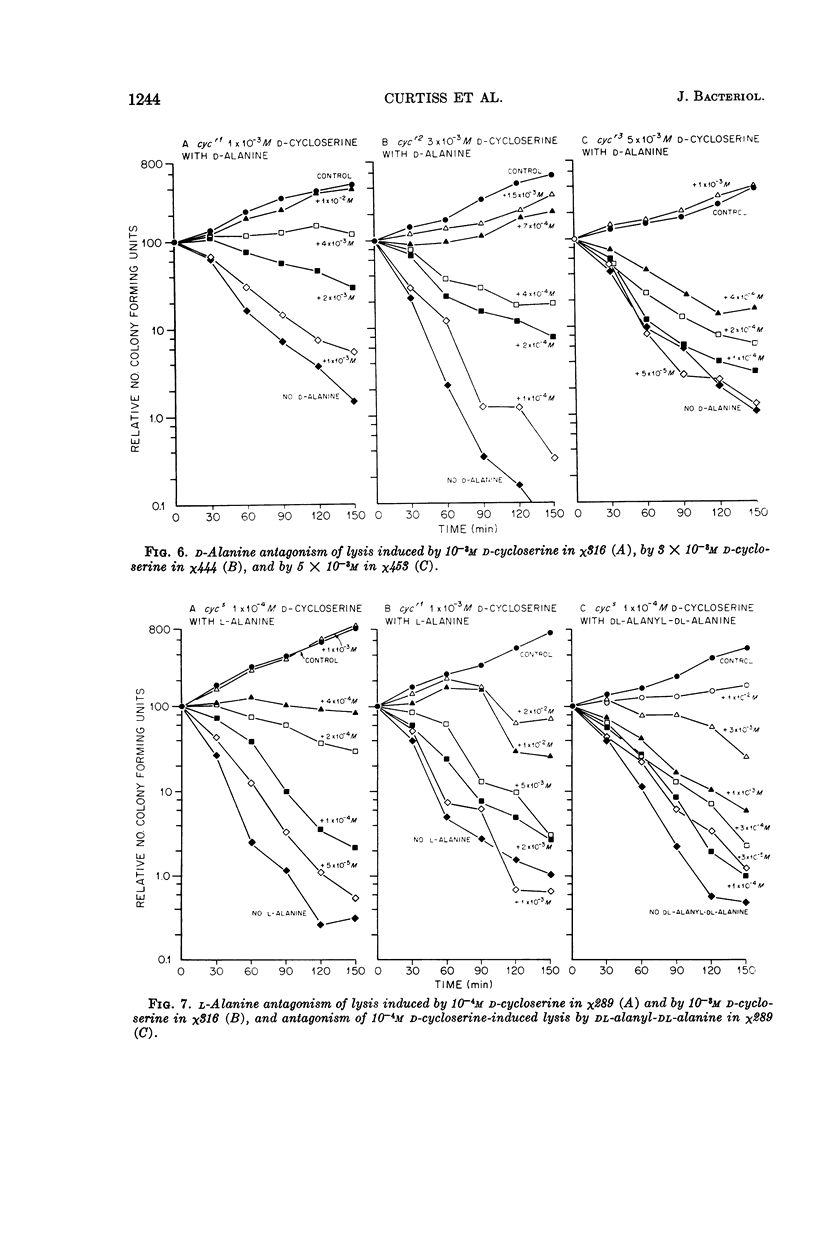
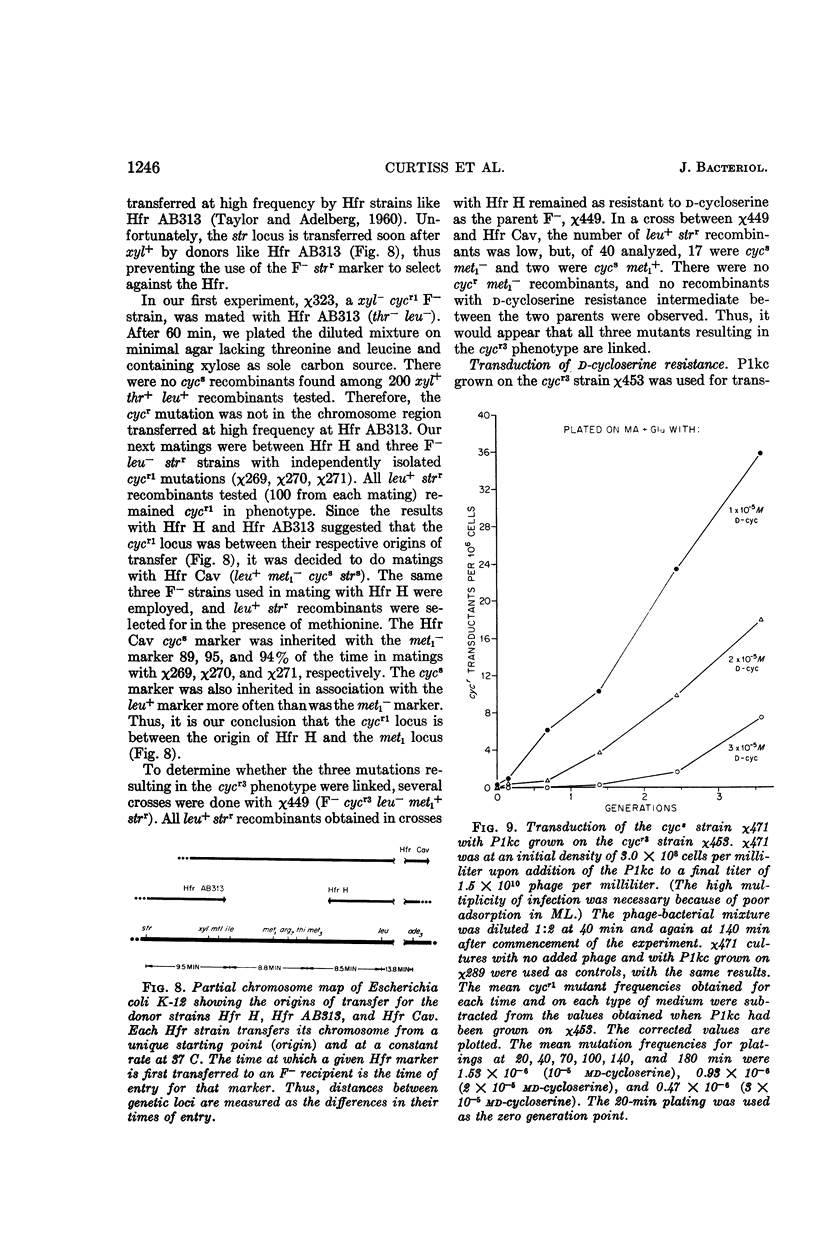
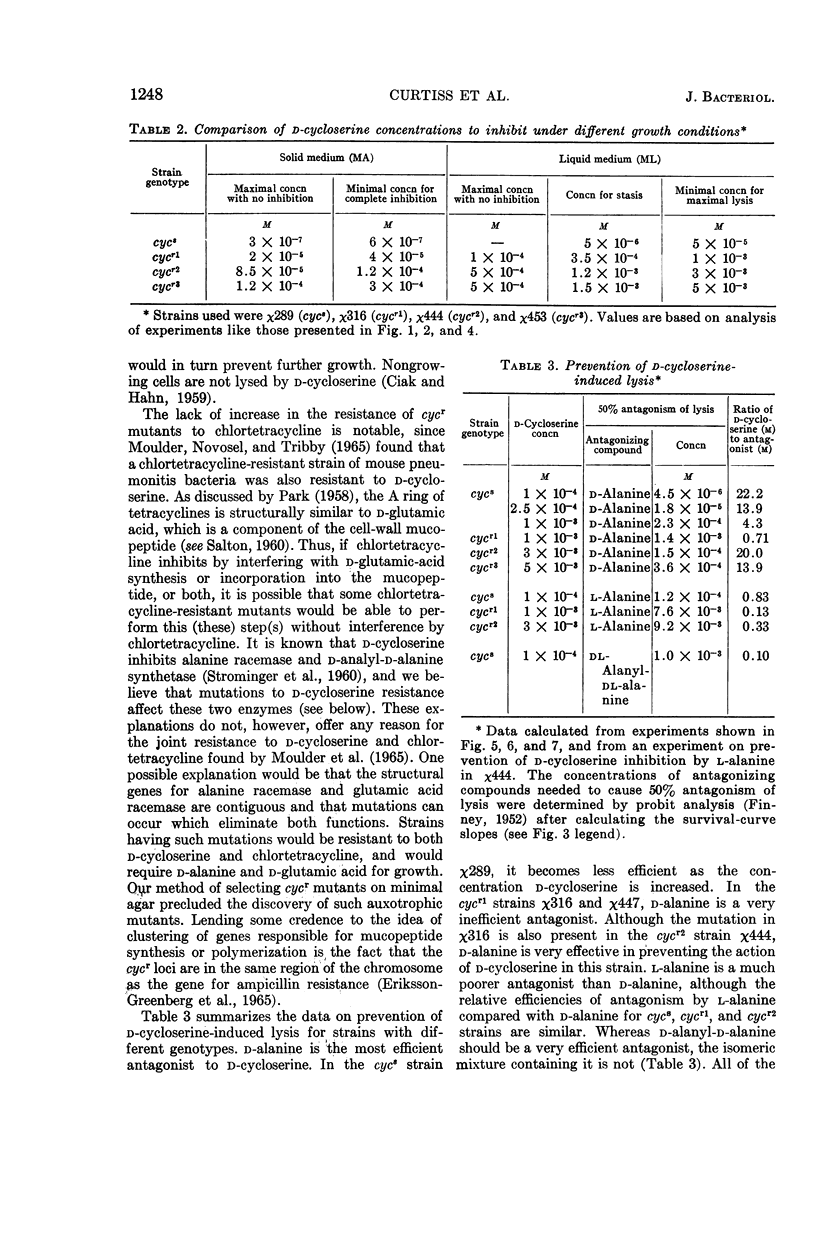
Abstract
Curtiss, Roy, III (Oak Ridge National Laboratory, Oak Ridge, Tenn.), Leigh J. Charamella, Claire M. Berg, and Paula E. Harris. Kinetic and genetic analyses of d-cycloserine inhibition and resistance in Escherichia coli. J. Bacteriol. 90:1238–1250.1965.—Wild-type cells of Escherichia coli growing at 37 C in mineral salts-glucose medium with vigorous aeration were lysed at maximal exponential rates by 10−4 to 10−2md-cycloserine. At concentrations above 2 × 10−2m, d-cycloserine was bacteriostatic. Low levels of d-cycloserine (10−5m) and pencillin G (10 units per ml) interacted synergistically to cause a rapid exponential rate of lysis. Spontaneous mutations to d-cycloserine resistance occurred in discrete steps at frequencies of 10−6 to 10−7 for each step. First-, second-, and third-step d-cycloserine-resistant mutants were lysed at maximal exponential rates by d-cycloserine concentrations of 10−3, 3 × 10−3, and 5 × 10−3m, respectively. d-Alanine, l-alanine, and dl-alanyl-dl-alanine reversed d-cycloserine-induced lysis, in that order of effectiveness. On the basis of these observations, a d-cycloserine-enrichment cycling technique was developed for isolation of auxotrophic mutants. d-Cycloserine at 2 × 10−3m was as efficient as penicillin G (1,000 units per ml) for mutant enrichment in E. coli and should be useful for isolation of mutants in penicillin-resistant microorganisms. Bacterial conjugation experiments indicated that all three mutations conferring d-cycloserine resistance were linked to the met1 locus. Transduction experiments showed that the mutation conferring first-step resistance was at least 0.5 min away from the mutations conferring second- and third-step resistance. The latter two mutations possibly occurred in the same gene, since they were sometimes carried in the same transducing phage. Studies on expression of d-cycloserine resistance indicated that these mutations were neither dominant nor recessive to each other nor to the d-cycloserine-sensitivity allele. Each allelic state exerted its influence on the phenotype independently of the others. These results are discussed in terms of the known inhibition of alanine racemase and d-alanyl-d-alanine synthetase by d-cycloserine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADDILEY J., NEUHAUS F. C. The enzymic activation of D-alanine. Biochem J. 1960 Jun;75:579–587. doi: 10.1042/bj0750579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARBIERI P., DI MARCO A., FUOCO L., JULITA P., MIGLIACCI A., RUSCONI A. Investigation on the mode of action of cycloserine upon protein synthesis of E. coli and animal cells. 2. Action of L-cycloserine on protein metabolism of alanine and on enzymic preparations. Biochem Pharmacol. 1960 Jul;3:264–271. doi: 10.1016/0006-2952(60)90090-3. [DOI] [PubMed] [Google Scholar]
- BONDI A., KORNBLUM J., FORTE C. Inhibition of anitbacterial activity of cycloserine by alpha-alanine. Proc Soc Exp Biol Med. 1957 Oct;96(1):270–272. doi: 10.3181/00379727-96-23452. [DOI] [PubMed] [Google Scholar]
- BRAUNSHTEIN A. E., AZARKH R. M., HSU Ts. [On kinetics of cycloserine inhibition of enzymatic transmination reactions]. Biokhimiia. 1961 Sep-Oct;26:882–896. [PubMed] [Google Scholar]
- CURTIS S. R., 3rd A STABLE PARTIAL DIPLOID STRAIN OF ESCHERICHIA COLI. Genetics. 1964 Oct;50:679–694. doi: 10.1093/genetics/50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Boman H. G., Jansson J. A., Thorén S. Resistance of Escherichia coli to Penicillins I. Genetic Study of Some Ampicillin-Resistant Mutants. J Bacteriol. 1965 Jul;90(1):54–62. doi: 10.1128/jb.90.1.54-62.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., KAUFMAN H. Selecting bacterial mutants by the penicillin method. Science. 1960 Feb 26;131(3400):604–605. doi: 10.1126/science.131.3400.604. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LUBIN M. Enrichment of auxotrophic mutant populations by recycling. J Bacteriol. 1962 Mar;83:696–697. doi: 10.1128/jb.83.3.696-697.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., OFFICER J. E. INHIBITION OF THE GROWTH OF AGENTS OF THE PSITTACOSIS GROUP BY D-CYCLOSERINE AND ITS SPECIFIC REVERSAL BY D-ALANINE. J Bacteriol. 1963 Mar;85:707–711. doi: 10.1128/jb.85.3.707-711.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., TRIBBY I. I. CHANGES IN MOUSE PNEUMONITIS AGENT ASSOCIATED WITH DEVELOPMENT OF RESISTANCE TO CHLORTETRACYCLINE. J Bacteriol. 1965 Jan;89:17–22. doi: 10.1128/jb.89.1.17-22.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. Studies on the inhibition of D-alanyl-D-alanine synthetase by the antibiotic D-cycloserine. Biochem Biophys Res Commun. 1962 Aug 7;8:377–382. doi: 10.1016/0006-291x(62)90011-6. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- Taylor A L, Adelberg E A. Linkage Analysis with Very High Frequency Males of Escherichia Coli. Genetics. 1960 Sep;45(9):1233–1243. doi: 10.1093/genetics/45.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELCH H., PUTNAM L. E., RANDALL W. A. Antibacterial activity and blood and urine concentrations of cycloserine, a new antibiotic, following oral administration. Antibiotic Med Clin Ther (New York) 1955 Feb;1(2):72–79. [PubMed] [Google Scholar]
- ZYGMUNT W. A. ANTAGONISM OF D-CYCLOSERINE INHIBITION OF MYCOBACTERIAL GROWTH BY D-ALANINE. J Bacteriol. 1963 Jun;85:1217–1220. doi: 10.1128/jb.85.6.1217-1220.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt W. A. REVERSAL OF d-CYCLOSERINE INHIBITION OF BACTERIAL GROWTH BY ALANINE. J Bacteriol. 1962 Jul;84(1):154–156. doi: 10.1128/jb.84.1.154-156.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]



