Abstract
Background:
Ventilator-associated bacterial pneumonia (VAP) is a important intensive care unit (ICU)-acquired infection in mechanically ventilated patients. Early and correct diagnosis of VAP is difficult but is an urgent challenge for an optimal antibiotic treatment. The aim of the study was to evaluate the incidence and microbiology of ventilator-associated pneumonia and to compare three quantitative bronchoscopic methods for diagnosis.
Methods:
A prospective, open, epidemiological clinical study was performed in a surgical ICU. In a prospective study, 279 patients admitted to a 14-bed surgical ICU during a 1-year period were evaluated with regard to VAP. Three quantitative culture bronchoscopic techniques for identifying the etiological agent were compared [bronchoalveolar lavage (BAL), protected specimen brush (PSB) and bronchoscopic tracheobronchial secretion (TBS)].
Results:
Among 103 long-term ventilated patients, 49 (48%) developed one or more VAPs (a total of 60 VAPs). The incidence was 24 VAPs per 100 ventilated patients or 23 VAPs per 1000 ventilator days. BAL, PSB and TBS with quantitative measurements were equivalent in identifying the bacterial etiology. The VAP was caused predominantly by Staphylococcus aureus in 38% of cases, followed by Pseudomonas aeruginosa in 10%, Haemophilus influenzae in 10% and Klebsiella sp. in 9%. We did not find an increased mortality rate in patients undergoing long-term ventilation who acquired VAP in comparison with patients without VAP.
Conclusion:
For the identification of the microbiological etiology of VAP, one of three available bronchoscopic methods analysed by quantitative measurements is sufficient. In our study, quantitative bronchoscopic tracheal secretion analysis was very promising. Before accepting this method as a standard technique, other studies will have to confirm our results.
Keywords: bronchoscopic techniques, microbial etiology, surgical intensive care unit, ventilator-associated pneumonia
Introduction
Ventilator-associated bacterial pneumonia (VAP) is a major threat to the recovery of patients receiving mechanical ventilation, and is one of the most important intensive care unit (ICU)-acquired infections in mechanically ventilated patients. In the European Prevalence of Infection in Intensive Care (EPIC) study, VAP was the most frequent infection, with about 45% of all infections in ICUs in Europe [1]. The incidence of bronchopulmonary infections in ICUs reported in a review by George et al [2] was 12.5 cases per 1000 patient days, but device-related incidence was 20.5 cases per 1000 patient ventilator days. Today there is no standard test for the diagnosis of VAP and no standard method to exclude pulmonary infections in mechanically ventilated patients with fever and multiorgan dysfunction syndrome or multiorgan failure. Even the post-mortem histological diagnosis of VAP is uncertain [3]. The lack of certainty in the diagnosis of VAP has led to many different clinical and microbiological approaches in the past.
The bronchoscopic methods bronchoalveolar lavage (BAL) and protected specimen brush (PSB) are well-standardised and widely accepted invasive diagnostic techniques for identifying the etiological pathogen of VAP [4]. In a recent study, Heyland et al [5] found that invasive diagnostic testing might increase the confidence of physicians in the diagnosis and management of VAP as well as decreased antibiotic usage and lower mortality.
In the past the impracticability of bronchoscopic methods in ICUs, and also their cost, has led to other strategies in clinical use such as nonbronchoscopic techniques with catheters or mini-lavage [6, 7].
The early and accurate diagnosis of VAP is difficult, but because of the increasing problem of multi-resistant pathogens in many ICUs it constitutes an urgent challenge as well as a rational basis for an optimal antibiotic treatment. It is especially difficult to distinguish VAP from other causes of fever and inflammatory reaction in ICU patients [4]. During treatment in an ICU, the accuracy of VAP diagnosis has to be checked and if necessary amended.
A fatal outcome is frequently observed in patients requiring mechanical ventilation for more than 48 h. VAP is a frequent complication in these cases. Several investigators have assumed that VAP has a direct causal influence on mortality [8]. However, it has not been clearly demonstrated that VAP is directly responsible for mortality [9]. In a matched controlled cohort study, Papazian et al [10] found no significant difference in mortality between mechanically ventilated patients with and without VAP.
In a prospective clinical approach, we examined the epidemiology and microbiological findings of VAP in a surgical ICU and compared the results of three different bronchoscopic methods with quantitative measurements to identify the etiological pathogens. In our ICU bronchoscopy is performed quite frequently in patients suspected of having VAP, and we were interested in a simple specimen sampling method.
Materials and methods
In a prospective study we evaluated the epidemiology and microbiological etiology of VAP in a 14-bed surgical ICU of a 1000-bed general hospital during a one-year period (from 1 January to 31 December 1996). We collected demographic data, duration of ventilator therapy, length of ICU stay, APACHE (Acute Physiology And Chronic Health Evaluation) II score at the time of admission, and occurrence of VAP.
With the use of the clinical and X-ray criteria described below we divided the patients into two groups: (1) long-term ventilated patients (more than 48 h of mechanical ventilation) who developed VAP (case group) and (2) long-term ventilated patients without VAP (control group). Reasons for admission were principally acute respiratory failure with requirement of mechanical ventilation; coma; or, rarely, disorders of other basic vital functions such as renal failure, coagulopathy and shock. The surgical discipline for the treatment of underlying disease, duration of treatment and mechanical ventilation, demographic and physiological data, outcome and, if necessary, cause of death were recorded and compared (Table 1).
Table 1.
Characteristics of the 103 patients studied
| Variables | Patients with VAP (n = 49) | Patients without VAP (n = 54) |
| Sex (M/F) | 39/10 | 35/19 |
| Age (years) | 51 ± 18.5 | 56 ± 18 (n.s.)† |
| APACHE II score | 11.5 ± 5 | 13.6 ± 6 (n.s.)† |
| Ventilator duration (days) (mean ± SD) | 1519 (31 ± 27) | 783 (14.5 ± 14)*† |
| Treatment on ICU (days) (mean ± SD) | 1849 (37.7 ± 27) | 979 (18.1 ± 15.6)*† |
| Cause of admission (n): | ||
| Abdominal surgery | 21 | 25 |
| Thoracic surgery | 1 | 2 |
| Aortic surgery | 2 | 2 |
| Other surgery | 18 | 15 |
| Multiple trauma | 5 | 6 |
| No surgery | 2 | 4 |
| Mortality (n) | 11 | 25*‡ |
*Significant with P < 0.05; n.s., not significant but because 0.1 > P > 0.05 a trend is perceptible, which points to a distinction. †U test (Wilcoxon, Mann and Witney). ‡Χ2 test. VAP, ventilator-associated pneumonia; ICU, intensive care unit.
Definition
The VAP was defined by means of clinical and radiological criteria and by using the Clinical Pulmonary Infection Score (CPIS) [7]. These criteria were as follows:
1. A new and persistent infiltration in the chest X-ray in patients mechanically ventilated for more than 48 h.
2. Body temperature above 38.5 or below 36°C.
3. White cell count above 12,000/μl or below 4000/μl.
4. Purulent tracheobronchial secretion (TBS).
5. Impairment of pulmonary function as defined by the PaO2/FiO2 ratio.
6. The absence of alternative sources of infection such as urinary tract infection or peritonitis.
A score of 6 or more points using the CPIS criteria is needed to define pneumonia.
Bronchoscopy
In each case of definite pneumonia by CPIS score we performed bronchoscopy on the anaesthetized and paralysed patient to collect samples from the respiratory tract for quantitative microbiological examination in a specific, constant sequence and with the following thresholds: TBS, 105and 106 colony-forming units (CFU)/ml); PSB, 103 CFU/ml; BAL, 104 CFU/ml.
After these procedures, patients with CPIS criteria for pneumonia who had not received antibiotic therapy were given antibiotics. The antibiotic treatment was changed when appropriate depending on the resistance of the bacteria.
First, TBS was aspirated through the working channel of the bronchoscope (Olympus Optical Co. Ltd, Tokyo, Japan), diluted 1:1 with sterile physiological saline solution and vortex-mixed. Then the brush of the PSB (microbiology specimen brush; Boston Scientific Corporation, Watertown, MA, USA) was severed aseptically into 1 ml of transport medium (sterile physiological saline solution). After this procedure, BAL was performed in the wedge position with 120 ml of sterile physiological saline solution in six aliquots, of which the first recovered aliquot was used for rinsing the working channel of the bronchoscope and then rejected.
Microbiological analysis
Acquired specimens were transferred to the microbiological laboratory within 45 min. Direct microscopic analysis of respiratory samples is the first and very important component of microbiological diagnosis of VAP. We used Gram stain to detect microorganisms. By using a modified twofold dilution scheme we determined the quantitative culture in CFU/ml. The media and incubation conditions were appropriate for the cultivation of the most probable respiratory pathogens. The set-up included blood agar as a general non-selective medium, chocolate agar for Haemophilus, bile-chrysoidine agar for enterics, and anaerobic media [11]. The direct examination of smears was not performed in this study.
Statistics
The results are presented as medians or means ± standard deviation. Differences in parameters and in the frequency of characteristic features were assessed with the U test of Wilcoxon, Mann and Whitney, and with the Χ2 test. The grade of concordance of the different sampling methods was established with the concordance index of Cohen's Kappa, κ = (P0 - Pe)/(1- Pe), P0 =(a + d)/n and Pe = [(a + b)(a + c)+(c + d)(b + d)]/n2.
For all statistical tests used, P < 0.05 was considered statistically significant. The data were processed with commercially available statistical software (SPSS 6.1 for Windows).
Results
Patient demographics
Over the 1-year study period, 103 patients (of a total of 279 admitted patients) required mechanical ventilation for more than 48 h; 245 of the 279 patients were mechanically ventilated.
The reasons for ICU admission were major abdominal or thoracic surgery in 87 cases (31.2%), multiple trauma or severe head injury in 58 cases (20.8%), sepsis in 29 cases (10.3%), aortic surgery in 21 cases (7.5%) and others, including postoperative monitoring, pulmonary embolism, cerebral function disorders or haemodynamic instability. Eight patients (2.8%) were admitted for non-surgical reasons only. Data on the 103 patients with mechanical ventilation for longer than 48 h are presented in Table 1. The patients with VAP (the case group) and without VAP (the control group) who were ventilated for more than 48 h were comparable with regard to age, sex and APACHE II scores. In the U test the APACHE II score had a P value of 0.08, which points to a trend of higher values in the group without VAP. Patients with VAP had to be ventilated for a significantly longer period (20 compared with 9 days). Their treatment in ICU was also longer (37 compared with 18 days). In contrast with these findings, the mortality rate was significantly higher in the group without VAP: 25 of 54 (46.3%) patients died in the non-VAP group, whereas only 11 of 49 (22.4%) patients with VAP died (Χ2 test, P < 0.05).
Incidence and microbiology
According to CPIS criteria, a total of 60 VAPs in 49 patients were observed among 103 of the patients who were ventilated for more than 48 h. During treatment in the ICU, 39 patients had one VAP, nine patients had two VAPs and one patient had three VAPs.
The incidence was thus 24 VAPs per 100 ventilated patients (60 of 245) or 23 VAPs per 1000 ventilator days (60 of 2574).
Through bronchoscopic samples we found one or more microorganisms in an amount above the threshold of the respective methods in 54 of the 60 VAPs.
The etiological agents of the VAPs are presented in Table 2. In 34 of the VAPs one microorganism was identified, in 18 of the VAPs two different pathogens were found and in two cases three agents were found. Staphylococcus aureus (29 cases, 37.7%), Haemophilus influenzae and Pseudomonas aeruginosa (each 8, 10.4%), Klebsiella sp. (7, 9.1%) and Escherichia coli (6, 7.8%) were the leading aetiologies of pulmonary infections. In six cases the bacterial cultures did not match any of the definitions. In three of these six cases without a positive culture result, antibiotics were given at the time of examination. All other patients were not on antibiotics at the time of the bronchoscopy.
Table 2.
Bacteriology of VAP
| Species | Number of cases; n = 77 |
| Gram-positive: | |
| Staphylococcus aureus | |
| (among them 1 methicillin-resistant) | 29 (37.7%) |
| Streptococcus pneumoniae | 4 (5.2%) |
| Other streptococci | 5 (6.5%) |
| Gram-negative: | |
| Haemophilus influenzae | 8 (10.4%) |
| Pseudomonas aeruginosa | 8 (10.4%) |
| Klebsiella sp. | 7 (9.1%) |
| Escherichia coli | 6 (7.8%) |
| Enterobacter sp. | 3 (3.9%) |
| Proteus sp. | 3 (3.9%) |
| Acinetobacter sp. | 1 (1.3%) |
| Morganella morganii | 1 (1.3%) |
| Citrobacter freundii | 1 (1.3%) |
| Branhamella catarrhalis | 1 (1.3%) |
VAP, ventilator-associated pneumonia.
The elapsed times between the beginning of ventilator treatment and the appearance of the first VAP for Gram-positive, Gram-negative and some special microorganisms are presented in Figure 1. Our data did not show any difference between Gram-positive and Gram-negative pathogens with regard to the onset of infection (early-onset compared with late-onset pneumonia). From our data, staphylococci had an important role even in late-onset cases and Pseudomonas aeruginosa could appear even in early-onset cases.
Figure 1.
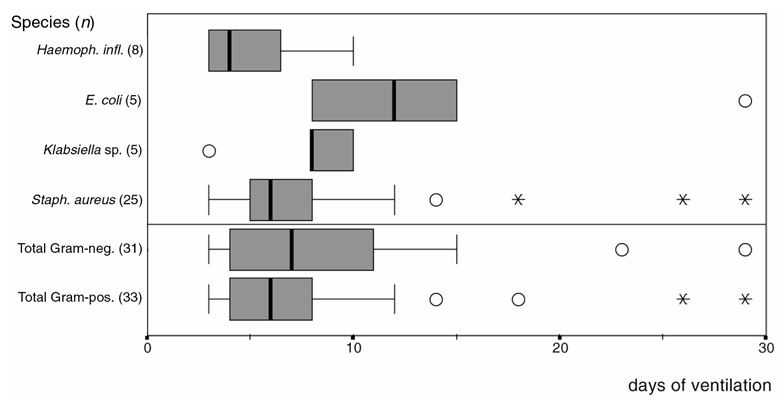
Times until the start of ventilator-associated pneumonia (always the first episode). Circles, runaway; asterisks, extreme case.
For the nine patients who developed a second VAP during their prolonged ICU stay, Pseudomonas was most frequently observed (four VAPs) followed by Staphylococcus aureus (three VAPs), Klebsiella (two VAPs), Citrobacter freundii (one VAP) and Streptococcus pneumoniae (one VAP).
Bronchoscopic sampling methods
Evaluation of the microbiological results for BAL and PSB coincided with the accepted thresholds (104 CFU/ml and 103 CFU/ml). Two different thresholds were tested for the TBS (105 CFU/ml and 106 CFU/ml) to determine which limit was more useful.
The following comparative results were found for the quantitative microbiological data: in 54 VAP cases (90%) both the BAL (threshold 104CFU/ml) and the TBS (threshold 105 CFU/ml) showed the etiological pathogens, and the PSB (threshold 103 CFU/ml) in 50 cases (83%). Only 30 of the 60 infections (50%) with the higher threshold value in the TBS (106 CFU/ml) fulfilled the quantitative microbiological criteria for a VAP (Table 3).
Table 3.
Positive results of bronchoscopic methods in 60 VAPs
| Method | Threshold (CFU/ml) | Positive cultures; n = 60 VAPs |
| BAL | 104 | 56 (90%) |
| TBS | 105 | 56 (90%) |
| PSB | 103 | 50 (83%) |
| TBS | 106 | 30 (50%) |
BAL, bronchoalveolar lavage; CFU, colony-forming units; PSB, protected specimen brush; TBS, tracheobronchial secretion; VAP, ventilator-associated pneumonia.
Statistical evaluation of the results of all three methods revealed an almost complete coincidence in the results of BAL and TBS with the lower threshold (105 CFU/ml). With the higher threshold of 106 CFU/ml for TBS the coincidence between TBS and BAL or PSB is weak. The difference in κ between PSB - TBS (105 CFU/ml) (κ = 0.571) and BAL-PSB (κ = 0.741) is attributed to the different distribution in the four-square table needed for calculation (Table 4).
Table 4.
Grade of concordance by κ index
| Method 1 (threshold, CFU/ml) | Method 2 (threshold, CFU/ml) | κ | Concordance | |
| TBS (105) | - | BAL (104) | 1.000 | Nearly complete |
| PSB (103) | - | BAL (104) | 0.714 | Strong |
| TBS (105) | - | PSB (103) | 0.571 | Clear |
| TBS (106) | - | PSB (103) | 0.216 | Weak/poor |
| TBS (106) | - | BAL (104) | 0.200 | Weak/poor |
BAL, bronchoalveolar lavage; CFU, colony-forming units; PSB, protected specimen brush; TBS, tracheobronchial secretion; VAP, ventilator- associated pneumonia.
Discussion
VAP is a common and serious infection in ICU patients, and is often difficult to diagnose. The difficulties of diagnosis are mostly a result of the following factors:
1. The possibility of multiple other causes of systemic inflammatory reactions in these patients [10].
2. Pre-existing antibiotic usage in ICU patients.
3. The absence of a standard test to detect and diagnose VAP [3].
In general the methods used in our study were accompanied by a usually accepted clinical-radiological diagnosis of VAP [2, 7]. This is the deciding factor for pneumonia, because in this setting, and perhaps generally, microbiological findings or infiltrations in the chest X-ray alone, without clinical signs of infection of the lung, means that there is no pneumonia. In the control group we found definite signs of an infection or sepsis but no signs of pulmonary infection (CPIS <6).
The incidence of VAP in our study tallies with results reported previously [2, 12, 13].
The high mortality rate with regard to the APACHE II score is probably attributable to the time of determination at admission. The score is affected by treatment and therapeutic interventions and we did not investigate APACHE scores of the patients during their ICU stay. We found a lower mortality rate among patients with VAP (case group) than in patients without pneumonia (control group) during their stay in the ICU (Table 5). Opinions are divided on the idea that VAP increases mortality rates. In a cohort study, Bregeon et al [9] found no increase in mortality rate among ICU patients with VAP in comparison with those without VAP. Other authors showed an increased mortality caused by pneumonia [14].
Table 5.
Mortality
| Case group | Control group | |
| Cause of death | (VAP); n = 49 | (no VAP); n = 54 |
| Sepsis with multi-organ failure | 6 (12%) | 15 (27%) |
| caused by pancreatitis and | ||
| peritonitis | ||
| Multi-organ failure without sepsis | 1 (2%) | 4 (9%) |
| Trauma of the brain | 3 (6%) | 3 (5%) |
| Multiple trauma | - | 1 (2%) |
| Pulmonary embolism | - | 1 (2%) |
| Gas gangrene | 1 (2%) | - |
| Rupture of an aneurysm of the | - | 1 (2%) |
| aorta abdominalis | ||
| Total | 11 | 25 |
VAP, ventilator-associated pneumonia.
We believe that the basic disease - the reason for ICU admission - must be considered to be the essential factor responsible for mortality. This opinion is supported by the study of Rello et al [15], who showed that severity of illness is the most important predictor for survival of patients in an ICU when pneumonia is diagnosed. In the control group, more patients suffered from ultimately fatal diseases than in the case group. In addition to the severity of the underlying disease, 29 patients in the control group received antibiotic therapy; it is certainly not reported, but it is plausible, that antibiotic therapy of non-pulmonary infections decreases the incidence of pneumonia. In these circumstances our lower mortality rates in patients with VAP are primarily a result of the lower severity of underlying illness with adequate therapy of VAP with antibiotics.
The etiological pathogens found in our study represented the microbiological situation of our ICU. Comparison with the results of other authors is difficult because each ICU has its specific patient population and also a specific antibiotic policy. We find no increase in the number of VAPs caused by typically Gram-negative bacteria with multi-drug resistance such as Enterobacter spp. or Pseudomonas aeruginosa.
One fundamental and contemporary aspect of our study is noteworthy. The vast majority of our patients with VAP were not on antibiotics at the time of bronchoscopy. This is especially important because it is often not mentioned in published studies. Perhaps the reason for the high frequency of Gram-positive microorganisms was a more restrictive policy on the use of antibiotics, a policy not in place during a previous study [16] on our ICU in 1988, when Pseudomonas aeruginosa was the predominant microorganism.
Our results indicate that one must consider the total spectrum of etiological agents during the entire period of treatment. Differentiation between early-onset and late-onset pneumonia does not seem to be justifiable [17]. However, in second and third cases of pneumonia one must assume a dominance of Pseudomonas aeruginosa.
Bronchoscopy has established itself as the preferred method for collecting representative samples in our ICU, although there is no consensus in the literature on these invasive methods [18, 19].
BAL (threshold 104 CFU/ml), PSB (threshold 103 CFU/ml) and TBS (threshold 105 CFU/ml) were comparable in their quantitative results. A higher threshold for TBS (106 CFU/ml) led to an unacceptable decrease in comparability of results.
In conclusion, our study showed that VAP occurred 23 times for every 1000 days of mechanical ventilation among surgical ICU patients; the prognosis of VAP was not worse in terms of mortality in comparison with non-infected ICU patients. Staphylococcus aureus was the main etiological pathogen of VAP. After quantitative analysis, bronchoscopic measurements including the BAL, PSB and TBS methods produced comparable results with a high detection rate of 90%. TBS sampling is an easy and cheap bronchoscopic procedure that should be investigated further.
Abbreviations
BAL = bronchoalveolar lavage; CFU = colony-forming units; CPIS = Clinical Pulmonary Infection Score; ICU = intensive care unit; PSB = protected specimen brush; TBS = tracheobronchial secretion; VAP = ventilator-associated pneumonia.
References
- Vincent JL, Bihari DJ, Suter PM, Bruining HA, White J, Nicolas-Chanoin MH, Wolff M, Spencer RC, Hemmer M. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. J Am Med Assoc. 1995;278:639–644. [PubMed] [Google Scholar]
- George DL, Falk PS, Wunderink RG, Leeper KV, Jr, Meduri GU, Steere EL, Corbett CE, Mayhall CG. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Resp Crit Care Med. 1998;156:1839–1847. doi: 10.1164/ajrccm.158.6.9610069. [DOI] [PubMed] [Google Scholar]
- Corley DE, Kirtland SH, Winterbauer RH, Hammar SP, Dail DH, Bauermeister DE, Bolen JW. Reproducibility of the histologic diagnosis of Pneumonia among a panel of four pathologists. Chest. 1997;112:458–465. doi: 10.1378/chest.112.2.458. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Mauldin GL, Wunderink RG, Leeper KV, Jr, Jones CB, Tolley E, Mayhall G. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994;106:221–235. doi: 10.1378/chest.106.1.221. [DOI] [PubMed] [Google Scholar]
- Heyland DK, Cook DJ, Marshall J, Heule M, Guslits B, Lang Jeff, Jaeschke R. The clinical utility of invasive diagnostic techniques in the setting of ventilator-associated pneumonia. Chest. 1999;115:1076–1084. doi: 10.1378/chest.115.4.1076. [DOI] [PubMed] [Google Scholar]
- el-Ebiary M, Torres A, Gonzalez J, de la Bellacasa JP, Garcia C, Jimenez de Anta MT, Ferrer M, Rodriguez-Roisin R. Quantitative cultures of endotracheal aspirates for the diagnosis of ventilator-associated pneumonia. Am Rev Respir Dis. 1993;148:1552–1557. doi: 10.1164/ajrccm/148.6_Pt_1.1552. [DOI] [PubMed] [Google Scholar]
- Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic 'blind' bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- Bregeon F, Papazian L, Visconti A, Gregoire R, Thirion X, Gouin F. Relationship of microbiologic diagnostic criteria to morbidity and mortality in patients with ventilator-associated pneumonia. J Am Med Assoc. 1997;277:655–662. [PubMed] [Google Scholar]
- Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis JP, Perin G, Charrel J, Dumon JF, Affray JP, Gouin F. Effect of ventilator-associated pneumonia on mortality and morbidity. Am J Respir Crit Care Med. 1996;154:91–97. doi: 10.1164/ajrccm.154.1.8680705. [DOI] [PubMed] [Google Scholar]
- Baselski V. Microbiologic diagnosis of ventilator-associated pneumonia. Infect Dis Clin North Am. 1993;7:331–357. [PubMed] [Google Scholar]
- Jiménez P, Torres A, Rodriguez-Roisin R, Bellacasa JP, Aznar R, Gatell JM, Agusti-Vidal A. Incidence and etiology of pneumonia acquired during mechanical ventilation. Crit Care Med. 1989;17:882–886. doi: 10.1097/00003246-198909000-00007. [DOI] [PubMed] [Google Scholar]
- George DL, Falk PS, Wunderink RG, Leeper KV, Meduri GU, Steere EL, Corbett CE, Mayhall CG. Epidemiology of ventilator-acquired pneumonia based on protected bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:1839–1847. doi: 10.1164/ajrccm.158.6.9610069. [DOI] [PubMed] [Google Scholar]
- Greenway CA, Embill J, Orr PH, McLeod J, Dyck B, Nicolle LE. Nosocomial pneumonia on general medical and surgical wards in a tertiary-care hospital. Infect Control Hosp Epidemiol. 1998;18:749–756. doi: 10.1086/647529. [DOI] [PubMed] [Google Scholar]
- Rello J, Rue M, Jubert P, Musses G, Sonora R, Valles J, Niederman MS. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med. 1997;25:1862–1867. doi: 10.1097/00003246-199711000-00026. [DOI] [PubMed] [Google Scholar]
- Gastmeier K. Prospektive Studie zur Erfassung nosokomialer Infektionen auf einer multidiszipinären Intensivtherapiestation. Anaesthesiol Reanimat. 1988;13:324–332. [PubMed] [Google Scholar]
- Langer M, Cigada M, Mandelli M, Mosconi P, Tognoni G. Early onset pneumonia - a multicentre study in intensive care units. Intens Care Med. 1987;13:342–346. doi: 10.1007/BF00255791. [DOI] [PubMed] [Google Scholar]
- Chastre J, Fagon JY. Invasive diagnostic testing should be routinely used to manage ventilated patients with suspected pneumonia. Am J Respir Crit Care Med. 1994;150:570–574. doi: 10.1164/ajrccm.150.2.8049850. [DOI] [PubMed] [Google Scholar]
- Niederman MS, Torres A, Summer W. Invasive diagnostic testing is not needed routinely to manage suspected ventilator-associated pneumonia. Am J Respir Crit Care Med. 1994;150:565–569. doi: 10.1164/ajrccm.150.2.8049849. [DOI] [PubMed] [Google Scholar]


