Abstract
Background
RovA is a global transcriptional regulator of gene expression in pathogenic Yersinia. RovA levels are kept in check by a sophisticated layering of distinct transcriptional and post-transcriptional regulatory mechanisms. In the enteropathogen Y. pseudotuberculosis, we have previously reported that the extracytoplasmic stress sensing CpxA-CpxR two-component regulatory system modulates rovA expression.
Methodology/Principal Findings
In this study, we characterized CpxR phosphorylation (CpxR∼P) in vitro, and determined that phosphorylation was necessary for CpxR to efficiently bind to the PCR-amplified upstream regulatory region of rovA. The precise CpxR∼P binding site was mapped by a nuclease protection assay and directed mutagenesis confirmed that in vivo binding to the rovA promoter inhibits transcription. Reduced RovA production was most pronounced following CpxR∼P accumulation in the Yersinia cytoplasm during chronic Cpx pathway activation and by the indiscriminate phosphodonor action of acetyl phosphate.
Conclusions/Significance
Cpx pathway activation restricts levels of the RovA global regulator. The regulatory influence of CpxR∼P must therefore extend well beyond periplasmic quality control in the Yersinia envelope, to include genes involved in environmental survival and pathogenicity.
Introduction
Extracytoplasmic stress (ECS) has a deleterious effect on bacterial fitness, since it impacts on cell envelope integrity, protein folding and function. Bacteria have consequently evolved multiple signaling pathways to counter these situations [1]. One of these is a two-component regulatory system (TCRS) composed of CpxA and CpxR (for conjugative plasmid expression). In response to diverse stresses (such as high pH, detergents, EDTA, altered membrane lipid composition and bacterial adhesion to surfaces) that ultimately leads to protein misfolding in the periplasm, the Cpx pathway induces synthesis of periplasmic protein folding and degradation factors that aid in the (re)folding of extracytoplasmic proteins and protein complexes [2], [3], [4].
Spanning the inner membrane, CpxA is a histidine kinase possessing three distinct activities in E. coli – autokinase, CpxR kinase and CpxR phosphatase activity [5]. In the absence of inducing signal, CpxA activity is subdued by the binding in the periplasm of the accessory factor CpxP [6]. In recognition of ECS signals however, CpxP is titrated away from CpxA and is targeted for degradation by the DegP serine protease [7], [8]. This enables CpxA to become auto-phosphorylated presumably at the conserved histidine residue at position 249 [5]. This permits the phosphate to be relayed to the CpxR response regulator that, based on homology to other response regulators, would occur on the aspartate residue at position 51 [9]. At least in E. coli, CpxR∼P is then capable of up- or down-regulating the transcription of many genes that function to alleviate the effects of ECS [2], [3], [4]. Once this has occurred, status quo is restored by the phosphatase activity of CpxA, which de-phosphorylates CpxR [5]. Thus, a consequence of generating CpxA phosphatase deficient E. coli, as would result from a full length cpxA deletion mutant or so-called gain-of-function mutants (designated cpxA*), would be to accumulate CpxR∼P [5], [10], [11], [12], [13], [14]. In these situations, acetyl phosphate (acetyl∼P), a small molecular weight phosphodonor, can also elevate CpxR∼P levels [5], [15], [16], [17]. This CpxA-independent phosphorylation of CpxR by acetyl∼P is potentially a global signal reflecting the status of bacterial growth and central metabolism [18].
Homologues to components of the Cpx pathway exist in a number of clinically important bacterial pathogens. We have recently studied the function of the Cpx TCRS in the enteropathogen Yersinia pseudotuberculosis [19], [20], a bacterium causing self-limiting gastroenteritis in infected individuals [21]. Intriguingly, loss of CpxA in this bacterium caused a down-regulation of several determinants known to be important in the pathogenesis of disease in mouse infection models; most notably, the plasmid-encoded Ysc-Yop type III secretion system (T3SS), an integral outer membrane adhesin, termed invasin, and RovA, a member of the MarR/SlyA family of transcriptional regulators [19], [20]. These studies provided the first indications that CpxR∼P could act as a negative transcriptional regulator of Y. pseudotuberculosis virulence factors. The fact that we could demonstrate in vitro CpxR∼P binding to inv and rovA promoters supported this view, although typical consensus CpxR∼P binding sites in these regions were never located [20]. Moreover, it was assumed from this earlier work that Y. pseudotuberculosis lacking CpxA phosphatase activity must accumulate CpxR∼P, serving as the fundamental basis for transcriptional repression of virulence gene expression in this mutant background [19], [20]. However, this critical notion is unproven.
RovA was first identified in Yersinia because it was required for transcription of the inv gene encoding for invasin [22], [23]. Whole-genome analyses has since indicated a global regulatory role for RovA in pathogenic Yersinia [24], [25], [26]. Additional virulence strategies among the RovA regulon include the prominent pH 6 antigen chaperone-usher system, the Ysc-Yop T3SS, and other uncharacterized potential secretion systems. To regulate these virulence determinants, RovA levels are therefore tightly controlled by multiple pathways in accordance with the prevailing environmental growth conditions. Dynamic regulatory mechanisms are obviously paramount for RovA to impart significant global control on virulence gene transcription in Yersinia. Thus, this study therefore seeks to substantiate the contribution of the Cpx TCRS to control of RovA-dependent virulence gene regulation in Yersinia.
Materials and Methods
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids can be viewed in Table S1. We consistently used Y. pseudotuberculosis YPIII/pIB102 (serotype III) as the parental strain. pIB102 is a virulence plasmid encoding for the Ysc-Yop T3SS and is a variant of cryptic pIB1. It differs only by a kanamycin resistance cartridge inserted into the yadA gene, which does not reduce the pathogenicity of YPIII/pIB102 in mouse models [27]. The strain also harbors an internal duplication within phoP located on the chromosome. This is predicted to generate an inactive truncated variant of the PhoP response regulator that impairs bacteria growth and survival inside macrophages [28]. Bacteria were normally cultivated in Luria-Bertani (LB) agar or broth at either 26°C (Y. pseudotuberculosis) or 37°C (E. coli) with aeration. When examining transcription and translation from rovA and inv, bacteria were grown at both 26°C and 37°C in LB broth to late stationary phase. These conditions were selected on the basis that reports from other laboratories have clearly established the peak rovA and inv expression occurs in bacteria grown at low temperature to stationary phase [22], [24]. Where required, antibiotics were added at the final concentrations of carbenicillin (Cb; 100 µg per ml), kanamycin (Km; 50 µg per ml), Trimethoprim (Tp; 10 µg per ml) and chloramphenicol (Cm; 25 µg per ml).
Mutant construction
To construct individual in-frame deletions, site-directed amino acid substitutions and nucleotide ‘shuffle’ mutations, we applied the standard overlap PCR technique using the relevant primer combinations listed in Table S2. To facilitate the sequencing process (performed by MWG Biotech AG, Ebersberg, Germany), all amplified fragments were initially cloned into pCR®4-TOPO TA (Invitrogen AB, Stockholm, Sweden). Confirmed fragments were then lifted into the mutagenesis vector, pDM4. These mutagenesis constructs were then conjugated by E. coli S17-1λpir into Y. pseudotuberculosis. Mutated alleles were initially introduced into the recipient genome by a single cross-over event. A second single cross-over event followed to secure the complete allelic exchange, which was initially screened for on the basis of sacB-dependent sucrose sensitivity [19], [29]. The presence of the mutated allele in the Y. pseudotuberculosis genome was then confirmed by diagnostic PCR and sequence analysis of the amplified regions flanking the mutation.
Western blotting
Protein derived from lysates of the bacterial pellet was fractionated by 12% (for invasin) or 15% (for RovA) SDS-PAGE. Protein was then transferred to Schleicher and Schuell Protran® nitrocellulose (GE Healthcare) using a Hoefer semi-dry transfer assembly. Proteins of interest were bound with specific rabbit polyclonal antibodies that were a gift from Petra Dersch (anti-RovA), Hans Wolf-Watz (anti-invasin), Thomas Silhavy (anti-MBP-CpxR) or Shu-ichi Nakayama (anti-CpxR). These were then detected with an anti-rabbit monoclonal antibody conjugated with horse radish peroxidase (GE Healthcare) and a homemade chemiluminescent solution.
nlpE cloning and expression
The primer combination for PCR amplification of Y. pseudotuberculosis nlpE is listed in Table S2. The DNA fragment was cloned into pBAD18 [30] using EcoRI/XbaI restriction to generate pJF027. This placed nlpE expression under arabinose control. The nlpE allele from E. coli cloned under arabinose control in pBAD18 (termed pND18) was a gift from Thomas Silhavy. These two constructs along with the vector control were electroporated into Yersinia. To induce nlpE expression, overnight bacterial cultures were used to seed fresh growth medium supplemented with 0.2% (w/v) L-arabinose and appropriate antibiotic selection.
Cloning, expression and purification of CpxR variants
Alleles encoding CpxR wild type and the CpxRD51A and CpxRM199A variants were amplified with gene specific primers (Table S2) using template DNA derived from parental Y. pseudotuberculosis or the respective mutants and cloned with NdeI and XhoI in front of the IPTG inducible promoter of pET22b(+). These expression plasmids were maintained in E. coli BL21(DE3) plysS allowing for the IPTG-inducible expression of individual CpxR variants fused to a C-terminal His(6) tag. Recombinant protein was purified as previously described [20].
Electrophoretic mobility shift assay (EMSA)
32P end-labeled forward primers listed in Table S2 were used for the PCR amplification of the promoter regions of cpxR/P, rovA, ppiA, ail, ail-like, inv, psaA and psaE. The amplified DNA fragments were purified by agarose gel electrophoresis. Purified CpxR was mixed with the DNA fragments (∼2 to 5 nM) in a 20 µL reaction volume containing 20 mM Tris-HCl pH 7.0, 30 mM Acetyl phosphate, 125 mM KCl, 10 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 0.25 mg per ml BSA and 5 µg per ml sonicated salmon sperm DNA. BSA corresponding to the highest CpxR concentration (1.5 µM) was used as a negative control, and about 7 fold excess of unlabeled PCR amplified DNA was used in a competition reaction. After incubation at 30°C for 1 h and addition of the DNA dye solution (40% glycerol, 0.05% bromophenol blue, 0.05% xylene cyanol), the mixture was loaded directly onto a pre-run 5% polyacrylamide gel. Gel electrophoresis was performed in 1× TBE at 100 V for 2 h at 4°C. After gel drying, signals were detected by autoradiography with a Storm 860 PhosphorImager (Molecular Dynamics).
DNase 1 footprinting assay
The primers pE-rovAfor3, pE-ppiAfor, pcpxRb and pcpxRfor were radioactively labeled with 32P using γ32P-ATP (Perkin Elmer) and T4 polynucleotide kinase (Fermentas). The labeled pE-rovAfor3, pE-ppiAfor and pcpxRb were paired with unlabeled pE-rovArev3, pE-ppiArev and pcpxPb respectively and used to PCR amplify promoter regions of rovA (314 bp), ppiA (276 bp) and the divergent cpxR-cpxP (245 bp). For a control, the labeled pcpxRfor was paired with unlabeled pcpxRrev for the PCR amplification of an internal region of cpxR (389 bp). Subsequently, 1.5 nM of the amplified DNA fragments and 0, 50, 100, 200 and 400 nM acetyl∼P phosphorylated CpxRwt::His6 were mixed in a 40 µL reaction containing 25 mM Hepes (pH 8), 100 mM potassium glutamate, 0.5 mg/mL BSA. After incubation of the reaction mixture at room temperature for 10 minutes, 4 µl DNase 1 (0.0005 mg/mL; Sigma) was added and the reaction was left for 80 seconds at 37°C. The DNase 1 digestion was stopped by addition of phenol/chloroform and 200 µL DNase 1 STOP (0.4 M sodium acetate (pH 5), 2.5 mM EDTA, 10 ug/mL herring sperm DNA), and then the DNA was precipitated using ethanol. Samples were analyzed on a 7% denaturing polyacrylamide gel and visualized with a Storm 860 PhosphorImager (Molecular Dynamics).
Visualization of phosphorylated CpxR (CpxR∼P)
Purified CpxR variants labeled with acetyl∼P were mixed with 2× loading buffer (0.02% (w/v) Bromophenol blue, 20% (v/v) Glycerol). For analysis, samples were either fractionated on a SDS-12%-PAGE or a Manganese(II)-Phos-tag™ (33 µM) 12.5% acrylamide gel. Purified CpxR∼P was detected by the Pro-Q® Diamond phosphoprotein gel staining method as described by the manufacturer (Invitrogen). To confirm equal loading, subsequent detection of total protein present on the same gel was revealed by SYPRO® Ruby staining (Invitrogen). In both cases, fluorescent output was recorded using a Fluor-S™ MultiImager (BioRad) and band intensity quantified with Quantity One® quantitation software version 4.2.3 (BioRad). The presence of recombinant purified CpxR∼P or that which is produced endogenously and present in bacterial lysates was also assessed by coupling Manganese(II)-Phos-tag™ acrylamide gel fractionation to immunoblotting with monospecific polyclonal anti-CpxR antiserum. The Manganese(II)-Phos-tag™ approach is an affinity based system for the recognition and separation of anionic substrates, such as phosphorylated proteins, during polyacrylamide electrophoresis [31]. For example, characteristic separation patterns for phosphoprotein isoforms can be generated according to the number and or site of the phosphate group. As a result, it later proved to be routinely applicable to in vitro and in vivo visualization of TCRS response regulator aspartate phosphorylation [32]. Samples for this analysis were generated from pelleted bacteria vigorously resuspended in 1.2 M formic acid preceding a very brief incubation at room temperature. Prior to fractionation, lysates were solubilized by addition of 4× loading buffer (250 mM Tris-HCl pH 6.8, 8% SDS, 40% glycerol, 4% BME, and 0.08% Bromophenol Blue,) and neutralized by the addition of minuscule amounts of 5 M NaOH.
Biophysical analysis of intact and digested CpxR∼P
The reduction of CpxR∼P using sodium borohydride was performed as described previously [33], [34]. Before the reaction, CpxR∼P was desalted using C18 micro columns and dried by vacuum centrifugation [35], [36]. Controls were performed using unphosphorylated CpxR under identical conditions.
Intact mass determination of CpxR and CpxR∼P utilized purified recombinant CpxR, and this CpxR phosphorylated with acetyl∼P. Duplicate samples of both phosphorylated and non-phosphorylated CpxR were then desalted on homemade C18 columns [35], [36]. For analysis by ESI-MS, the proteins were dissolved in 50% (v/v) acetonitrile containing 0.5% (v/v) formic acid. ESI-MS spectra of intact CpxR and CpxR∼P were acquired by ESI-MS in the positive ion mode using a Q-Tof ultima mass spectrometer (Waters, Manchester, UK). Spectra were acquired off-line using nano spray capillaries (Q-tof) from Proxeon (Odense, Denmark) and deconvoluted using the MassLynx 4.0 software.
Peptides of CpxR and CpxR-P were prepared using trypsin and pepsin. Protein was desalted using C18 micro columns and dried by vacuum centrifugation [35], [36]. In-solution digestion using trypsin was performed for 40 min at 37°C in 10 µl of fresh 50% ammonium bicarbonate containing 10 ng/µl of sequencing grade trypsin (Promega Biotech AB, Nacka, Sweden). In-solution digestion using pepsin (pepsin from porcine gastric mucosa, Sigma Life Sciences) was performed for 40 min at 37°C in 20 µl of 5% (v/v) formic acid containing 20 ng/µl of pepsin. As an alternative protocol, in-solution digestion using pepsin was performed for 10 min at 37°C in 20 µl of 5% (v/v) formic acid containing 100 ng/µl of pepsin. The generated peptides were then purified using C18 micro-columns [35], [36] loaded with Poros R3 C18 material (Applied Biosystems, Stockholm, Sweden) and dried by vacuum centrifugation. In addition, enrichment and purification of phosphorylated peptides was performed by affinity chromatography on titanium dioxides as described [37], [38], [39].
MALDI-MS of peptides were acquired using a Voyager DE-STR mass spectrometer (AB SIEX. Stockholm, Sweden) in the reflector mode and 2,5 dihydroxybenzoic acid (DHB) solution containing 0.5% (v/v) phosphoric acid as a matrix (2,5 dihydroxybenzoic acid solution G2039A) from Agilent Technologies, Dalco Chromtech AB, Sollentuna, Sweden. LC-MS/MS combined with ESI-TRAP and ETD-TRAP was performed using a HCT ultra ETD II mass spectrometer from Bruker linked to Easy nano LC from Proxeon. Spectra were acquired using the enhanced scanning mode covering a mass range from m/z 200 to m/z 1300.
Analysis of gene transcription by Reverse Transcription (RT)-PCR
Protocols detailing the isolation of total RNA, the reverse transcription of mRNA into cDNA and its use as template for subsequent PCR amplification with the gene specific primers listed in Table S2 are described in detail elsewhere [19], [20].
Results
Phosphorylation of the CpxR residue Asp51 by acetyl∼P
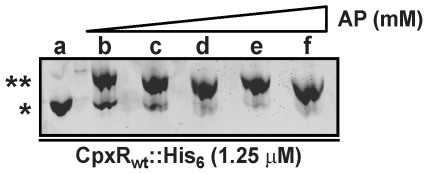
Phosphorylation of response regulators is thought to stimulate structural changes leading to formation of functional dimers – a prerequisite for efficient binding to target DNA. Thus, to determine if CpxR∼P truly mediates repression of Yersinia virulence, we first wanted to characterize CpxR phosphorylation in vitro using a high energy phosphate donor, acetyl∼P. We initially determined the minimal concentration of acetyl∼P needed to sufficiently in vitro phosphorylate CpxR. Purified CpxRwt::His6 was incubated with increasing concentrations of acetyl∼P. Aliquots were then fractionated on Manganese(II)-Phos-tag™ 12.5% acrylamide gels to monitor the extent of CpxR∼P. As little as 6.25 mM acetyl∼P was sufficient to phosphorylate the majority of CpxR (Figure 1). Moreover, the maximal achievable amount of CpxR∼P was reached in the presence of 25 mM acetyl∼P (Figure 1). Hence, in vitro phosphorylation of CpxR by acetyl∼P is a robust method for generating a high proportion of CpxR∼P molecules.
Figure 1. In vitro phosphorylation of CpxR by acetyl∼P.
Wild type CpxR (CpxRwt::His6) was purified by metal affinity chromatography. Purified protein (1.25 µM) was incubated in sequentially increasing concentrations of acetyl∼P (AP). Samples were fractionated on a Manganese(II)-Phos-tag™ 12.5% acrylamide gel and then visualized by the SYPRO® Ruby staining method. Lanes: a, no addition of AP; b, 6.25 mM AP; c, 12.5 mM AP; d, 25 mM AP; e, 50 mM AP; f, 100 mM AP. Phosphorylated CpxR is indicated by a double asterisk and unphosphorylated CpxR by a single asterisk.
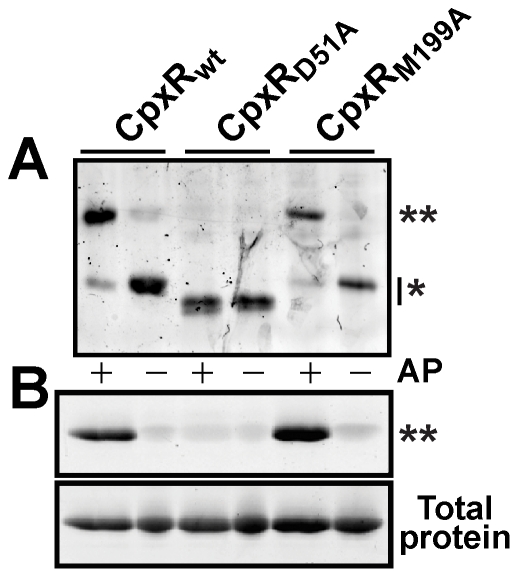
On the basis of homology to the OmpR/PhoB response regulator family, the conserved aspartate at position 51 (Asp51) is the predicted phosphorylation site of CpxR [9]. We therefore established a cpxR mutation in which Asp51 was replaced with alanine. As a control, another mutant was constructed in which methionine at position 199 (Met199) was replaced with alanine. Based on the helix-turn-helix DNA-binding motif prediction algorithm of Dodd and Egan [40], this substitution is predicted to disrupt a helix-turn-helix motif needed for DNA binding – a characteristic of the OmpR/PhoB family [41] (data not shown). C-terminal His6-tagged fusions of CpxRwt and the two CpxRD51A and CpxRM199A variants were then purified by affinity chromatography from E. coli. Acetyl∼P was used to investigate which of these purified proteins could still be phosphorylated. The Manganese(II)-Phos-tag™ acrylamide system was used to visualize phosphorylated protein. Only CpxRwt::His6 and CpxRM199A::His6 were deemed to be phosphorylated in vitro by acetyl∼P (Figure 2B). Given that CpxRD51A::His6 exhibited altered mobility in the Manganese(II)-Phos-tag™ acrylamide gel, we also confirmed the absence of phosphorylation using conventional SDS-12%-PAGE followed by visualization with the independent Pro-Q® Diamond phosphoprotein stain (Figure 2B). As expected therefore, Asp51 appears to be a genuine site of CpxR phosphorylation.
Figure 2. Phosphorylation of the CpxR residue Asp51.
Wild type (CpxRwt::His6) and defined variants with the amino acid substitutions Asp51Ala (CpxRD51A::His6) and Met199Ala (CpxRM199A::His6) were purified by metal affinity chromatography. Purified proteins (1.25 µM) were incubated in the presence of 50 mM acetyl∼P (AP) (+). In A, samples were fractionated by a Manganese(II)-Phos-tag™ 12.5% acrylamide gel and then stained by the SYPRO® Ruby staining method. In B, samples were separated in a conventional SDS 12% polyacrylamide gel. Phosphorylated protein (upper panel) was specifically detected by the Pro-Q® Diamond phosphoprotein gel stain technique, while total protein (lower panel) was visualized by the SYPRO® Ruby staining method. Phosphorylated CpxR is indicated by a double asterisk and unphosphorylated CpxR by a single asterisk. The reason for the enhanced mobility of CpxRD51A::His6 when fractionated by Manganese(II)-Phos-tag™ acrylamide (Panel A) is uncertain.
It is possible that substitution at Asp51 may cause an extreme allosteric effect that prevents CpxR phosphorylation at an alternative site. As phosphorylation on Asp residues is considered to be unstable, our initial approach had the goal to reduce phosphorylated Asp51 of CpxR∼P using sodium borohydride to a stable homoserine [33], [34] and to confirm this conversion by mass spectrometry. However, the yield of this reaction was very low so the approach was not continued. Instead, in vitro phosphorylation of CpxR was confirmed by intact mass determinations using ESI-MS (Figure S1). These experiments showed that the major product of in vitro phosphorylation of CpxR carried only one phospho group (Table 1). This is consistent with the model that Asp51 is the principal phosphorylation site of CpxR. In addition, the intact mass determination showed that CpxR∼P was sufficiently stable to be analyzed without converting Asp51 to homoserine by sodium borohydride reduction.
Table 1. Summary of mass determination of CpxR and CpxR∼P by ESI-MS.
| Sample | Experimental mass | Theoretical mass |
| CpxR | 27561±4 | 27560 (unmodified) |
| 27564±3 | ||
| 27556±4 | ||
| CpxR∼P | 27640±3 | 27640 (singularly phosphorylated) |
| 27641±2 |
Phosphorylation-dependent binding of CpxR∼P to virulence gene promoters in vitro
Having established that CpxR∼P carries only one phospho group that is likely to be at Asp51, we now wanted to gain insight into the role of CpxR∼P in virulence gene regulation in Yersinia. In a previous study, we noted that the expression of genes encoding the potential adhesins Ail (ail – YPTB2867) and Ail-like (YPTB2113), as well as the confirmed adhesins pH6 antigen (psaA) and invasin (inv), are all influenced by loss of CpxA [20]. We made use of the purified CpxRwt and CpxRD51A His6-tagged variants derived from Yersinia CpxR and performed an EMSA to examine if CpxR∼P bound to radiolabeled PCR- amplified DNA control regions upstream of these Yersinia virulence genes. All regions of amplified target DNA included a minimum of ∼300 bp upstream and 40 bp downstream of the translational start codon. Our experiments were controlled by incorporating DNA encompassing the cpxR/cpxP divergent promoter and the ppiA promoter, both of which are known to bind CpxR∼P either from Y. pseudotuberculosis [20] or E. coli [12], [17], [42]. We also included the rovA gene encoding the transcriptional activator of invasin [22], [23], and the psaE gene, which encodes a regulator for pH6 antigen expression [43]. As anticipated from data obtained in our earlier study [20], in vitro phosphorylated CpxRwt bound the promoters of inv and rovA, as well as those known CpxR∼P regulon members ppiA and cpxR/cpxP (Figure 3). Interestingly, at least two distinct migration shifts of rovA, ppiA and cpxR/cpxP template was observed when using different concentrations of CpxR∼P. This could suggest that these particular promoters contain multiple CpxR∼P binding sites of differential affinity so that several CpxR molecules bind cooperatively. This would be reminiscent of how CpxR∼P is thought to bind the promoter of csgD involved in curli biogenesis in E. coli [44]. Here, we also demonstrate for the first time a mobility shift of DNA specific to the promoter regions of psaA and psaE. Crucially, identical unlabeled (‘cold’) template DNA could compete for CpxR∼P binding. Although the degree of successful competition varied for each template, the addition of cold DNA did reduce the amount of radiolabeled (‘hot’) cpxR/P, ppiA, rovA, inv, psaA and psaE template DNA being retarded in migration during electrophoresis (Figure 3). Quite possibly the ail (YPTB2867) promoter also represents a target of CpxR∼P considering that the higher CpxR∼P concentration (1.5 µM) bound to all available free DNA resulting in the complete retardation of the hot template (the unbound signal at the bottom of the gel disappears). This is distinct from the reduced amount of ail-like (YPTB2113) promoter template shifted by equivalent amounts of CpxR∼P. In this case, significant signal representing free unbound ail-like hot template still exists at the bottom of the gel (Figure 3). In fact, this degree of ‘non-specific’ CpxR∼P binding was also observed in the negative control represented by an internal fragment of cpxR encompassing the nucleotides +32 through to +420 downstream of the translational start (Figure 3). Finally, binding by the non-phosphorylated CpxRD51A variant was not observed in our assay conditions as evidenced by the absence of a migration shift for any hot DNA template (Figure 3). Taken together, this demonstrates that CpxR phosphorylation at position 51 is required for direct and efficient binding of CpxR∼P to the rovA, inv, psaA and psaE (and possibly also the ail) virulence gene promoters. On the other hand, modulation of expression of the Ail-like homologue (YPTB2113) by CpxR∼P is apparently not via direct binding, but must presumably involve at least one other unknown regulatory intermediate.
Figure 3. Phosphorylation-dependent binding of CpxR to DNA upstream of Yersinia virulence genes.
Mobility shift assays were performed with purified CpxRwt::His6, and the non-phosphorylated mutant CpxRD51A::His6. Target DNAs were radiolabeled PCR fragments harboring the regulatory regions of cpxR/cpxP, ppiA, rovA, inv, ail (YPBT2867), ail-like (YPBT2113), psaA and psaE. An internal fragment of the cpxR gene was used as a negative control. The approximate size of each amplified PCR fragment is given in parentheses. Where indicated, the purified CpxR-His tagged variants were incubated with ‘hot’ DNA templates at the concentrations of 0.75 and 1.5 µM. All reactions were performed in the presence of the phosphodonor acetyl∼P and binding specificity was aided by the constant presence of BSA and non-specific single stranded DNA. EMSAs were further controlled by a reaction containing 1.5 µM BSA instead of CpxR or competition reactions in which excess ‘cold’ DNA template competed with ‘hot’ DNA for available CpxR∼P. The electrophoretic mobility of the ‘hot’ DNA fragments in the absence of bound protein is highlighted by an arrow, while DNA-CpxR∼P complexes are signified with an asterisk (*). In some cases, the extent of mobility shifted DNA was dependent on the CpxR∼P concentration. Two asterisks (**) indicate residual non-specific background binding noise and are not believed to represent bona fide binding targets of CpxR∼P.
Mapping the DNA binding site of CpxR∼P in the rovA promoter
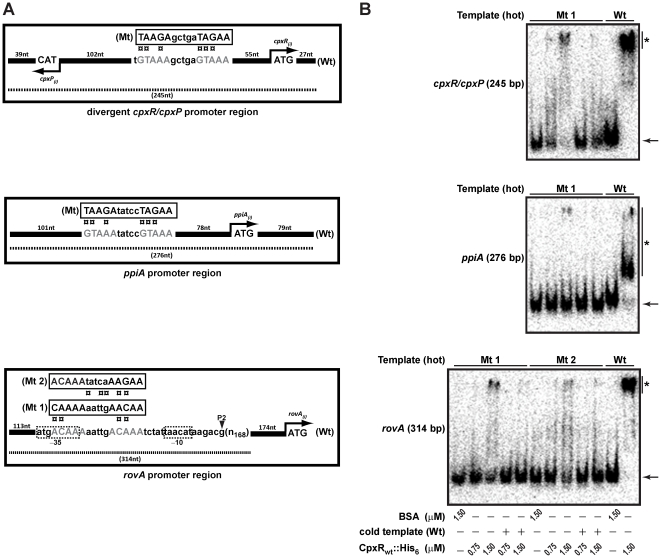
RovA is a global regulator of Yersinia gene expression, being responsible for fine-tuning expression of a multitude of genes involved in general housekeeping, environmental survival and pathogenicity [24], [25], [26]. Levels of RovA need to be strictly controlled; dissecting these control mechanisms will therefore benefit our general understanding of how this pathogenic bacterium responds and adapts to its prevailing environment. In order to determine how CpxR∼P controls the levels of rovA transcription, we performed a nuclease protection (footprinting) analysis to map the DNA binding site of CpxR∼P in the rovA promoter. A protected sequence of the sense strand estimated to be 5′-gcgtgctaacgataatgacaaaaattgacaaatcta-3′ was identified within the P2 promoter region of rovA (Figure 4). We could also identify protected regions in the sense strand of ppiA promoter (5′-ttctgttacgtaaatatccgtaaatgggtgg-3′) and the divergent cpxR-cpxP promoter (5′-gtcagggcatgtaaagctga-3′) (Figure 4). As expected, a control sequence residing downstream of the cpxR start codon was not protected from DNase1 digestion. In E. coli, the consensus CpxR∼P DNA binding sequence is represented by 5′-GTAAA(N)4–8GTAAA-3′ [4], [42]. We manually inspected these DNase1 protected sequences for consensus CpxR∼P DNA binding motifs. A poorly conserved putative CpxR∼P binding site may lie in the protected region derived from the rovA promoter, while more obviously conserved consensus CpxR∼P binding motifs were observed in the protected sequence upstream of ppiA and between cpxP/cpxR (Figure 4).
Figure 4. Mapping the CpxR∼P DNA binding site upstream of rovA by nuclease protection (footprinting) analysis.
DNase I footprinting assays were performed to investigate the binding of CpxR∼P to a region within the rovA, ppiA and cpxR-cpxP promoters. These 314 base pair (bp), 276 bp and 245 bp fragments respectively, were labeled on the sense strand before being incubated with CpxR∼P at the following final concentrations: 50 nM, lane c; 100 nM, lane d; 200 nM, lane e; 400 nM, lane f. The absence of CpxR∼P in lanes a and b is indicated by ‘–’. Reactions were resolved by denaturing PAGE and analyzed with a Molecular Dynamics PhosphorImager. Labeled pBR322 DNA digested with MspI (New England Biolabs) was used as a size marker (lane a). An estimation of the protected sequence is given on the right hand side of the panels. Based upon the E. coli consensus sequence of 5′-GTAAA(N)4–8GTAAA-3, a putative CpxR∼P consensus binding site is highlighted in a gray box. A labeled internal fragment (389 bp) within the cpxR open reading frame served as a non-protected control.
Based on the analysis of these ‘footprints’, we used sited-directed mutagenesis to shuffle the order of the nucleotides predicted to compose the CpxR∼P binding sites upstream of ppiA, cpxR (and cpxP) and rovA (Figure 5A). Since the CpxR∼P binding site upstream of rovA might overlap with the −35 box of the P2 promoter [45], we generated two scramble mutants in this region. The first was designated Mt 1, which included alteration of the −35 box sequence. In contrast, the second mutant (Mt 2) left the −35 box sequence intact. Significantly, an EMSA revealed a clear reduction in the retardation of PCR amplified fragments of the mutagenized DNA by CpxR∼P (Figure 5B; designated ‘Mt’). Collectively, these data indicate that the sequence 5′-ACAAA(N)5ACAAA-3′ overlapping with the −35 box of the P2 promoter and roughly located 360 nucleotides upstream of the rovA ATG start codon contributes to CpxR∼P binding. In addition, the 5′-GTAAA(N)5GTAAA-3′ sequences situated ∼80 and ∼55 nucleotides upstream of ppiA and cpxR respectively are also bona fide CpxR∼P binding sites.
Figure 5. Site-directed mutagenesis of the CpxR∼P DNA binding site upstream of rovA.
(A) As identified by DNase 1 foot-printing (see Figure 4), the potential CpxR∼P binding site sequence and position is shown relative to each ATG start codon within the regulatory regions of rovA and ppiA and the divergent cpxR/cpxP promoter (designated as ‘Wt’). Site-directed mutagenesis was performed to ‘shuffle’ the nucleotide sequence of each potential CpxR∼P binding site (designated as ‘Mt’). Since the initial mutation in the rovA sequence (‘Mt 1’) would potentially disrupt RNA polymerase binding because of an altered −35 region within promoter P2, a second mutation (‘Mt 2’) was performed in which the −35 region was left untouched. (B) Mobility shift assays with purified CpxRwt::His6 as outlined in the legend to Figure 3 were performed on radiolabled amplified DNA from these mutated templates. Once again, the electrophoretic mobility of the ‘hot’ DNA fragments in the absence of bound protein is highlighted by an arrow, while DNA-CpxR∼P complexes are signified with a single asterisk (*).
In vivo accumulation of CpxR∼P in the Yersinia cytoplasm reduces levels of RovA
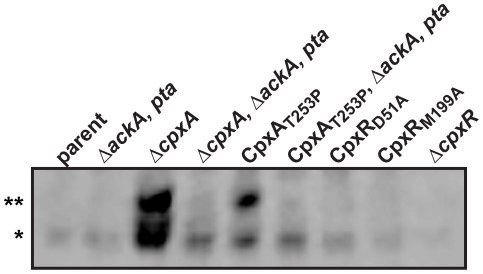
We predict that binding of CpxR∼P to the rovA promoter restricts transcription. Therefore, elevating available CpxR∼P in the Yersinia cytoplasm in turn should reduce detectable RovA levels. To test this, we took advantage of work performed in E. coli, whereby phosphatase deficient CpxAT253P encoded by the allelic variant designated cpxA101* generally lead to a constitutively active Cpx pathway [5], [14]. Although never directly tested, such a mutant should accumulate unusually high levels of CpxR∼P. To test this in Yersinia, we constructed an in cis cpxA101* mutation in Y. pseudotuberculosis. We then utilized the Manganese(II)-Phos-tag™ acrylamide gel system to measure in vivo levels of CpxR∼P. Formic acid-lysed bacteria grown in LB broth to early stationary phase (OD600 in the range of 0.85 to 0.95) at 26°C were fractionated on a Manganese(II)-Phos-tag™ 12.5% acrylamide gel and blotted with affinity purified anti-CpxR antiserum. Impressively high levels of both CpxR and CpxR∼P was detected in the cytoplasm of the Yersinia mutant completely lacking the cpxA allele (Figure 6). These two CpxR isoforms were also present in the cpxA101* background, although not to the same extent. Moreover, it seems that the vast majority of detectable CpxR is actually phosphorylated in this cpxA101* background. At present, reasons for why different levels and ratios of the two CpxR isoforms accumulate in these independent cpxA mutants are not clear. In contrast, CpxR was generally undetectable in parental bacteria or the cpxR mutants encoding the non-phosphorylated CpxRD51A or the CpxRM199A variant predicted to possess a defective winged helix-turn-helix domain (Figure 6). Given the noted toxicity of elevated CpxR∼P levels [4], [19], [20], accumulation of cytoplasmic CpxR∼P in the ΔcpxA and cpxA101* mutants does correlate with an inferior rate of growth (Figure S2A).
Figure 6. In vivo detection of phosphorylated and unphosphrylated CpxR.
The Manganese(II)-Phos-tag™ acrylamide gel system was used to measure in vivo levels of CpxR∼P. Bacteria were cultured at 26°C in LB broth to stationary phase. After harvesting by centrifugation, bacteria were lysed with formic acid and samples rapidly fractionated on a Manganese(II)-Phos-tag™ 12.5% acrylamide gel and blotted with affinity purified anti-CpxR antiserum. Strains: parent, YPIII/pIB102; ackA, pta null mutant, YPIII69/pIB102; cpxA null mutant, YPIII07/pIB102; cpxA, ackA, pta null mutant, YPIII49/pIB102; cpxA101* encoding CpxAT253P, YPIII51/pIB102; cpxA101*, ackA, pta null mutant, YPIII74/pIB102; mutated cpxR encoding CpxRD51A, YPIII52/pIB102; mutated cpxR encoding CpxRM199A, YPIII46/pIB102; cpxR null mutant, YPIII08/pIB102. The single asterisk (*) reflects non-phosphorylated CpxR, while the double asterisk (**) indicates phosphorylated CpxR accumulated in the Yersinia cytoplasm.
In the absence of CpxA phosphatase activity, CpxR∼P may generate via non-specific low molecular weight phosphodonors such as acetyl∼P [5], [15], [16], [17]. Acetyl∼P is derived from the phosphotransacetylase (Pta) – acetate kinase (AckA) pathway [46]. Therefore, to investigate if this phosphodonor contributes to accumulated CpxR∼P in the ΔcpxA null mutant and the gain-of-function cpxA101* mutant producing CpxAT253P, a full length ΔackA, pta null mutation was introduced into these bacteria. Since mutants lacking both ackA and pta genes should be incapable of synthesizing acetyl∼P [47], this second-site mutation should therefore reverse the build-up of CpxR∼P exhibited by the ΔcpxA and cpxA101* mutants. Indeed, CpxR∼P no longer accumulated in the ΔcpxA and cpxA101* mutants also lacking the ability to synthesize acetyl∼P (Figure 6), which to some extent suppressed the inferior growth rate of the single ΔcpxA and cpxA101* mutants (Figure S2A). The singular ΔackA, pta null mutant was also modestly growth restricted consistent with reports in E. coli (Figure S2A) [18], [48], [49]. However, it did not accumulate either non-phosphorylated or phosphorylated CpxR, being equivalent to parental bacteria where CpxR also remained below the level of detection in our assay (Figure 6). Hence, the Cpx pathway of Yersinia is subject to feedback inhibition that tightly restricts available CpxR∼P levels. However, in the absence of CpxA phosphatase activity, CpxR∼P readily accumulates by virtue of sensor kinase activity and/or small molecular weight phosphodonors associated with central metabolism.
Having established Yersinia mutants in which the in vivo levels of CpxR∼P were characterized, we used these to address whether elevated CpxR∼P levels restrict production of the RovA global regulator. RovA production from stationary phase bacteria grown in LB broth was analyzed by western blotting. Elevated levels of CpxR∼P accumulated in the ΔcpxA and cpxA101* mutants dramatically restricted RovA production (Figure 7). However, production to parental levels was restored in both mutants by the introduction of a second-site deletion of the ackA and pta genes (Figure 7). We also analyzed invasin, whose production is under RovA positive control. Not surprisingly therefore, invasin levels mirrored the pattern of RovA production in all strain backgrounds. Removal of the ackA and pta genes from parental Yersinia did not affect RovA or invasin production (Figure 7). We therefore conclude that cytoplasmic CpxR∼P accumulation is an important mediator of Yersinia virulence factor expression. The data also suggests that Yersinia low molecular weight metabolic intermediates, such as acetyl∼P, have the capacity to transfer high-energy phospho-groups to CpxR, and perhaps even other TCRS response regulators. Thus, this could be a way for Yersinia to effectively fine-tune global gene expression depending upon the prevailing environmental conditions such as nutrient supply.
Figure 7. Cpx pathway activation influences the production of the RovA global regulator.
RovA and invasin production was determined from protein isolated from early stationary phase bacteria grown in LB broth at either 26°C or 37°C. Protein was separated on a 12% SDS-PAGE and then identified by immunoblot analysis using polyclonal rabbit antiserum raised against RovA or invasin. Strains: parent, YPIII/pIB102; ΔackA, pta null mutant, YPIII69/pIB102; ΔcpxA null mutant, YPIII07/pIB102; ΔcpxA, ΔackA, pta null mutant, YPIII49/pIB102; cpxA101* encoding CpxAT253P, YPIII51/pIB102; cpxA101*, ΔackA, pta null mutant, YPIII74/pIB102. Molecular weights shown in parentheses are deduced from primary sequence. The single asterisk (*) reflects typical invasin degradation products, while two asterisks (**) indicates a non-specific protein band recognized by the anti-RovA antisera that also serves as a convenient loading control.
Elevated levels of the NlpE lipoprotein reduce invasin and RovA levels
Examination of the Cpx response in the absence of a functioning CpxA protein is artificial. We therefore wanted to examine the repressive effect of CpxR∼P on RovA production following activation of the Cpx response in which the signal transduction cascade is still intact. Simulating natural Cpx TCRS responsiveness has been achieved by the over-production of the E. coli NlpE (NlpEE.c) lipoprotein [50], [51]. We therefore transformed parent and CpxR defective Yersinia with pBAD18 derivatives encoding E. coli nlpE (termed pND18) or Y. pseudotuberculosis nlpE (NlpEY.p – pJF027) under the control of an arabinose inducible promoter. Since NlpEY.p shares less than 50% identity at the amino acid level with NlpEE.c (Figure 8A), we considered that native NlpE could possibly be a better inducer of Cpx pathway activation in Yersinia. Bacteria were grown in the presence or absence of arabinose, and production of RovA and invasin was examined. Over-production of either NlpEE.c or NlpEY.p in parental Yersinia caused a reduction in RovA and invasin production that was dependent on functional CpxR, because neither lipoprotein caused this decrease in the ΔcpxR null mutant (Figure 8B). Interestingly, NlpEY.p appeared to restrict RovA and invasin production to a greater degree. These data reinforce how Cpx pathway activation and the presence of CpxR∼P can restrict virulence factor production in Y. pseudotuberculosis.
Figure 8. Inhibitory effect of NlpE overexpression.
(A) A ClustalW2 alignment colored by Boxshade (ver. 3.21) between NlpEY.p (NCBI annotation YPK_1090) from Y. pseudotuberculosis YPIII and NlpEE.c (b0192) from E. coli K-12 strain MG1655. (B) Sampling and detection of invasin and RovA at stationary phase in LB broth at 26°C followed the procedure detailed in the legend to Figure 7. Additionally, NlpEE.c (derived from E. coli) or NlpEY.p (Y. pseudotuberculosis) over-production was controlled by the presence (+) or absence (−) of 0.2% (w/v) L-arabinose. The pBAD18 empty expression vector served as a control. The strains used were: parent with empty vector, YPIII/pIB102, pBAD18; parent with NlpEE.c, YPIII/pIB102, pND18; parent with NlpEY.p, YPIII/pIB102, pJF027; cpxR null mutant with empty vector, YPIII08/pIB102, pBAD18; cpxR null mutant with NlpEE.c, YPIII08/pIB102, pND18; cpxR null mutant with NlpEY.p, YPIII08/pIB102, pJF027. Molecular weights shown in parentheses are deduced from primary sequence.
CpxR∼P DNA binding is required for RovA repression in vivo
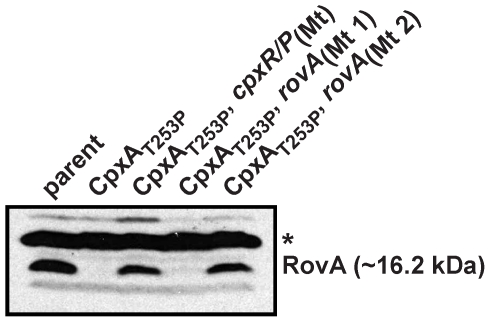
We have identified a CpxR∼P binding site in the regulatory regions of three genes of Yersinia; ppiA, cpxP/cpxR and rovA. We have also shown the accumulated CpxR∼P levels result in restricted RovA production. Therefore, we wondered whether RovA repression by CpxR∼P could be relieved by modulating the CpxR∼P binding site within the rovA promoter. The same rovA ‘shuffle’ mutations (Mt 1 and Mt 2) used to identify CpxR∼P binding in vitro (see Figure 5) were introduced in cis into the chromosome of the cpxA101* mutant of Y. pseudotuberculosis. This background was chosen because the Cpx pathway is constitutively active, CpxR∼P accumulation is relatively high and the integrity of the cpxRA transcriptional unit remains intact. Bacteria were grown to early-stationary phase and the extent of RovA production was examined by immunoblot. RovA levels were essentially undetectable in the cpxA101* mutant and this mutant also harboring rovA (Mt 1) (Figure 9). Significantly however, RovA levels were restored in the cpxA101*, rovA (Mt 2) double mutant indicating that the ‘Mt 2’ mutation made the rovA promoter non responsive to Cpx pathway activation. Similarly, RovA levels were also restored in the cpxA101*, cpxR/P (Mt) double mutant (Figure 9). In this strain, cpxR transcription is not induced limiting the accumulation of CpxR∼P (data not shown). To confirm these data, we performed semi-quantitative RT-PCR analysis to gain some insight into rovA transcription levels. Compared to the single cpxA101* mutant, rovA transcription was elevated in both cpxA101*, rovA (Mt 2) and cpxA101*, cpxR/P (Mt) double mutants (Figure S3). Interestingly, rovA transcription in the cpxA101*, rovA (Mt 1) mutant was still low, which explains the absence of RovA detection by western blotting (Figure 9). This indicates that the ‘Mt 1’ nucleotide shuffling within the putative −35 region (see Figure 5A) predictably affected RNA polymerase promoter recognition and transcriptional output from the ‘P2’ rovA promoter [45]. Collectively, these data therefore identify nucleotides upstream of rovA that are critical for the in vivo recognition by CpxR∼P and the subsequent repression of rovA transcription. Despite our EMSA results indicating multiple CpxR∼P binding sites, we believe that this sequence in the ‘P2’ rovA promoter is primarily responsible for governing CpxR∼P-dependent rovA regulatory control.
Figure 9. RovA produced from a mutant allele lacking the upstream CpxR∼P DNA binding site is no longer restricted by Cpx pathway activation.
Y. pseudotuberculosis was grown until entry into early stationary phase at 26°C in LB broth. Bacterial cells were harvested and lysates prepared in loading buffer. Protein was separated on a 15% SDS-PAGE and then identified by immunoblot analysis using polyclonal rabbit antiserum raised against RovA. The molecular weight shown in parenthesis was deduced from primary sequence. The single asterisk (*) indicates a non-specific protein band recognized by the anti-RovA antisera. Strains: parent, YPIII/pIB102; cpxA101* encoding CpxAT253P, YPIII51/pIB102; cpxR/cpxP (Mt) (shuffle mutation of the CpxR∼P binding site identified in Figure 4) placed in the cpxA101* background, YPIII115/pIB102; rovA (Mt 1) placed in cpxA101*, YPIII120/pIB102; rovA (Mt 2) placed in cpxA101*, YPIII121/pIB102.
Discussion
Pathogenic bacteria experience diverse environmental conditions both inside and outside of a host. Multiple regulatory pathways are required to incorporate these diverse signals that then converge to fine tune spatial and temporal virulence gene expression. Herein, we report that the effect of CpxA-CpxR pathway activation in the enteropathogen Y. pseudotuberculosis is two-fold; not only does CpxR∼P serve to directly up-regulate the expression of genes encoding for periplasmic quality control factors, but it also down-regulates genes encoding virulence associated determinants – especially the global regulator RovA. In Y. pseudotuberculosis, the CpxA-CpxR TCRS therefore serves to coordinate the maintenance of bacterial envelope integrity with a reduction in virulence gene expression to ensure that vital energy resources are used to maximize the chances of survival in the prevailing environment.
How can CpxR∼P both induce and repress the expression of target genes in pathogenic Yersinia? A review of studies on other OmpR/PhoB family members provides some insight into the many assorted possibilities. CpxR∼P binding to target DNA may physically recruit the RNA polymerase. This principle is well-established for the phosphate starvation regulon, whereby PhoB∼P facilitates specific interactions between RNA polymerase and the pho promoters [52], [53]. CpxR∼P binding to target DNA may also alter DNA curvature that in turn modulates exposure of additional DNA binding sequences to provide access to secondary transcriptional activators or antagonistic repressors. This is no better illustrated than by the reciprocal interactions of OmpR∼P and integration host factor within the regulatory region of the ompF porin gene induced by changes in osmolarity [54], [55]. The orientation or the proximity of the CpxR∼P binding site relative to the transcriptional start may also influence activation or repression, especially if this influences CpxR∼P positioning with respect to the RNA polymerase or the binding sites for other activators or repressors. A strong correlation between genes under heavy CpxR-regulatory influence and the positioning of CpxR∼P binding sites within 100 base pairs upstream of the transcription start supports this notion [3]. Differences in relative binding affinities or the actual number of distinct binding sites within each promoter could also determine the degree of CpxR∼P influence. This has been used to explain the inverse regulation of the porin genes ompC and ompF by OmpR∼P [56]. It is also plausible that intrinsic promoter activity may influence CpxR∼P regulatory control. Indeed, such a mechanism may in part determine whether the RcaC response regulator activates or represses transcription of genes encoding products of cyanobacterial photosynthetic light harvesting antenna in response to ambient light color changes [57]. At this stage the mechanisms of CpxR∼P action are unclear, but future investigation will aim to reveal whether any of these complex scenarios dictate how CpxR∼P mediates both activation and repression of Yersinia gene expression.
Response regulator activation is thought to first require phosphorylation for the triggering of dimerization that is necessary for target DNA binding [58]. However, it is apparent that among the OmpR/PhoB subfamily, some members can bind DNA in the absence of initial phosphorylation. In fact, non-phosphorylated OmpR is proposed to first bind to DNA as a monomer [34], [59]. Phosphorylation then occurs to promote dimerization and facilitate DNA binding by the second monomer [59], [60]. The CovR response regulator of virulence and stress survival gene expression in Streptococcus spp. can also bind DNA as a non-phosphorylated monomer, although binding is enhanced by phosphorylation-dependent dimerization [61]. It is also notable that PhoP, a response regulator of Salmonella enterica that senses environmental Mg2+, dimerizes and binds DNA independent of phosphorylation [62]. In our own study, the non-phosphorylated CpxRD51A variant was not able to bind DNA under the assay conditions used here. However, our previous results suggested the possibility of weak binding when nonphosphorylated CpxR was used in EMSAs with rovA and inv [20]. Thus, while phosphorylation significantly enhances CpxR binding to target DNA, we are unable to unequivocally conclude that binding cannot occur without it.
Mechanisms of rovA transcriptional regulation are complex (Figure S4). It chiefly involves a thermoregulated auto-amplification loop that overcomes H-NS/YmoA mediated silencing [22], [45], [63], [64]. However, an additional layer of positive regulation might incorporate LeuO, a LysR-like regulator [63], while negative regulation does involve another LysR-type protein, RovM [63], [65]. This negative regulatory loop probably senses nutritional status since its output is refined by participation of a carbon storage regulator system controlling rovM expression [66]. In addition, RovA activity is affected post-transcriptionally; temperature-dependent conformational changes render RovA less able to bind to target DNA sequences and to resist proteolysis by endogenous proteases [67]. Our data demonstrates another layer of RovA control; as a result of sensing ECS, accumulated CpxR∼P represses rovA transcription by a mechanism that involves direct binding to the rovA promoter. We will endeavor to pry apart this mechanism in order to understand its contribution to RovA control in relation to other regulatory players, especially those that converge on the 5′-UTR of rovA to fine-tune expression (Figure S5). Based on the CpxR∼P binding site overlapping with the −35 region of the ‘P2’ promoter, we currently speculate that CpxR∼P bound to the DNA may occlude appropriate positioning of the RNA polymerase holoenzyme and/or open promoter complex formation. Nevertheless, CpxR∼P has the ability to affect the expression of multiple genes [2], [3], [4], [68]. Thus, it also remains possible that CpxR∼P-dependent repression of rovA transcription involves additional indirect mechanisms; for example, by modulating leuO or rovM expression.
Y. pseudotuberculosis requires close eukaryotic cell contact in order to establish a host infection. Crucial to this are various adhesins located at the bacterial surface. CpxR∼P accumulation affects the expression levels of genes encoding several of these adhesins. While, CpxR∼P binds directly to the inv (invasin) promoter, the cornerstone of invasin control is positive regulation by RovA. We currently have no explanation for why CpxR∼P-dependent invasin regulation requires two mechanisms – one direct via binding to the inv promoter and the second indirect through binding to the rovA promoter. These two mechanisms may be a way to unite multiple overlapping signals to establish exquisite spatial and temporal control of inv expression – an important Yersinia virulence determinant. With respect to the control of other Yersinia surface adhesins, our DNA binding data suggests CpxR∼P might repress ail expression directly. Additionally, the controlled production of the pH 6 antigen appears to involve a complex mechanism requiring direct binding of CpxR∼P to the promoter of the tip adhesin gene psaA as well as to the promoter of psaE, encoding a membrane-associated protein required for transcriptional activation of psaA [43]. How PsaE mediates regulation of psaA transcription is currently unknown. Intriguingly, CpxR activation must also influence pH 6 antigen levels through an effect on RovA, since RovA binds to both psaA and psaE promoter regions [24].
In several respects the cpxA101*-encoding CpxAT253P variant duplicates the effects of completely removing cpxA. In this context, it is curious that the cpxA101* and ΔcpxA full length mutants lacking ackA and pta both similarly failed to accumulate detectable CpxR∼P (see Figure 6). Unlike a complete cpxA deletion, one could have expected the gain-of-function cpxA* mutant to accumulate CpxR∼P, even in the absence of the Pta-AckA pathway, because it retains a genetically intact kinase component that can still phosphorylate CpxR [5], [11]. Significantly however, T253P substitution is known to hamper endogenous CpxA autokinase and CpxR phosphotransfer activity [5]. Thus, disruption of the Pta-AckA pathway in this background severely restricts an alternative means of CpxR∼P accumulation. In this context, it is worth questioning whether acetyl∼P is actually a bona fide in vivo phosphodonor responsible for kinase-independent activation of CpxR in Yersinia. While our data points in this direction, it was recently discovered that an intact Pta-AckA pathway, rather than acetyl∼P per se, may actually be more critical to CpxR activation [18]. Hence, removal of pta and ackA from Yersinia may conceivably erase a critical intracellular signal needed for CpxR activation. In E. coli, loss of the Pta-AckA pathway results in a plethora of diverse phenotypes and deficiencies ranging from defects in central metabolism, stress survival, proteolysis and chaperone function [46], [69]. Any one of these could potentially occur in Yersinia and thereby contribute to CpxR activation.
We made use of NlpE lipoprotein over-production, either from E. coli (NlpEE.c) or Y. pseudotuberculosis (NlpEY.p) to stimulate an intact Cpx pathway. NlpEY.p and NlpEE.c are about 49% identical at the amino acid level. Even though both proteins activated the Cpx pathway, NlpEY.p did so to a greater extent. Exactly how the Cpx pathway is induced by NlpE is unknown, but it likely involves sensing of bacterial adhesion to surfaces [70]. Relay of this signal probably involves one or more structural changes in NlpE caused by external environmental stresses imposed during the adhesion process [71], [72]. Presumably this is mimicked in part by NlpE over-expression; an activating signal may result from erroneously assembled NlpE no longer transported to the outer membrane by the Lol system [71], [73]. Whatever the mechanism, it is striking for its molecular conservation and specificity. Of all the many lipoproteins produced by E. coli, only the over-expression of NlpE and one other – the inner membrane located YafY of unknown function – caused Cpx pathway activation [73]. Thus, comparative structure-function analyses of NlpEY.p and NlpEE.c could potentially provide important insight into the activating mechanism of the Cpx pathway.
In an effort to provide experimental evidence for a phosphorylation of CpxR on Asp51, peptides were generated by fast enzymatic digestion. Commonly used proteases such as trypsin, chymotrypsin, and the endoproteinases AspN and GluC, were not suitable to generate peptides for a study of Asp51. All produced theoretically predicted Asp51-containing peptides with masses above 4000, which is relatively high for successful structural analyses by tandem mass spectrometry (MS/MS). Nevertheless, a protocol for fast digestion with trypsin and pepsin was developed. While MALDI-MS and ion trap MS combined with ESI and ETD ionization [74] both confirmed the identity of digested CpxR and CpxR∼P with high confidence, no peptides containing phosphorylated Asp51 or any other phosphorylation sites were detected (data not shown). This was even the case after enrichment of phosphopeptides using titanium dioxide [37], [38], [39]. Thus, we were unable to analyze phosphorylation of CpxR∼P by mass spectrometry. In part, this can be explained by the inability to generate specific peptides in the optimal mass range between 1200 and 2000 that would facilitate MS analysis.
In this study we have described how CpxR∼P accumulation has a direct negative effect on rovA expression in Yersinia. RovA is a global regulator targeting in excess of 60 genes in each of Y. enterocolitica and Y. pestis [24], [25]. As several of these genes may represent novel virulence determinants in these bacteria, the direct and indirect impact of Cpx pathway activation on Yersinia pathogenicity can be widespread. We see CpxR∼P as an ‘over-ride mechanism’ capable of a quick response to repress virulence gene expression in over-committed, fully-induced bacteria that suddenly find themselves in an unfavorable environment exposed to ECS where virulence factors are no longer useful for their survival – to continue to express them would waste precious energy resources.
Supporting Information
Bacterial strains and plasmids used in this study.
(RTF)
Oligonucleotides used in this study.
(RTF)
Mass determination of intact CpxR and CpxR∼P by MALDI-MS. The respective CpxR and CpxR∼P peaks are indicated along with the experimental mass (Daltons).
(TIF)
Growth curves of various Y. pseudotuberculosis bacteria. Overnight cultures of parental and mutant bacteria were sub-cultured into fresh LB broth (time point 0 hours) and their growth during aerobic culturing with agitation at 26°C was monitored over a period of 9 hours by optical density measurement at 600 nm (A and B). Parent, YPIII/pIB102; YPIII07, ΔcpxA; YPIII08, ΔcpxR; YPIII46, mutated cpxR encoding CpxRM199A; YPIII49, ΔcpxA, ΔackA, pta null mutant; YPIII51, cpxA101* encoding CpxAT253P; YPIII52, mutated cpxR encoding CpxRD51A; YPIII69, ΔackA, pta null mutant; YPIII74, cpxA101*, ackA, pta null mutant; YPIII112, cpxR/cpxP (Mt) placed in the parent background; YPIII113, rovA (Mt 1) placed in the parent background; YPIII114, rovA (Mt 2) placed in the parent background; YPIII115, cpxR/cpxP (Mt) placed in the cpxA101* background; YPIII120, rovA (Mt 1) placed in the cpxA101* background; YPIII121, rovA (Mt 2) placed in the cpxA101* background. The asterisk (*) signifies modest to severe growth restriction in those bacteria with defects in the Pta-AckA biosynthetic pathway [18], [48], [49] (A) or in the CpxA sensor kinase that would be expected to accumulate toxic levels of CpxR∼P [4], [19], [20] (A and B). Note that despite being in a cpxA101* background, strain YPIII115/pIB102 grows quite well (B). Presumably, this is because CpxR∼P does not accumulate in this strain consistent with poor cpxR expression (data not shown) resulting from a shuffle mutation that makes the divergent promoter of cpxR/cpxP unable to bind CpxR∼P.
(TIF)
CpxR∼P DNA binding is required for repression of rovA transcription in vivo. For transcription analysis, crude semi-quantitative RT-PCR was performed on mRNA isolated from Y. pseudotuberculosis. Given the limitations of semi-quantitative RT-PCR, we attempted to increase the robustness of our assay by reverse transcribing mRNA isolated from bacteria grown at 26°C in LB broth to two different growth phases as measured by optical density at 600 nm; the first being an OD600 value between 0.45 to 0.55 (mid-logarithmic phase) and the second being an OD600 measurement between 0.85 to 0.95 (early-stationary phase). This was necessary to control for the altered growth rate of those bacteria expected to accumulate toxic levels of CpxR∼P (Figure S2B). Samples were subjected to RT-PCR with primers specific for rovA (plus RT). As an mRNA loading control, we analyzed the transcription of rpoA encoding for the α-subunit of RNA polymerase, which remained the same regardless of genetic background or phase of growth. To confirm the purity of the RNA isolated, PCRs with rpoA and rovA primer pairs was performed on template derived from RT reactions in which the enzyme was intentionally excluded (minus RT). PCR analysis on these samples indicated that the RNA isolation was essentially free of genomic DNA contamination. All images were acquired with a Fluor-S MultiImager (Bio-Rad) and analyzed with Quantity One software version 4.2.3 (Bio-Rad). DNA fragment sizes in base pairs are given in parentheses. Strains: parent, YPIII/pIB102; cpxA101* encoding CpxAT253P, YPIII51/pIB102; cpxR/cpxP (Mt) (shuffle mutation of the CpxR∼P binding site identified in Figure 4) placed in the cpxA101* background, YPIII115/pIB102; rovA (Mt 1) placed in cpxA101*, YPIII120/pIB102; rovA (Mt 2) placed in cpxA101*, YPIII121/pIB102.
(TIF)
Established and potential mechanisms of Cpx-dependent modulation of rovA and inv expression. The Cpx pathway is a sensor of extracytoplasmic stress (ECS) [1]. However, the role of CpxR∼P as a central regulator of Y. pseudotuberculosis pathogenicity is also becoming apparent (this study) [19], [20]. CpxR∼P levels in the bacterial cytoplasm are manipulated by cognate CpxA kinase and phosphatase activity. This is even further affected by the CpxA-independent phosphorylation of CpxR via second messenger phosphodonors; the levels of which are somehow influenced by an intact AckA/Pta pathway. In turn, CpxR∼P binds directly to the rovA and inv promoters repressing transcriptional output (red line). This influence may also be augmented indirectly through as yet unknown (indicated by a dashed line and a ‘?’ symbol) regulatory links to other positive (green) and negative (red) factors controlling rovA and/or inv expression [22], [23], [45], [63], [64], [65], [66], [75], [76], [77]. Recently, H-NS was described to be a part of the CpxR∼P regulon [2]. However, our in vitro electrophoretic mobility shift analysis did not reveal any CpxR∼P bound to the hns or ymoA promoters (Liu and Francis, unpublished). Not shown in this diagram is the influence of CpxR∼P on lon expression [78], but this connection is still relevant given how RovA is subject to proteolysis by the Lon protease [67]. Elevated CpxR∼P levels also diminishes efficient T3S and the production of other ‘non-invasin’ adhesins by a mechanism(s) that are not yet understood (red dotted line).
(TIF)
Regulator binding sites in the upstream flanking sequence of rovA. Y. pseudotuberculosis rovA transcription is initiated from two sites (P1 and P2) upstream of the translational start codon (TTG – green) [45]. DNA sequences flanking P1 and P2 serve as binding sites for an array of regulators including H-NS (purple), RovM (dark blue) and RovA (red) [45], [65]. We have now shown herein that CpxR∼P (brown) also binds to DNA sequences that incorporate the −35 region of the P2 promoter. Based upon the binding site in the −35 region however, CpxR∼P could prevent access to the P2 promoter by the RNA polymerase holoenzyme. It is not yet known if or how CpxR∼P binding influences the binding of the other rovA DNA-binding regulators.
(TIF)
Acknowledgments
This work was performed within the virtual framework of the Umeå Centre for Microbial Research (UCMR) Linnaeus Program (LP). We express gratitude to Hans Wolf-Watz (Umeå University, Umeå, Sweden), Petra Dersch (Braunschweig University of Technology, Braunschweig, Germany) and Shu-ichi Nakayama (National Institute of Infectious Diseases, Tokyo, Japan) for their generous gifts of antiserum and to Thomas Silhavy (Princeton University, Princeton, NJ) for his kind gifts of plasmid pND18 and antiserum. We are also most grateful for the expert technical advice regarding our DNase I footprinting and gel shift experiments that was provided by Teresa Del Peso Santos and Samir El Qaidi (Umeå University, Umeå, Sweden).
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was principally supported by grants 2006–3869 and 2009–3660 from the Swedish Research Council (http://www.vr.se/) and 04∶110 and 06∶141 from Carl Tryggers Foundation for Scientific Research (http://www.carltryggersstiftelse.se). Partial funding was also received from the Medical Research Foundation at Umeå University. JL was sponsored in part by a Carl Tryggers Post-doctoral fellowship and IRO is a Post-doctoral fellow supported by Umeå Centre for Microbial Research (UCMR) Linnaeus Program (LP) (http://www.ucmr.umu.se/). The authors also thank the Wallenberg foundation (http://www.wallenberg.com/) and Kempe foundation (http://www.kempe.com/index_start.html) for their support of the instruments and bioinformatics infrastructure at the Umeå Protein Analysis Facility. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Macritchie DM, Raivio TL. Envelope stress responses. In: Böck A, Curtiss R III, Kaper JB, Karp PD, Neidhardt FC, et al., editors. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology, http://wwwecosalorg. Washington, DC.: ASM Press; 2009. [Google Scholar]
- 2.Bury-Mone S, Nomane Y, Reymond N, Barbet R, Jacquet E, et al. Global analysis of extracytoplasmic stress signaling in Escherichia coli. PLoS Genet. 2009;5:e1000651. doi: 10.1371/journal.pgen.1000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price NL, Raivio TL. Characterization of the Cpx regulon in Escherichia coli strain MC4100. J Bacteriol. 2009;191:1798–1815. doi: 10.1128/JB.00798-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Wulf P, McGuire AM, Liu X, Lin EC. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J Biol Chem. 2002;277:26652–26661. doi: 10.1074/jbc.M203487200. [DOI] [PubMed] [Google Scholar]
- 5.Raivio TL, Silhavy TJ. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J Bacteriol. 1997;179:7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raivio TL, Popkin DL, Silhavy TJ. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J Bacteriol. 1999;181:5263–5272. doi: 10.1128/jb.181.17.5263-5272.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isaac DD, Pinkner JS, Hultgren SJ, Silhavy TJ. The extracytoplasmic adaptor protein CpxP is degraded with substrate by DegP. Proc Natl Acad Sci U S A. 2005;102:17775–17779. doi: 10.1073/pnas.0508936102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buelow DR, Raivio TL. Cpx signal transduction is influenced by a conserved N-terminal domain in the novel inhibitor CpxP and the periplasmic protease DegP. J Bacteriol. 2005;187:6622–6630. doi: 10.1128/JB.187.19.6622-6630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volz K. Structural conservation in the CheY superfamily. Biochemistry. 1993;32:11741–11753. doi: 10.1021/bi00095a001. [DOI] [PubMed] [Google Scholar]
- 10.Pogliano J, Dong JM, De Wulf P, Furlong D, Boyd D, et al. Aberrant cell division and random FtsZ ring positioning in Escherichia coli cpxA* mutants. J Bacteriol. 1998;180:3486–3490. doi: 10.1128/jb.180.13.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Wulf P, Lin EC. Cpx two-component signal transduction in Escherichia coli: excessive CpxR-P levels underlie CpxA* phenotypes. J Bacteriol. 2000;182:1423–1426. doi: 10.1128/jb.182.5.1423-1426.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Wulf P, Kwon O, Lin EC. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J Bacteriol. 1999;181:6772–6778. doi: 10.1128/jb.181.21.6772-6778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danese PN, Snyder WB, Cosma CL, Davis LJ, Silhavy TJ. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 1995;9:387–398. doi: 10.1101/gad.9.4.387. [DOI] [PubMed] [Google Scholar]
- 14.Cosma CL, Danese PN, Carlson JH, Silhavy TJ, Snyder WB. Mutational activation of the Cpx signal transduction pathway of Escherichia coli suppresses the toxicity conferred by certain envelope-associated stresses. Mol Microbiol. 1995;18:491–505. doi: 10.1111/j.1365-2958.1995.mmi_18030491.x. [DOI] [PubMed] [Google Scholar]
- 15.Danese PN, Silhavy TJ. CpxP, a stress-combative member of the Cpx regulon. J Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danese PN, Murphy CK, Silhavy TJ. Multicopy suppression of cold-sensitive sec mutations in Escherichia coli. J Bacteriol. 1995;177:4969–4973. doi: 10.1128/jb.177.17.4969-4973.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogliano J, Lynch AS, Belin D, Lin EC, Beckwith J. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 1997;11:1169–1182. doi: 10.1101/gad.11.9.1169. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol. 2008;190:2314–2322. doi: 10.1128/JB.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlsson KE, Liu J, Edqvist PJ, Francis MS. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect Immun. 2007;75:3913–3924. doi: 10.1128/IAI.01346-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson KE, Liu J, Edqvist PJ, Francis MS. Influence of the Cpx Extracytoplasmic-stress-responsive pathway on Yersinia sp.-eukaryotic cell contact. Infect Immun. 2007;75:4386–4399. doi: 10.1128/IAI.01450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naktin J, Beavis KG. Yersinia enterocolitica and Yersinia pseudotuberculosis. Clin Lab Med. 1999;19:523–536, vi. [PubMed] [Google Scholar]
- 22.Nagel G, Lahrz A, Dersch P. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol Microbiol. 2001;41:1249–1269. doi: 10.1046/j.1365-2958.2001.02522.x. [DOI] [PubMed] [Google Scholar]
- 23.Revell PA, Miller VL. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol Microbiol. 2000;35:677–685. doi: 10.1046/j.1365-2958.2000.01740.x. [DOI] [PubMed] [Google Scholar]
- 24.Cathelyn JS, Crosby SD, Lathem WW, Goldman WE, Miller VL. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc Natl Acad Sci U S A. 2006;103:13514–13519. doi: 10.1073/pnas.0603456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cathelyn JS, Ellison DW, Hinchliffe SJ, Wren BW, Miller VL. The RovA regulons of Yersinia enterocolitica and Yersinia pestis are distinct: evidence that many RovA-regulated genes were acquired more recently than the core genome. Mol Microbiol. 2007;66:189–205. doi: 10.1111/j.1365-2958.2007.05907.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, Ke Y, Tan Y, Bi Y, Shi Q, et al. Cell membrane is impaired, accompanied by enhanced type III secretion system expression in Yersinia pestis deficient in RovA regulator. PLoS One. 2010;5:e12840. doi: 10.1371/journal.pone.0012840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bölin I, Wolf-Watz H. Molecular cloning of the temperature-inducible outer membrane protein 1 of Yersinia pseudotuberculosis. Infect Immun. 1984;43:72–78. doi: 10.1128/iai.43.1.72-78.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect Immun. 2004;72:4973–4984. doi: 10.1128/IAI.72.9.4973-4984.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francis MS, Wolf-Watz H. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol Microbiol. 1998;29:799–813. doi: 10.1046/j.1365-2958.1998.00973.x. [DOI] [PubMed] [Google Scholar]
- 30.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kinoshita E, Kinoshita-Kikuta E, Takiyama K, Koike T. Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol Cell Proteomics. 2006;5:749–757. doi: 10.1074/mcp.T500024-MCP200. [DOI] [PubMed] [Google Scholar]
- 32.Barbieri CM, Stock AM. Universally applicable methods for monitoring response regulator aspartate phosphorylation both in vitro and in vivo using Phos-tag-based reagents. Anal Biochem. 2008;376:73–82. doi: 10.1016/j.ab.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purich DL. Use of sodium borohydride to detect acyl-phosphate linkages in enzyme reactions. Methods Enzymol. 2002;354:168–177. doi: 10.1016/s0076-6879(02)54013-1. [DOI] [PubMed] [Google Scholar]
- 34.Head CG, Tardy A, Kenney LJ. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J Mol Biol. 1998;281:857–870. doi: 10.1006/jmbi.1998.1985. [DOI] [PubMed] [Google Scholar]
- 35.Kussmann M, Lassing U, Sturmer CA, Przybylski M, Roepstorff P. Matrix-assisted laser desorption/ionization mass spectrometric peptide mapping of the neural cell adhesion protein neurolin purified by sodium dodecyl sulfate polyacrylamide gel electrophoresis or acidic precipitation. J Mass Spectrom. 1997;32:483–493. doi: 10.1002/(SICI)1096-9888(199705)32:5<483::AID-JMS502>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 36.Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75:663–670. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]
- 37.Jensen SS, Larsen MR. Evaluation of the impact of some experimental procedures on different phosphopeptide enrichment techniques. Rapid Commun Mass Spectrom. 2007;21:3635–3645. doi: 10.1002/rcm.3254. [DOI] [PubMed] [Google Scholar]
- 38.Thingholm TE, Jorgensen TJ, Jensen ON, Larsen MR. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc. 2006;1:1929–1935. doi: 10.1038/nprot.2006.185. [DOI] [PubMed] [Google Scholar]
- 39.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics. 2005;4:873–886. doi: 10.1074/mcp.T500007-MCP200. [DOI] [PubMed] [Google Scholar]
- 40.Dodd IB, Egan JB. Improved detection of helix-turn-helix DNA-binding motifs in protein sequences. Nucleic Acids Res. 1990;18:5019–5026. doi: 10.1093/nar/18.17.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galperin MY. Diversity of structure and function of response regulator output domains. Curr Opin Microbiol. 2010;13:150–159. doi: 10.1016/j.mib.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto K, Ishihama A. Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci Biotechnol Biochem. 2006;70:1688–1695. doi: 10.1271/bbb.60024. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, Isberg RR. Transcriptional regulation of the Yersinia pseudotuberculosis pH6 antigen adhesin by two envelope-associated components. Mol Microbiol. 1997;24:499–510. doi: 10.1046/j.1365-2958.1997.3511719.x. [DOI] [PubMed] [Google Scholar]
- 44.Jubelin G, Vianney A, Beloin C, Ghigo JM, Lazzaroni JC, et al. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J Bacteriol. 2005;187:2038–2049. doi: 10.1128/JB.187.6.2038-2049.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heroven AK, Nagel G, Tran HJ, Parr S, Dersch P. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis. Mol Microbiol. 2004;53:871–888. doi: 10.1111/j.1365-2958.2004.04162.x. [DOI] [PubMed] [Google Scholar]
- 46.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCleary WR, Stock JB. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem. 1994;269:31567–31572. [PubMed] [Google Scholar]
- 48.Wolfe AJ, Chang DE, Walker JD, Seitz-Partridge JE, Vidaurri MD, et al. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol Microbiol. 2003;48:977–988. doi: 10.1046/j.1365-2958.2003.03457.x. [DOI] [PubMed] [Google Scholar]
- 49.Mizrahi I, Biran D, Ron EZ. Requirement for the acetyl phosphate pathway in Escherichia coli ATP-dependent proteolysis. Mol Microbiol. 2006;62:201–211. doi: 10.1111/j.1365-2958.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 50.Snyder WB, Davis LJ, Danese PN, Cosma CL, Silhavy TJ. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suntharalingam P, Spencer H, Gallant CV, Martin NL. Salmonella enterica serovar typhimurium rdoA is growth phase regulated and involved in relaying Cpx-induced signals. J Bacteriol. 2003;185:432–443. doi: 10.1128/JB.185.2.432-443.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makino K, Amemura M, Kawamoto T, Kimura S, Shinagawa H, et al. DNA binding of PhoB and its interaction with RNA polymerase. J Mol Biol. 1996;259:15–26. doi: 10.1006/jmbi.1996.0298. [DOI] [PubMed] [Google Scholar]
- 53.Makino K, Amemura M, Kim SK, Nakata A, Shinagawa H. Role of the sigma 70 subunit of RNA polymerase in transcriptional activation by activator protein PhoB in Escherichia coli. Genes Dev. 1993;7:149–160. doi: 10.1101/gad.7.1.149. [DOI] [PubMed] [Google Scholar]
- 54.Ramani N, Huang L, Freundlich M. In vitro interactions of integration host factor with the ompF promoter-regulatory region of Escherichia coli. Mol Gen Genet. 1992;231:248–255. doi: 10.1007/BF00279798. [DOI] [PubMed] [Google Scholar]
- 55.Slauch JM, Silhavy TJ. cis-acting ompF mutations that result in OmpR-dependent constitutive expression. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoshida T, Qin L, Egger LA, Inouye M. Transcription regulation of ompF and ompC by a single transcription factor, OmpR. J Biol Chem. 2006;281:17114–17123. doi: 10.1074/jbc.M602112200. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Alvey RM, Bezy RP, Kehoe DM. Inverse transcriptional activities during complementary chromatic adaptation are controlled by the response regulator RcaC binding to red and green light-responsive promoters. Mol Microbiol. 2008;68:286–297. doi: 10.1111/j.1365-2958.2008.06151.x. [DOI] [PubMed] [Google Scholar]
- 58.Gao R, Stock AM. Molecular strategies for phosphorylation-mediated regulation of response regulator activity. Curr Opin Microbiol. 2010;13:160–167. doi: 10.1016/j.mib.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rhee JE, Sheng W, Morgan LK, Nolet R, Liao X, et al. Amino acids important for DNA recognition by the response regulator OmpR. J Biol Chem. 2008;283:8664–8677. doi: 10.1074/jbc.M705550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ames SK, Frankema N, Kenney LJ. C-terminal DNA binding stimulates N-terminal phosphorylation of the outer membrane protein regulator OmpR from Escherichia coli. Proc Natl Acad Sci U S A. 1999;96:11792–11797. doi: 10.1073/pnas.96.21.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gusa AA, Gao J, Stringer V, Churchward G, Scott JR. Phosphorylation of the group A Streptococcal CovR response regulator causes dimerization and promoter-specific recruitment by RNA polymerase. J Bacteriol. 2006;188:4620–4626. doi: 10.1128/JB.00198-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perron-Savard P, De Crescenzo G, Le Moual H. Dimerization and DNA binding of the Salmonella enterica PhoP response regulator are phosphorylation independent. Microbiology. 2005;151:3979–3987. doi: 10.1099/mic.0.28236-0. [DOI] [PubMed] [Google Scholar]
- 63.Lawrenz MB, Miller VL. Comparative analysis of the regulation of rovA from the pathogenic yersiniae. J Bacteriol. 2007;189:5963–5975. doi: 10.1128/JB.00528-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran HJ, Heroven AK, Winkler L, Spreter T, Beatrix B, et al. Analysis of RovA, a transcriptional regulator of Yersinia pseudotuberculosis virulence that acts through antirepression and direct transcriptional activation. J Biol Chem. 2005;280:42423–42432. doi: 10.1074/jbc.M504464200. [DOI] [PubMed] [Google Scholar]
- 65.Heroven AK, Dersch P. RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis. Mol Microbiol. 2006;62:1469–1483. doi: 10.1111/j.1365-2958.2006.05458.x. [DOI] [PubMed] [Google Scholar]
- 66.Heroven AK, Bohme K, Rohde M, Dersch P. A Csr-type regulatory system, including small non-coding RNAs, regulates the global virulence regulator RovA of Yersinia pseudotuberculosis through RovM. Mol Microbiol. 2008;68:1179–1195. doi: 10.1111/j.1365-2958.2008.06218.x. [DOI] [PubMed] [Google Scholar]
- 67.Herbst K, Bujara M, Heroven AK, Opitz W, Weichert M, et al. Intrinsic thermal sensing controls proteolysis of Yersinia virulence regulator RovA. PLoS Pathog. 2009;5:e1000435. doi: 10.1371/journal.ppat.1000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Labandeira-Rey M, Brautigam CA, Hansen EJ. Characterization of the CpxRA regulon in Haemophilus ducreyi. Infect Immun. 2010;78:4779–4791. doi: 10.1128/IAI.00678-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizrahi I, Biran D, Ron EZ. Involvement of the Pta-AckA pathway in protein folding and aggregation. Res Microbiol. 2009;160:80–84. doi: 10.1016/j.resmic.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 70.Otto K, Silhavy TJ. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc Natl Acad Sci U S A. 2002;99:2287–2292. doi: 10.1073/pnas.042521699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano Y, Hossain MM, Takeda K, Tokuda H, Miki K. Structural studies of the Cpx pathway activator NlpE on the outer membrane of Escherichia coli. Structure. 2007;15:963–976. doi: 10.1016/j.str.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto K, Ishihama A. Transcriptional response of Escherichia coli to external copper. Mol Microbiol. 2005;56:215–227. doi: 10.1111/j.1365-2958.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 73.Miyadai H, Tanaka-Masuda K, Matsuyama S, Tokuda H. Effects of lipoprotein overproduction on the induction of DegP (HtrA) involved in quality control in the Escherichia coli periplasm. J Biol Chem. 2004;279:39807–39813. doi: 10.1074/jbc.M406390200. [DOI] [PubMed] [Google Scholar]
- 74.Chi A, Huttenhower C, Geer LY, Coon JJ, Syka JE, et al. Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci U S A. 2007;104:2193–2198. doi: 10.1073/pnas.0607084104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellison DW, Miller VL. H-NS represses inv transcription in Yersinia enterocolitica through competition with RovA and interaction with YmoA. J Bacteriol. 2006;188:5101–5112. doi: 10.1128/JB.00862-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ellison DW, Young B, Nelson K, Miller VL. YmoA negatively regulates expression of invasin from Yersinia enterocolitica. J Bacteriol. 2003;185:7153–7159. doi: 10.1128/JB.185.24.7153-7159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brzostek K, Brzostkowska M, Bukowska I, Karwicka E, Raczkowska A. OmpR negatively regulates expression of invasin in Yersinia enterocolitica. Microbiology. 2007;153:2416–2425. doi: 10.1099/mic.0.2006/003202-0. [DOI] [PubMed] [Google Scholar]
- 78.Lau-Wong IC, Locke T, Ellison MJ, Raivio TL, Frost LS. Activation of the Cpx regulon destabilizes the F plasmid transfer activator, TraJ, via the HslVU protease in Escherichia coli. Mol Microbiol. 2008;67:516–527. doi: 10.1111/j.1365-2958.2007.06055.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bacterial strains and plasmids used in this study.
(RTF)
Oligonucleotides used in this study.
(RTF)
Mass determination of intact CpxR and CpxR∼P by MALDI-MS. The respective CpxR and CpxR∼P peaks are indicated along with the experimental mass (Daltons).
(TIF)
Growth curves of various Y. pseudotuberculosis bacteria. Overnight cultures of parental and mutant bacteria were sub-cultured into fresh LB broth (time point 0 hours) and their growth during aerobic culturing with agitation at 26°C was monitored over a period of 9 hours by optical density measurement at 600 nm (A and B). Parent, YPIII/pIB102; YPIII07, ΔcpxA; YPIII08, ΔcpxR; YPIII46, mutated cpxR encoding CpxRM199A; YPIII49, ΔcpxA, ΔackA, pta null mutant; YPIII51, cpxA101* encoding CpxAT253P; YPIII52, mutated cpxR encoding CpxRD51A; YPIII69, ΔackA, pta null mutant; YPIII74, cpxA101*, ackA, pta null mutant; YPIII112, cpxR/cpxP (Mt) placed in the parent background; YPIII113, rovA (Mt 1) placed in the parent background; YPIII114, rovA (Mt 2) placed in the parent background; YPIII115, cpxR/cpxP (Mt) placed in the cpxA101* background; YPIII120, rovA (Mt 1) placed in the cpxA101* background; YPIII121, rovA (Mt 2) placed in the cpxA101* background. The asterisk (*) signifies modest to severe growth restriction in those bacteria with defects in the Pta-AckA biosynthetic pathway [18], [48], [49] (A) or in the CpxA sensor kinase that would be expected to accumulate toxic levels of CpxR∼P [4], [19], [20] (A and B). Note that despite being in a cpxA101* background, strain YPIII115/pIB102 grows quite well (B). Presumably, this is because CpxR∼P does not accumulate in this strain consistent with poor cpxR expression (data not shown) resulting from a shuffle mutation that makes the divergent promoter of cpxR/cpxP unable to bind CpxR∼P.
(TIF)
CpxR∼P DNA binding is required for repression of rovA transcription in vivo. For transcription analysis, crude semi-quantitative RT-PCR was performed on mRNA isolated from Y. pseudotuberculosis. Given the limitations of semi-quantitative RT-PCR, we attempted to increase the robustness of our assay by reverse transcribing mRNA isolated from bacteria grown at 26°C in LB broth to two different growth phases as measured by optical density at 600 nm; the first being an OD600 value between 0.45 to 0.55 (mid-logarithmic phase) and the second being an OD600 measurement between 0.85 to 0.95 (early-stationary phase). This was necessary to control for the altered growth rate of those bacteria expected to accumulate toxic levels of CpxR∼P (Figure S2B). Samples were subjected to RT-PCR with primers specific for rovA (plus RT). As an mRNA loading control, we analyzed the transcription of rpoA encoding for the α-subunit of RNA polymerase, which remained the same regardless of genetic background or phase of growth. To confirm the purity of the RNA isolated, PCRs with rpoA and rovA primer pairs was performed on template derived from RT reactions in which the enzyme was intentionally excluded (minus RT). PCR analysis on these samples indicated that the RNA isolation was essentially free of genomic DNA contamination. All images were acquired with a Fluor-S MultiImager (Bio-Rad) and analyzed with Quantity One software version 4.2.3 (Bio-Rad). DNA fragment sizes in base pairs are given in parentheses. Strains: parent, YPIII/pIB102; cpxA101* encoding CpxAT253P, YPIII51/pIB102; cpxR/cpxP (Mt) (shuffle mutation of the CpxR∼P binding site identified in Figure 4) placed in the cpxA101* background, YPIII115/pIB102; rovA (Mt 1) placed in cpxA101*, YPIII120/pIB102; rovA (Mt 2) placed in cpxA101*, YPIII121/pIB102.
(TIF)
Established and potential mechanisms of Cpx-dependent modulation of rovA and inv expression. The Cpx pathway is a sensor of extracytoplasmic stress (ECS) [1]. However, the role of CpxR∼P as a central regulator of Y. pseudotuberculosis pathogenicity is also becoming apparent (this study) [19], [20]. CpxR∼P levels in the bacterial cytoplasm are manipulated by cognate CpxA kinase and phosphatase activity. This is even further affected by the CpxA-independent phosphorylation of CpxR via second messenger phosphodonors; the levels of which are somehow influenced by an intact AckA/Pta pathway. In turn, CpxR∼P binds directly to the rovA and inv promoters repressing transcriptional output (red line). This influence may also be augmented indirectly through as yet unknown (indicated by a dashed line and a ‘?’ symbol) regulatory links to other positive (green) and negative (red) factors controlling rovA and/or inv expression [22], [23], [45], [63], [64], [65], [66], [75], [76], [77]. Recently, H-NS was described to be a part of the CpxR∼P regulon [2]. However, our in vitro electrophoretic mobility shift analysis did not reveal any CpxR∼P bound to the hns or ymoA promoters (Liu and Francis, unpublished). Not shown in this diagram is the influence of CpxR∼P on lon expression [78], but this connection is still relevant given how RovA is subject to proteolysis by the Lon protease [67]. Elevated CpxR∼P levels also diminishes efficient T3S and the production of other ‘non-invasin’ adhesins by a mechanism(s) that are not yet understood (red dotted line).
(TIF)
Regulator binding sites in the upstream flanking sequence of rovA. Y. pseudotuberculosis rovA transcription is initiated from two sites (P1 and P2) upstream of the translational start codon (TTG – green) [45]. DNA sequences flanking P1 and P2 serve as binding sites for an array of regulators including H-NS (purple), RovM (dark blue) and RovA (red) [45], [65]. We have now shown herein that CpxR∼P (brown) also binds to DNA sequences that incorporate the −35 region of the P2 promoter. Based upon the binding site in the −35 region however, CpxR∼P could prevent access to the P2 promoter by the RNA polymerase holoenzyme. It is not yet known if or how CpxR∼P binding influences the binding of the other rovA DNA-binding regulators.
(TIF)