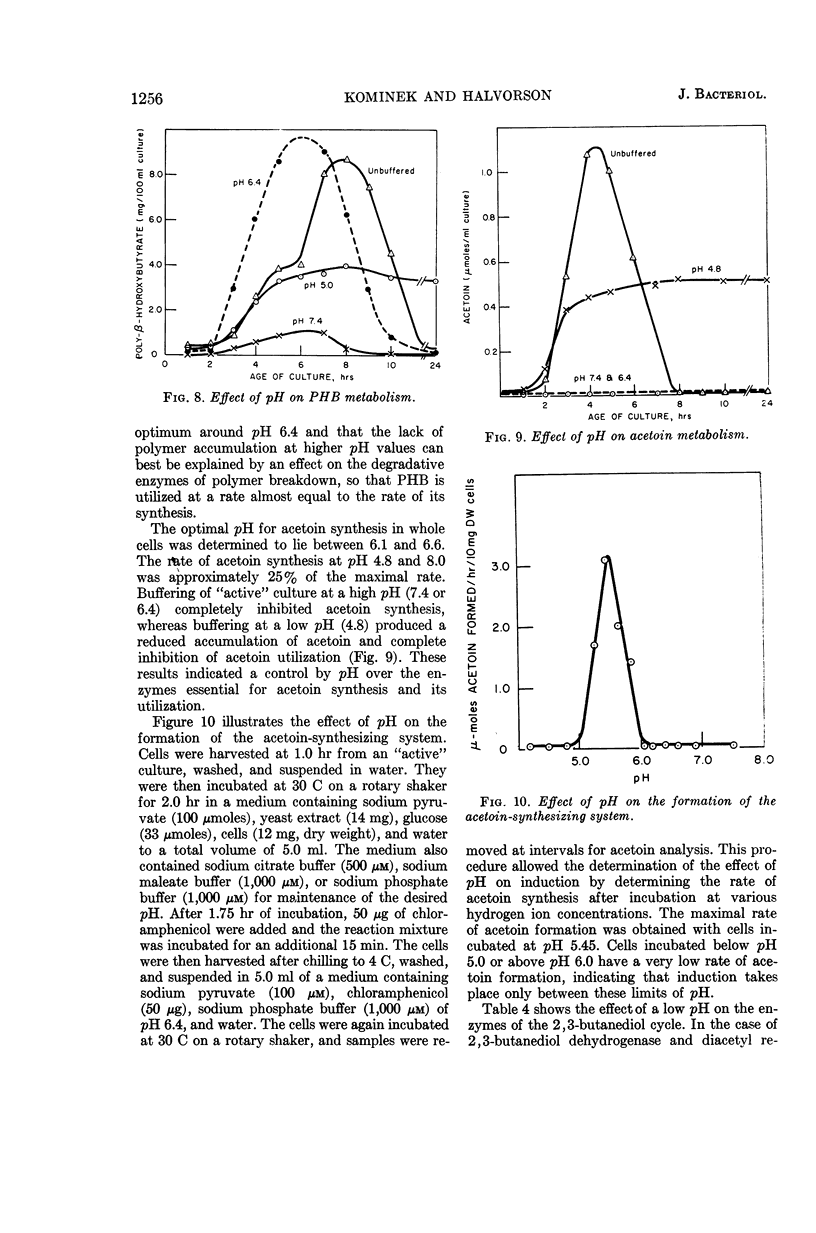
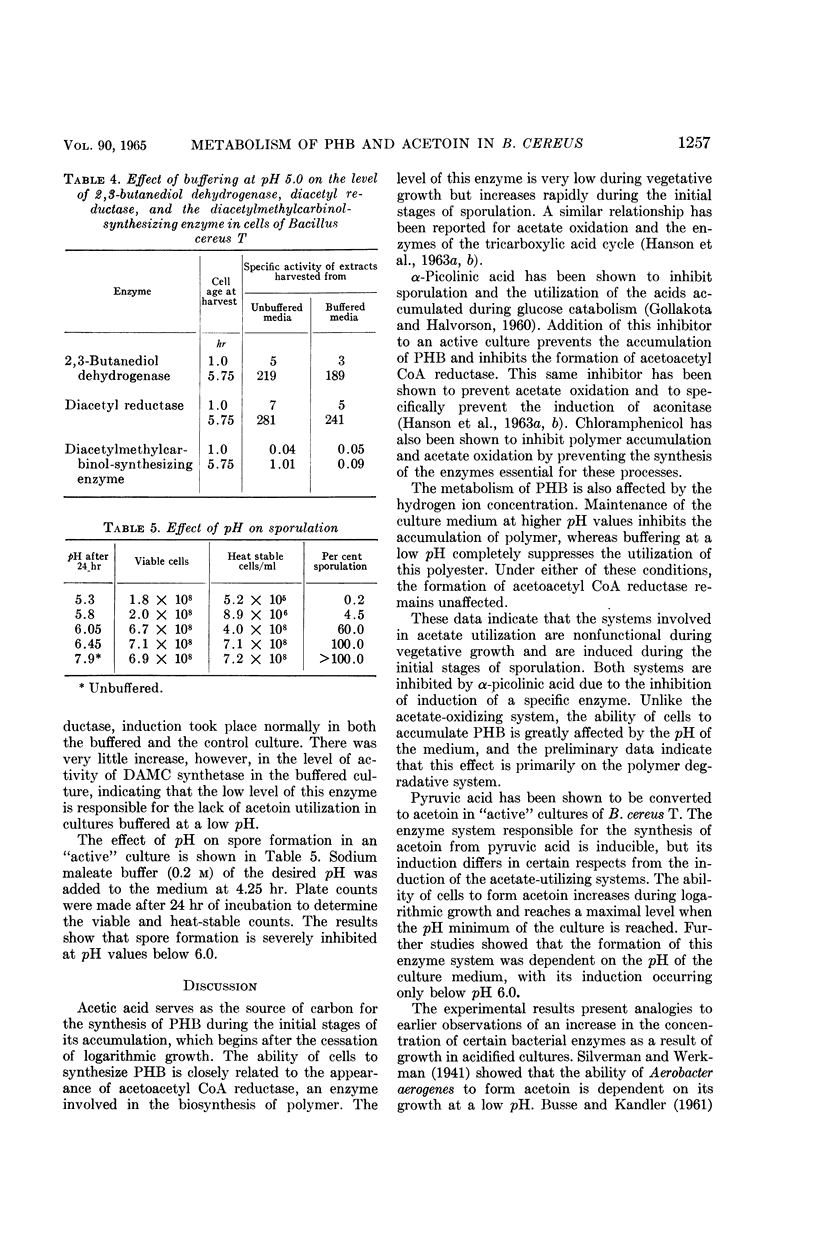
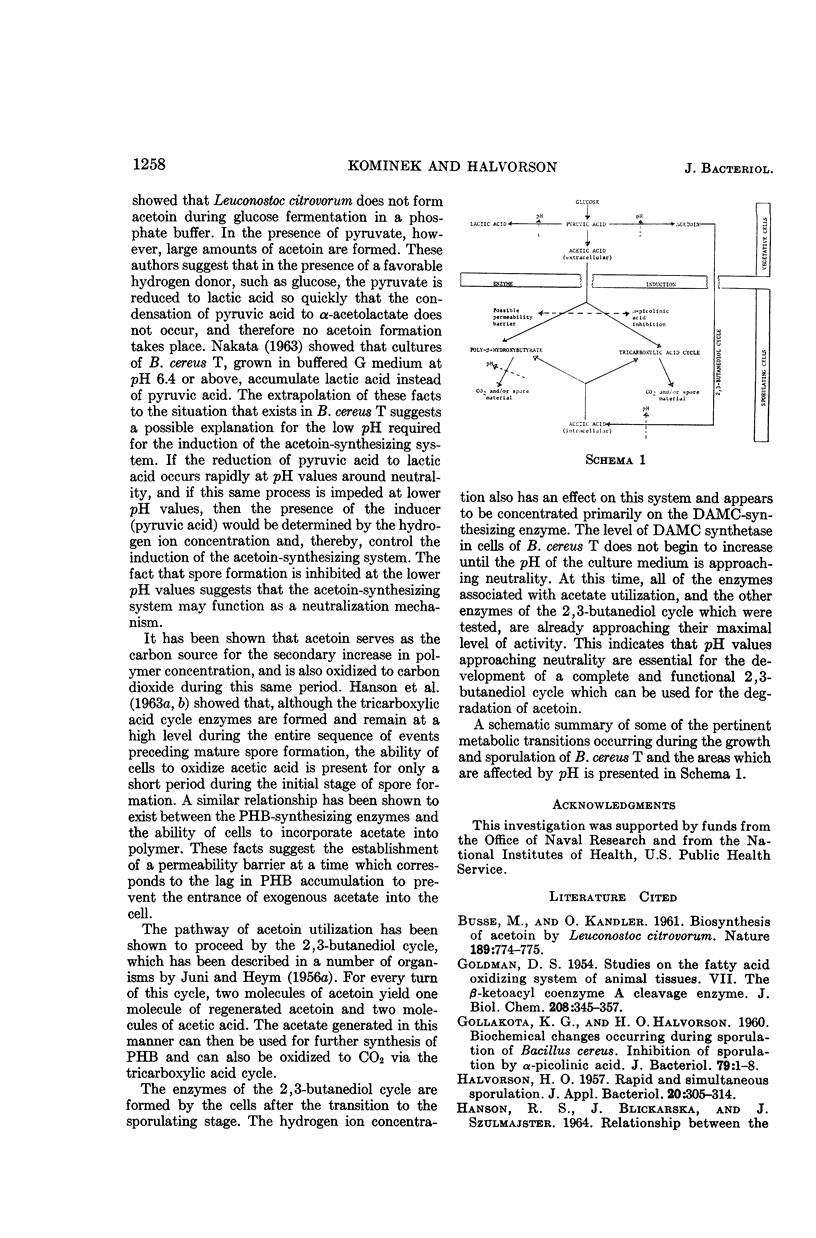
Abstract
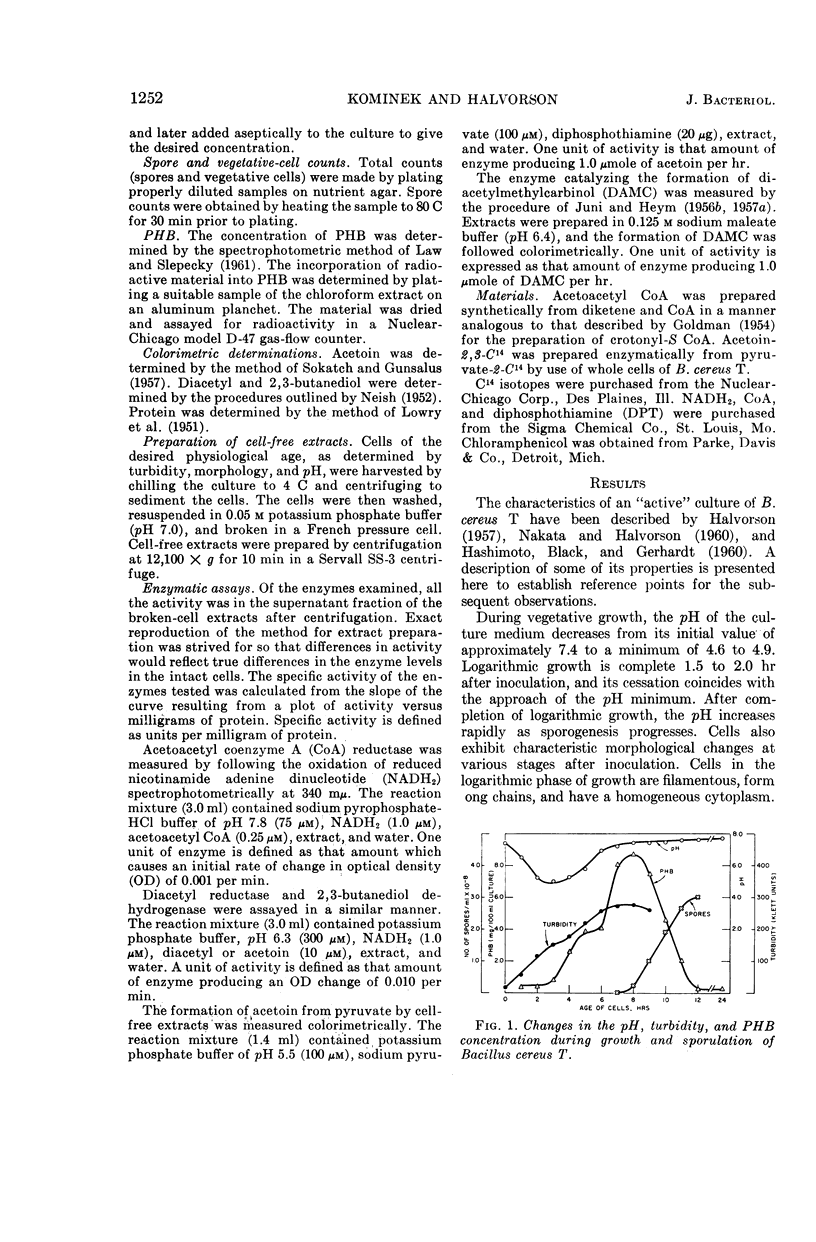
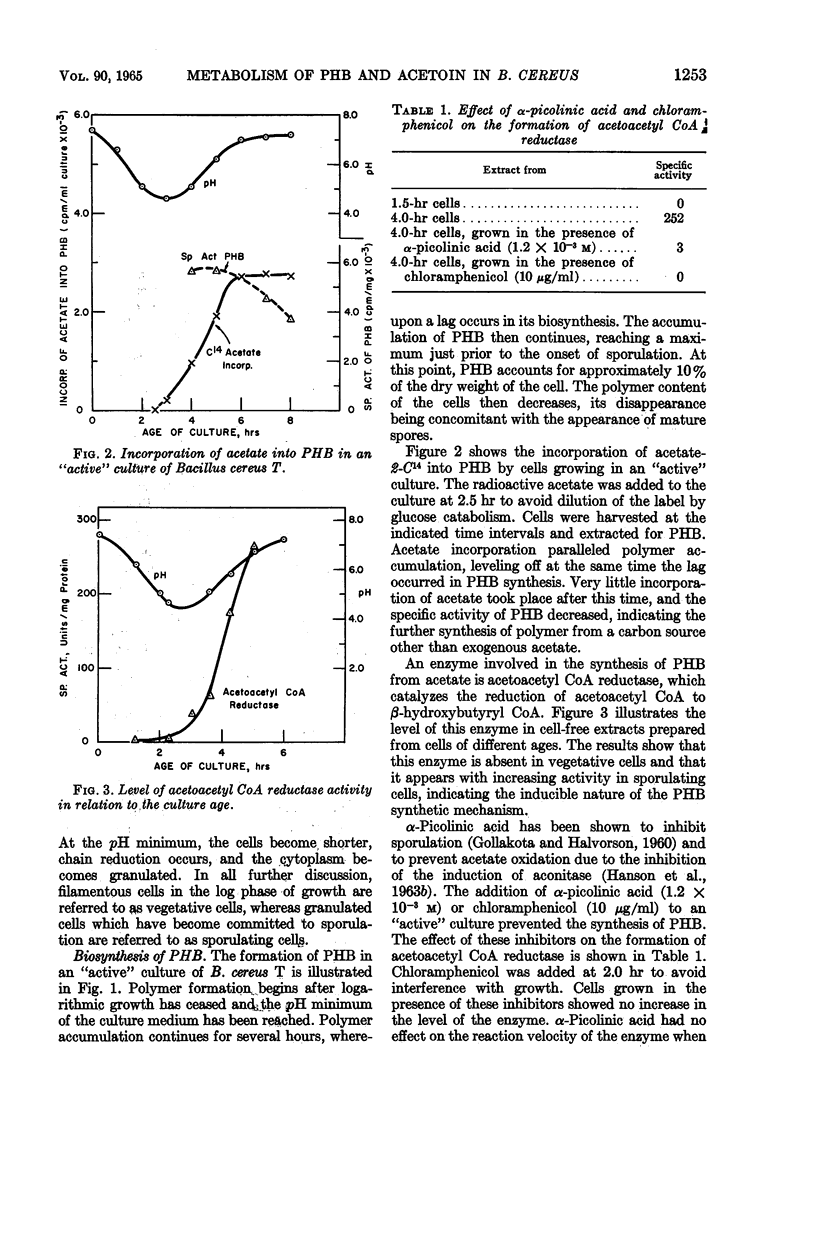
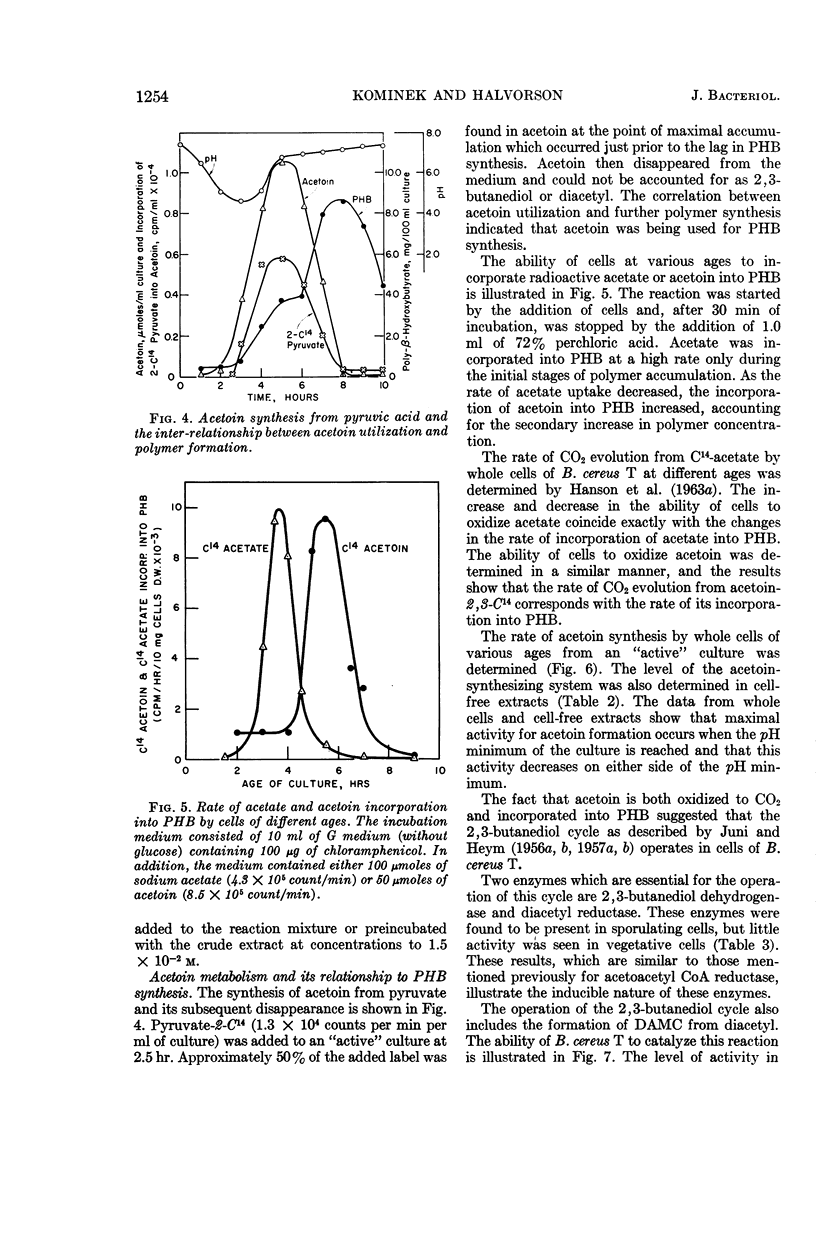
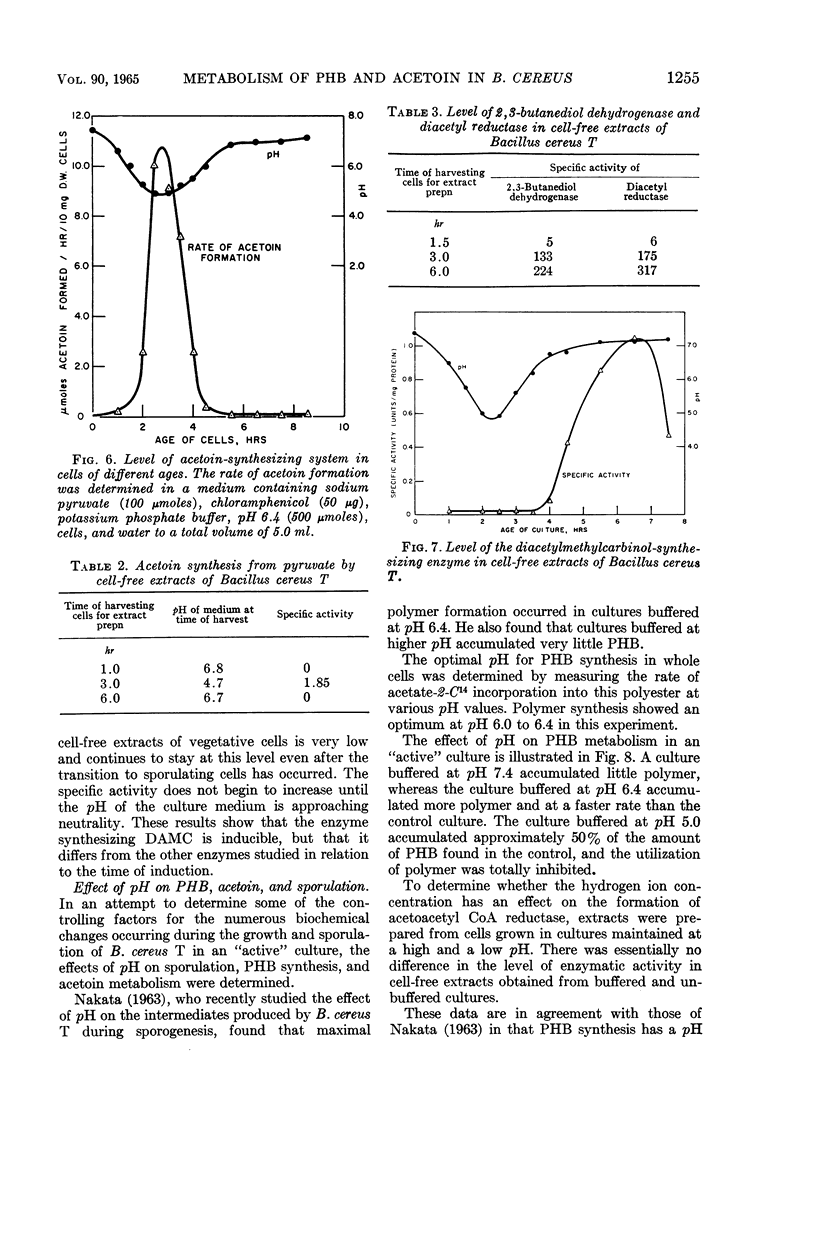
Kominek, Leo A. (University of Illinois, Urbana), and H. Orin Halvorson. Metabolism of poly-β-hydroxybutyrate and acetoin in Bacillus cereus. J. Bacteriol. 90:1251–1259. 1965.—The synthesis of poly-β-hydroxybutyrate (PHB) in Bacillus cereus strain T begins after the cessation of logarithmic growth. Its accumulation is preceded by the formation of acetoacetyl coenzyme A reductase, an enzyme used for its biosynthesis. Exogenous acetic acid present in the medium owing to incomplete glucose oxidation serves as the carbon source for polymer formation during the initial stages of its synthesis. Pyruvic acid is converted to acetoin by an enzyme system that is formed during vegetative growth. The formation of this enzyme system is dependent on a low pH in the medium. As the cells enter the sporulating stage, they lose the ability to form acetoin. The acetoin that accumulates is utilized via the 2,3-butanediol cycle which begins to function late in the sporulation stage. This cycle generates acetic acid which is used for PHB synthesis and is also oxidized to carbon dioxide. PHB accumulation reaches a maximum just prior to the formation of spores, and it is degraded during the process of sporulation. The effect of sporulation inhibitors and pH on PHB and acetoin metabolism are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUSSE M., KANDLER O. Biosynthesis of acetoin by Leuconostoc citrovorum. Nature. 1961 Mar 4;189:774–775. doi: 10.1038/189774b0. [DOI] [PubMed] [Google Scholar]
- GOLDMAN D. S. Studies on the fatty acid oxidizing system of animal tissues. VII. The beta-ketoacyl coenzyme A cleavage enzyme. J Biol Chem. 1954 May;208(1):345–357. [PubMed] [Google Scholar]
- GOLLAKOTA K. G., HALVORSON H. O. Biochemical changes occurring during sporulation of Bacillus cereus. Inhibition of sporulation by alpha-picolinic acid. J Bacteriol. 1960 Jan;79:1–8. doi: 10.1128/jb.79.1.1-8.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- HEYM G. A., JUNI E. A cyclic pathway for the bacterial dissimilation of 2, 3-butanediol, acetylmethylcarbinol and diacetyl. II. The synthesis of diacetylmethylcarbinol from diacetyl, a new diphosphothiamin catalyzed reaction. J Bacteriol. 1956 Dec;72(6):746–753. doi: 10.1128/jb.72.6.746-753.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. A cyclic pathway for the bacterial dissimilation of 2, 3-butanediol, acetylmethylcarbinol, and diacetyl. I. General aspects of the 2, 3-butanediol cycle. J Bacteriol. 1956 Apr;71(4):425–432. doi: 10.1128/jb.71.4.425-432.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. Cyclic pathway for the bacterial dissimilation of 2,3-butanediol, acetylmethylcarbinol, and diacetyl. III. A comparative study of 2,3-butanediol dehydrogenases from various microorganisms. J Bacteriol. 1957 Dec;74(6):757–767. doi: 10.1128/jb.74.6.757-767.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E., HEYM G. A. Preparation, properties, and colorimetric determination of diacetylmethylcarbinol. Arch Biochem Biophys. 1957 Apr;67(2):410–422. doi: 10.1016/0003-9861(57)90296-5. [DOI] [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOKATCH J. T., GUNSALUS I. C. Aldonic acid metabolism. I. Pathway of carbon in an inducible gluconate fermentation by Streptococcus faecalis. J Bacteriol. 1957 Apr;73(4):452–460. doi: 10.1128/jb.73.4.452-460.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]



