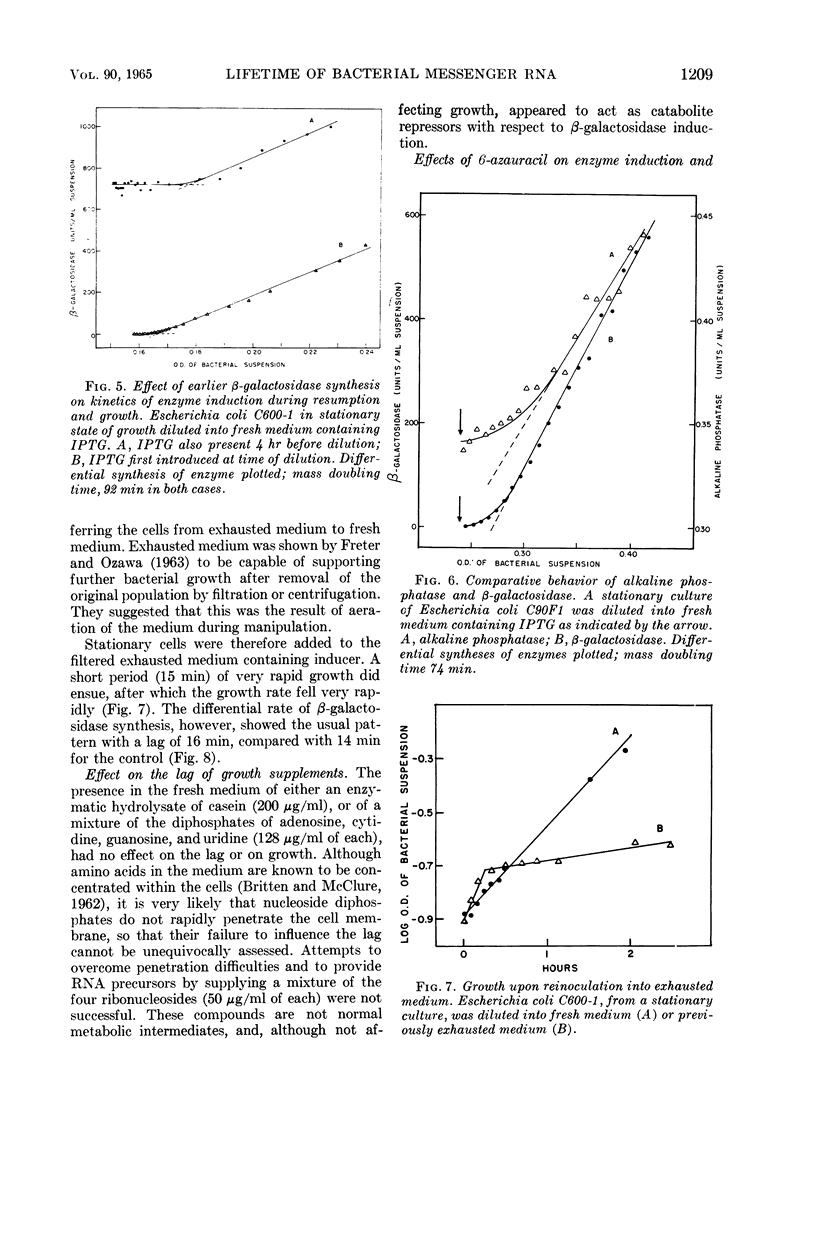
- PARDEE A. B., PRESTIDGE L. S. INACTIVATION OF BETA-GALACTOSIDASE INDUCTION BY ULTRAVIOLET LIGHT. Biochim Biophys Acta. 1963 Dec 20;76:614–621. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
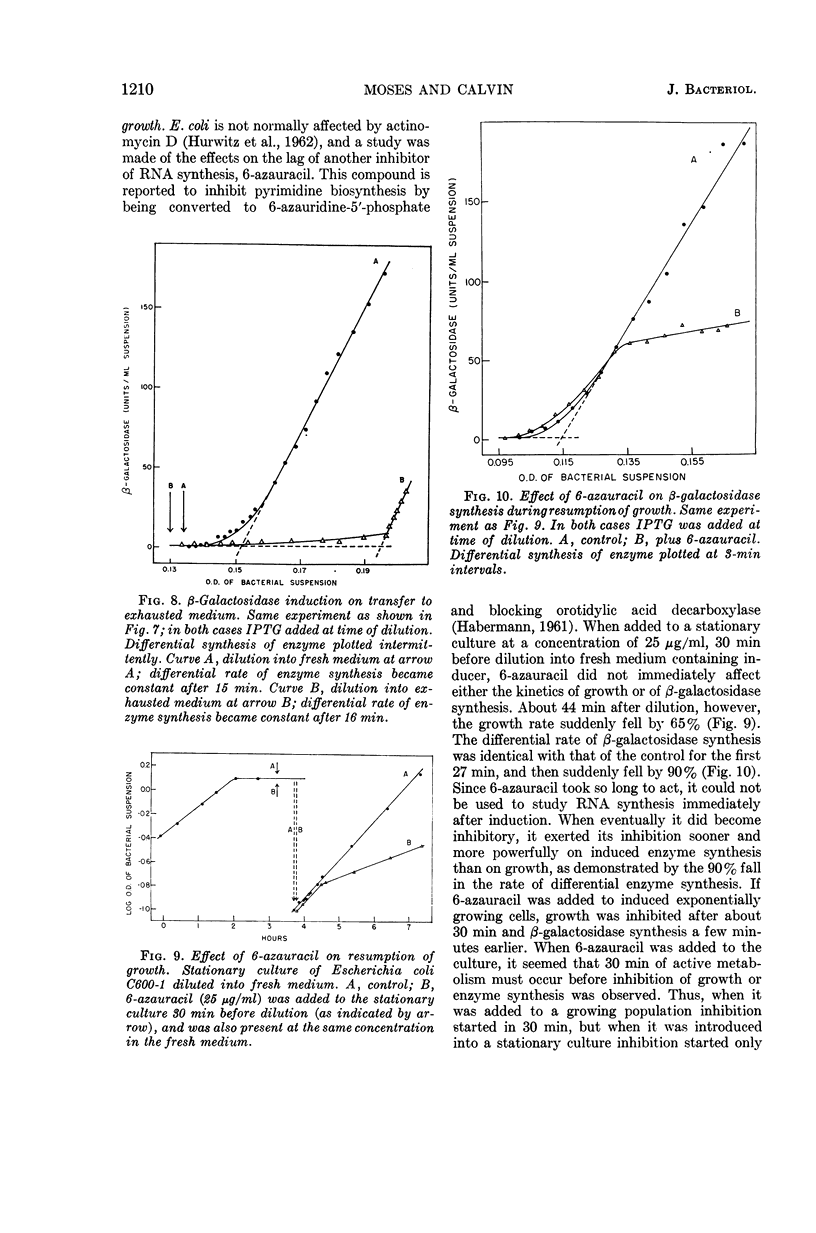
- PRESCOTT D. M. Nuclear synthesis of cytoplasmic ribonucleic acid in Amoeba proteus. J Biophys Biochem Cytol. 1959 Oct;6:203–206. doi: 10.1083/jcb.6.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESCOTT D. M. The nuclear dependence of RNA synthesis in Acanthamoeba sp. Exp Cell Res. 1960 Feb;19:29–34. doi: 10.1016/0014-4827(60)90034-3. [DOI] [PubMed] [Google Scholar]
- SELLS B. H. PUROMYCIN: EFFECT ON MESSENGER RNA SYNTHESIS AND BETA-GALACTOSIDASE FORMATION IN ESCHERICHIA COLI 15T. Science. 1965 Apr 16;148(3668):371–373. doi: 10.1126/science.148.3668.371. [DOI] [PubMed] [Google Scholar]
- SELLS B. H. RNA SYNTHESIS AND RIBOSOME PRODUCTION IN PUROMYCIN-TREATED CELLS. Biochim Biophys Acta. 1964 Feb 17;80:230–241. doi: 10.1016/0926-6550(64)90095-7. [DOI] [PubMed] [Google Scholar]
- SYPHERD P. S., DEMOSS J. A. THE STIMULATION BY CHLORAMPHENICOL OF "REPRESSOR" FORMATION IN ESCHERICHIA COLI. Biochim Biophys Acta. 1963 Dec 20;76:589–599. [PubMed] [Google Scholar]
- SYPHERD P. S., STRAUSS N. THE ROLE OF RNA IN REPRESSION OF ENZYME SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Dec;50:1059–1066. doi: 10.1073/pnas.50.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer T., Harris H. Regulation of enzyme synthesis in an enucleate cell. Biochem J. 1964 May;91(2):282–286. doi: 10.1042/bj0910282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sypherd P. S., Strauss N. CHLORAMPHENICOL-PROMOTED REPRESSION OF beta-GALACTOSIDASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Mar;49(3):400–407. doi: 10.1073/pnas.49.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON A. R., SCHWEET R. ROLE OF THE GENETIC MESSAGE IN INITIATION AND RELEASE OF THE POLYPEPTIDE CHAIN. Nature. 1964 May 2;202:435–437. doi: 10.1038/202435a0. [DOI] [PubMed] [Google Scholar]



