Abstract
The cladoniamides are bis-indole alkaloids isolated from Streptomyces uncialis, a lichen-associated actinomycete strain. The cladoniamides have an unusual, indenotryptoline structure rarely observed among bis-indole alkaloids. I report here the isolation, sequencing, and annotation of the cladoniamide biosynthetic gene cluster and compare it to the recently published gene cluster for BE-54017, a closely related indenotryptoline natural product. The cladoniamide gene cluster differs from the BE-54017 gene cluster in gene organization and in the absence of one N-methyltransferase gene but otherwise contains close homologs to all genes in the BE-54017 cluster. Both gene clusters encode enzymes needed for the construction of an indolocarbazole core, as well as flavin-dependent enzymes putatively involved in generating the indenotryptoline scaffold from an indolocarbazole. These two bis-indolic gene clusters exemplify the diversity of biosynthetic routes that begin from the oxidative dimerization of two molecules of l-tryptophan, highlight enzymes for further study, and provide new opportunities for combinatorial engineering.
Introduction
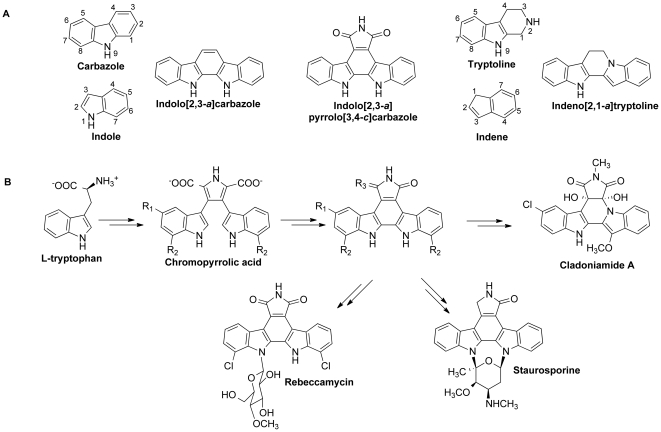
Streptomyces uncialis is an actinomycete bacterial strain isolated with the lichen Cladonia uncialis near the Pitt River in British Columbia. This bacterial strain is the source of the enediyne uncialamycin [1], [2], and the cladoniamides, a series of bis-indole alkaloids [3]. The cladoniamides are unusual among bis-indole natural products: most bis-indoles have an indolocarbazole structure [4], whereas the cladoniamides have a rarely observed indenotryptoline structure (Figure 1). The commonly observed indolocarbazole structure is found in a number of important molecules, including rebeccamycin, analogs of which are DNA-topoisomerase I inhibitors [5], [6], and staurosporine, a kinase inhibitor [7], [8]. Compounds related to rebeccamycin and staurosporine, including becatecarin and UCN-01, have been in multiple clinical trials against cancers (http://clinicaltrials.gov/ct2/home). The indolocarbazole core in rebeccamycin, staurosporine, and related natural product molecules derives biosynthetically from the face-to-face dimerization of two molecules of l-tryptophan [4], [9], [10], [11], [12]. By contrast, the indenotryptoline scaffold found in the cladoniamides cannot be derived in this manner, given that one indole ring is ‘flipped’ relative to the other in the final structure. Williams et al. have postulated that the cladoniamides are biosynthetically generated from the enzymatic degradation and rearrangement of an indolocarbazole intermediate [3].
Figure 1. Bis-indole biosynthesis.
(A) Chemical structures described in the text. (B) Overall biosynthetic pathways to rebeccamycin, staurosporine, and cladoniamide A. R1 = H, R2 = Cl, R3 = O in rebeccamycin pathway; R1,R2 = H, R3 = 2H in the staurosporine pathway; R1 = Cl, R2 = H, R3 = O in the cladoniamide A pathway.
Here the details of the cladoniamide (cla) gene cluster from Streptomyces uncialis are reported. The cla cluster is compared to the recently published BE-54017 (abe) gene cluster from environmental DNA [13]. Chemically, BE-54017 and its derivatives are identical to the cladoniamides, except for the presence of one additional N-methyl group and the lack of di-chloro derivatives (Figure 2). The BE-54017 and cladoniamide gene clusters differ in overall organization of genes and in the absence of one N-methyltransferase gene in the cladoniamide gene cluster. However, the gene clusters share other major features, including encoding enzymes needed for the construction of an indolocarbazole core and flavin-dependent enzymes putatively involved in the oxidative chemistry that facilitates formation of the indenotryptoline structure. The heterologous expression and transposon mutagenesis study reported with the BE-54017 gene cluster thus provides substantial experimental insight into the biosynthetic construction of not only BE-54017 but also the cladoniamides. The elucidation of both gene clusters supports the hypothesis that indenotryptoline cores are biosynthetically derived from the oxidative rearrangement of an indolocarbazole precursor. The availability of both gene clusters should enable further studies in combinatorial engineering of bis-indoles and exploration of the biochemistry and structural biology of the new enzymes identified in these studies.
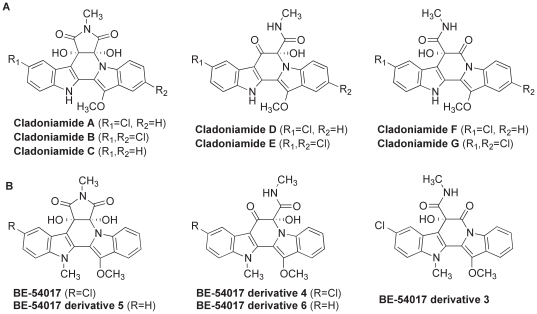
Figure 2. Structures of cladoniamides and BE-54017 and its derivatives.
(A) Cladoniamides from Streptomyces uncialis and (B) BE-54017 and its derivatives. Derivatives of BE-54017 are labelled to be consistent with the labels used by Chang and Brady [13]; the compounds are currently unnamed. The major cladoniamide from Streptomyces uncialis is cladoniamide A [3]; the major metabolite from the heterologous expression of the BE-54017 gene cluster in Streptomyces albus is BE-54017 [13].
Results and Discussion
Identification of the cladoniamide gene cluster
The cladoniamide producer Streptomyces uncialis [1], [3] was cultivated in YEME media and genomic DNA was isolated. Degenerate primers specific for conserved regions of the indolocarbazole gene rebC [10] and its homologs (Figure S1) [9], [14], [15], [16], [17] were used in the polymerase chain reaction to amplify a portion of the corresponding rebC homolog from Streptomyces uncialis. The sequence of this fragment was used for design of specific primers for screening of a cosmid library of Streptomyces uncialis genomic DNA by PCR. Five positive cosmids were identified, and following end sequencing, one cosmid predicted to contain the complete gene cluster was fully sequenced (see Materials and Methods).
The completely sequenced cosmid has an insert of ∼37.6 kilobasepairs (kbp). The cladoniamide gene cluster consists of 13 open reading frames (ORFs), spanning ∼20.7 kbp. These ORFs are in the central region of the cosmid, with ∼9.6 kbp of additional sequence upstream and ∼7.3 kbp of additional sequence downstream of the cluster.
Similarity of the cladoniamide and BE-54017 gene clusters
My annotation of individual genes in the cladoniamide (cla) cluster matches well with the independently reported BE-54017 (abe) cluster [13]. Each gene in the cla gene cluster has a homolog in the abe gene cluster, and encoded proteins are 46–74% identical on the amino acid level (Table 1). The abe cluster has one additional N-methyltransferase gene (abeM2) that is not present in the cla cluster. Correspondingly, the cladoniamides lack one methyl group when compared to BE-54017 and its derivatives (Figure 2). Overall, the similarity of the gene clusters suggests that cladoniamide and BE-54017 derive from highly related biosynthetic pathways. Genes in the cla cluster have been named according to the conventions established in the naming of the genes in the abe gene cluster (Table 1) [13].
Table 1. Enzymes encoded by the cladoniamide gene cluster.
| Enzyme | Sizea | Deduced function | BE-54017homologb | Sizea | % IDc | Rebeccamycin homologd | Sizea | % IDc |
| ClaH | 513 | Tryptophan 5-chlorinase | AbeH | 515 | 74 | RebH | 530 | 40 |
| ClaT | 422 | Na+/H+ antiporter | AbeT | 412 | 55 | RebT | 473 | 14 |
| ClaF | 177 | Flavin reductase | AbeF | 160 | 60 | RebF | 170 | 46 |
| ClaY | 278 | α/β hydrolase | AbeY | 264 | 56 | - | - | - |
| ClaO | 519 | l-tryptophan oxidase | AbeO | 513 | 66 | RebO | 473 | 51 |
| ClaC | 561 | Flavin-dependent oxygenase | AbeC | 533 | 69 | RebC | 529 | 59 |
| ClaP | 420 | Cytochrome P450 | AbeP | 392 | 64 | RebP | 397 | 54 |
| ClaX1 | 553 | Flavin-dependent oxygenase | AbeX1 | 533 | 71 | - | - | - |
| ClaM1 | 243 | N-Methyltransferase | AbeM1 | 231 | 73 | - | - | - |
| ClaD | 1090 | Chromopyrrolic acid synthase | AbeD | 1015 | 68 | RebD | 1013 | 53 |
| ClaX2 | 421 | Flavin-dependent oxygenase | AbeX2 | 407 | 73 | - | ||
| ClaM3 | 344 | O-methyltransferase | AbeM3 | 336 | 66 | - | ||
| ClaR | 981 | Transcriptional regulator | AbeR | 932 | 46 | RebR | 923 | 35 |
Gene cluster architecture
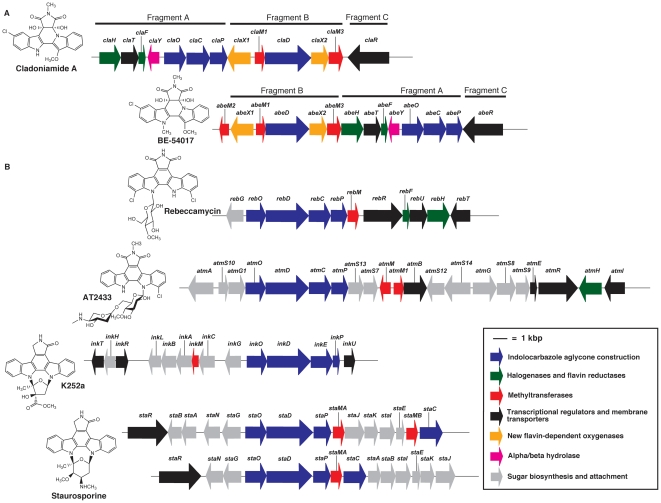
The gene organization differs between the cladoniamide and the BE-54017 gene clusters. For the purpose of comparative analysis, the entire cla cluster can be considered to be composed of three fragments of DNA (Figure 3A). Fragment ‘A’ consists of claH, claT, claF, claY, claO, claC, and claP. Fragment ‘B’ consists of claX1, claM1, claD, claX2, and claM3. Finally, fragment ‘C’ is claR. These fragments have a different organization in the abe cluster where the order is Fragment B (abeX1, abeM1, abeD, abeX2, and abeM3), Fragment A (abeH, abeT, abeF, abeY, abeO, abeC, and abeP), and Fragment C (abeR), with abeM2 appended upstream of Fragment B. This gene reorganization suggests that if the gene clusters were transferred between ancestral bacterial strains, a transposition of Fragment A occurred.
Figure 3. Comparison of indenotryptoline and indolocarbazole biosynthetic gene clusters.
(A) Indenotryptoline gene clusters. Top to bottom: Cladoniamide and BE-54017 [13] gene clusters. (B) Indolocarbazole gene clusters. Top to bottom: rebeccamycin [10], AT2433 [16], K252a [17], staurosporine (from Streptomyces sp. TP-A0274 and putative cluster from Streptomyces clavuligerus) [9], [19], and staurosporine (putative cluster from Salinispora arenicola) [15] gene clusters. Note that inkE is the homolog to rebC in the K252a gene cluster, and the Salinispora arenicola staurosporine gene cluster lacks a staMB homolog.
The cla cluster, from a Streptomyces strain, is 74% G+C, whereas the abe cluster, from an unknown source, is 71% G+C. High G+C content is a characteristic feature of actinomycete [18], and it is likely that the abe cluster, like the cla cluster, is derived from an actinomycete strain.
Cladoniamides are likely to derive from an indolocarbazole precursor
Although the cladoniamides and BE-54017 have a unique chemical skeleton relative to other known bis-indole alkaloids, their biosynthetic gene clusters contain genes encoding enzymes needed for the construction of the more common bis-indolic scaffold, indolo[2,3-a]pyrrolo[3,4-c]carbazole. All of the characterized indolo[2,3-a]pyrrolo[3,4-c]carbazole gene clusters, including rebeccamycin (reb) [10], [11], AT2433 (atm) [16], K252a (ink) [17], and staurosporine (sta) [9], [15], [19] have four conserved genes (Figure 3B). These are genes encoding an l-tryptophan oxidase (rebO homolog), a chromopyrrolic acid synthase (rebD homolog), a cytochrome P450 (rebP homolog), and a flavin-dependent monooxygenase (rebC homolog). These four encoded enzymes are thought to react in sequence to generate the indolo[2,3-a]pyrrolo[3,4-c]carbazole scaffold from two molecules of l-tryptophan and molecular oxygen [12]. The presence of homologs of these four genes in both the cladoniamide and the BE-54017 gene clusters suggests that in both pathways the indolo[2,3-a]pyrrolo[3,4-c]carbazole scaffold is generated on the route to the final indenotryptoline products.
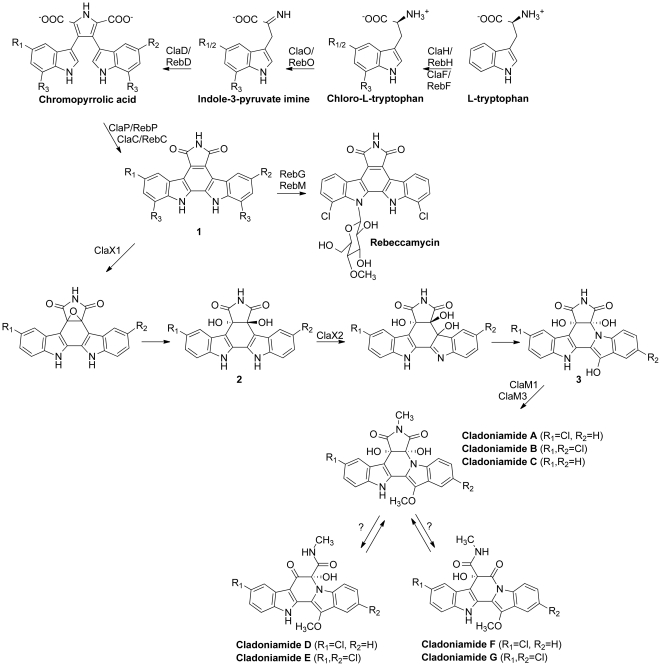
Two new flavin-dependent oxygenases are predicted in both the cladoniamide and BE-54017 biosynthetic pathways. Transposon mutagenesis of one of these, abeX1 in the heterologously expressed BE-54017 gene cluster, eliminates BE-54017 production and leads to the accumulation of the indolo[2,3-a]pyrrolo[3,4-c]carbazole scaffold 1 (R1 = Cl, R2,R3 = H) (Figure 4), demonstrating that 1 is the likely substrate for AbeX1 [13]. Disruption of the cladoniamide homolog claX1 is also anticipated to give accumulation of 1 because the encoded enzymes AbeX1 and ClaX1 are 71% identical. The presence of abeX1 and claX1 in both gene clusters strongly supports the role of an indolo[2,3-a]pyrrolo[3,4-c]carbazole intermediate in the biosynthesis of indenotryptolines.
Figure 4. Postulated biosynthetic route to the cladoniamides and similarities to biosynthetic route to rebeccamycin.
RebH, RebF, RebO, RebD, RebP, RebC, RebG, and RebM are enzymes from the rebeccamycin biosynthetic pathway (see Table 1) [10]. R1,R2 = H, R3 = Cl in rebeccamycin biosynthesis; R1 = Cl, R2,R3 = H in cladoniamide A, D, and F biosynthesis; R1,R2 = Cl, R3 = H in cladoniamide B, E, and G biosynthesis; R1,R2,R3 = H in cladoniamide C biosynthesis.
Although biosynthetic construction via an indolocarbazole intermediate appears to be conserved between indolocarbazole and indenotryptoline pathways, the core rebODCP genes have been reorganized. In the rebeccamycin [10], AT2433 [16], and K252a [17] gene clusters the corresponding homologs, rebODCP, are clustered. In the staurosporine gene clusters found in Streptomyces sp. TP-A0274 [9], Streptomyces clavuligerus [19], and Salinispora arenicola [15], the staODP genes are similarly located together, but the staC gene is moved downstream. However, in both the cla and abe [13] clusters, a different local arrangement is observed in that the rebD homologs (claD and abeD) are separated from the rebOCP homologs (claOCP and abeOCP) (Figure 3).
New enzymes in the indenotryptoline biosynthetic pathways
There are three enzymes unique to the indenotryptoline biosynthetic pathways. These are two putative flavin-dependent oxygenases (ClaX1 / AbeX1 and ClaX2 / AbeX2) and a putative α/β hydrolase (ClaY / AbeY). Comparative bioinformatics analysis and the genetic studies carried out on the abe cluster [13] provide considerable insight into the likely roles of the encoded enzymes. The two flavin-dependent oxygenases are described directly below, and discussion on the unknown role of the α/β hydrolase follows the description of the halogenase, flavin reductase, transcriptional regulator, membrane transporter, and methyltransferases identified in the cla cluster.
Oxygenase ClaX1
A transposon mutant of abeX1 in the BE-54017 heterologous expression system accumulates 1 (R1 = Cl, R2,R3 = H) and a transposon mutant of abeX2 accumulates a methylated derivative of 2 (R1,R2 = H) (Figure 4) [13]. This finding suggests that the likely substrate of ClaX1 (71% identity to AbeX1) is 1 and that the enzyme is likely to install two hydroxyl groups during catalysis to give 2 (Figure 4), the likely substrate of ClaX2 (73% identity to AbeX2).
Further insight into the role of ClaX1 comes from consideration of related proteins, all of which are putative flavin-dependent oxygenases. The closest characterized homologs to ClaX1 are OxyL (31% identity) from the anhydrotetracycline biosynthetic pathway [20] and SsfO2 (29% identity) from the tetracycline SF2575 biosynthetic pathway [21]. These functionally equivalent enzymes are flavin-dependent dioxygenases that are thought to install two oxygen atoms on two carbon atoms on opposite sides of an electron rich tetracycline substrate via two independent flavin-dependent hydroxylation reactions (Figure S2A). However, a similar mechanism for double flavin-based hydroxylation chemistry of 1 is not possible because the carbon atoms targeted for hydroxylation lack hydrogen atoms that can be removed in the catalytic cycle (Figure S2A–C). Instead, an epoxidation of the double bond, followed by hydrolysis, would install two hydroxyl groups to give two tertiary alcohols. Indeed, other characterized homologs to ClaX1 are MtmOII [22] (30% identity) and TcmG [23] (26% identity). While the precise mechanisms of these latter enzymes are still under investigation, it is thought that both act as flavin-dependent epoxidases. An epoxidation mechanism is sensible for ClaX1's putative substrate 1 and gives the suspected product 2 after epoxide hydrolysis (Figure S2D). While it is likely that ClaX1 catalyzes the epoxidation, it is unknown if subsequent hydrolysis to give 2 is spontaneous or enzyme-catalyzed.
Oxygenase ClaX2
A transposon mutant of abeX2 in the BE-54017 heterologous expression system leads to accumulation of a methylated derivative of 2 (R1,R2 = H). 2 is the postulated substrate for AbeX2 [13]. It is likely that an equivalent result would be obtained for a deletion mutant of claX2, as ClaX2 is 73% identical to AbeX2. The route to generate downstream metabolites, e.g. indenotryptoline-containing molecules such as 3, from this substrate 2, via the action of a flavin oxygenase such as ClaX2, is not resolved. A chemically reasonable mechanism, as proposed by Williams et al. [3] and Chang and Brady [13], is epoxidation across a double bond. Cleavage of the epoxide could be driven through ketone formation from one tertiary alcohol, causing sigma-bond rupture and epoxide hydrolysis, opening the indolocarbazole scaffold. This cleaved molecule could then close through attack on the ketone by the indolic nitrogen, restoring the tertiary alcohol and arriving at the indenotryptoline scaffold 3 (Figure S3A).
A related mechanism is suggested through bioinformatics analysis. The closest characterized homolog to ClaX2 is RemO (45% identity) from the resistoflavin biosynthetic pathway. This flavin-dependent enzyme catalyzes a single hydroxylation on the re face of resistomycin, causing loss of aromaticity to yield resistoflavin (Figure S3B) [24]. A mechanism for ClaX2/AbeX2 catalysis more consistent with the known role of the close homolog RemO is the single hydroxylation on the 3′ position of one indole ring, causing loss in the aromaticity of the substrate. Further chemistry, as described above, including sigma-bond rupture to restore aromaticity to the indole ring, could lead to cleavage of the indolocarbazole. Then the ring could close via attack on the ketone by the indolic nitrogen, giving 3 (Figure S3C). Further biochemical characterization of ClaX2 will resolve whether ClaX2 is an epoxidase or a hydroxylase, and if subsequent steps are spontaneous or enzyme-mediated.
Halogenase ClaH and flavin reductase ClaF
Both the cla and abe clusters encode a putative FADH2-dependent l-tryptophan chlorinase and an associated flavin reductase. These proteins are ClaH / AbeH (chlorinases) and ClaF / AbeF (reductases). The chlorinases have highest sequence identity to one another (74% identical) and to a number of established l-tryptophan chlorinases, including KtzR (65% identical to ClaH) and KtzQ (65% identity) from the kutzneride biosynthetic pathway [25], [26], PyrH (61% identity) from pyrroindomycin biosynthesis [27], RebH (40% identity) from rebeccamycin biosynthesis [10], [28], and PrnA (40% identity) from pyrrolnitrin biosynthesis [29], [30].
An abeH deficient mutant in the BE-54017 heterologous expression system accumulates the non-chlorinated BE-54017 derivative [13] and demonstrates that AbeH is a chlorinase. By extension, deletion of claH in Streptomyces uncialis would likely accumulate cladoniamide C, the non-chlorinated derivative of the major metabolite cladoniamide A. However, the stage in the biosynthetic pathway at which chlorine is added is not known: chlorine could be installed early in the pathway, with non-chlorinated substrates also accepted by all downstream enzymes, or the chlorine could be installed late in the pathway. Both chlorinated and non-chlorinated cladoniamides and BE-54017 derivatives are observed in extracts of Streptomyces uncialis and the heterologous expression system of BE-54017, respectively.
Given that AbeH and ClaH are highly related to characterized l-tryptophan chlorinases, and given that the chlorine is installed on the l-tryptophan substrate by RebH in the first step of the related rebeccamycin pathway, with all downstream enzymes also accepting non-chlorinated substrates [12], it is likely that chlorine is also installed early in the cladoniamide biosynthetic pathway, at the 5′ position on the l-tryptophan substrate, with downstream enzymes accepting both chlorinated and non-chlorinated substrates.
Methyltransferases ClaM1 and ClaM3
The cladoniamides have two methyl groups in their structures, whereas BE-54017 and its derivatives have three methyl groups. Correspondingly, the cla cluster contains two methyltransferase genes (claM1 and claM3), whereas the abe cluster contains three methyltransferase genes. claM1 has highest similarity to abeM1. The encoded enzymes are 73% identical. Genetic studies show that abeM1 encodes an N-methyltransferase that installs a methyl group on the succinimide nitrogen [13]. Both ClaM1 and AbeM1 also have moderate sequence identity (20% identity for ClaM1) with the corresponding N-methyltransferase AtmM1 from AT2433 biosynthesis [16]. This enzyme catalyzes the transfer of a methyl group to the succinimide nitrogen. claM3 has highest similarity to abeM3 (encoded enzymes are 66% identical), and genetic studies show that abeM3 encodes an O-methyltransferase that installs a methyl group on the hydroxyl group [13]. ClaM3 also has high identity (36%) with the O-methyltransferase PokMT3 from polyketomycin biosynthesis [31], ElmNII from elloramycin biosynthesis (32% identity) [32], FdmN from fredricamycin biosynthesis (35% identity) [33], and the C-terminal domain of TcmN from tetracenomycin D3 biosynthesis (30% identity). Each of these enzymes is thought to catalyze the transfer of a methyl group to a phenolic oxygen, consistent with the likely role of ClaM3 in cladoniamide biosynthesis of installing a methyl group on the appended hydroxyl group. abeM2, which encodes the N–methyltransferase that installs a methyl group on the indole nitrogen of the tryptoline in BE-54017 biosynthesis, lacks a homolog in the cladoniamide biosynthetic gene cluster. Correspondingly, the cladoniamides lack methylation on the indole nitrogen of the tryptoline.
Transcriptional regulator ClaR and membrane transporter ClaT
Both the cla and abe clusters contain genes encoding a membrane transporter and a transcriptional regulator. Among all published protein sequences, the transcriptional regulator ClaR found in the cladoniamide biosynthetic pathway has highest similarity to the corresponding transcriptional regulators from indolocarbazole biosynthetic pathways. This finding suggests that the pathways are all regulated through similar mechanisms. The transcriptional regulators with highest similarity are those from the published staurosporine biosynthetic gene clusters, including StaR from Streptomyces sp. TP-A0274 (35% identity), StaR from Streptomyces clavuligerus (34% identity), and StaR from Salinispora arenicola (Sare_2326; 33% identity). The protein also shares high similarity with the transcriptional regulator RebR from the rebeccamycin pathway (35% identity) and AtmR from the AT2433 pathway (34% identity). Like RebR, ClaR is a putative member of the Large ATP-binding regulators of the LuxR (LAL) family [10], [34].
ClaT is a putative membrane transporter, predicted to contain 11 transmembrane helices. Bioinformatics prediction suggests that it is a Na+/H+ antiporter, sharing moderate sequence similarity with the putative Na+/H+ antiporters AtmB from the AT2433 biosynthetic pathway (27% identity) [17] and RebT from the rebeccamycin biosynthetic pathway (14% identity) [10].
Unknown role for the α/β hydrolase
The putative α/β hydrolase AbeY is not essential to the biosynthesis of BE-54017 or its derivatives in a heterologous expression system. Nonetheless, new low molecular weight metabolites (identities not reported) appeared in culture broths of abeY transposon mutants [13]. Given the close identity of abeY and claY (encoded enzymes are 56% identical), it is likely that an identical result would be seen for a claY deletion mutant in Streptomyces uncialis. As suggested by Chang and Brady, it is possible that another gene from the heterologous host Streptomyces albus complemented the abeY mutation and/or AbeY plays a role as modulating the activity of other enzymes. Further characterization of AbeY and ClaY is therefore warranted.
The likely role of these enzymes is unclear from bioinformatics analysis. The three closest homologs of AbeY and ClaY are annotated from genome sequencing projects but are not characterized. Possible roles for an α/β hydrolase in the biosynthesis of indenotryptolines are numerous. Enzymes with this type of fold catalyze ester hydrolysis, epoxide hydrolysis, amide bond hydrolysis, and other reactions [35], [36]. Since no enzyme is identified for the opening of the N-methylsuccinimide to give rise to the minor compounds, i.e. cladoniamides D-G and the ‘opened’ BE-54017 derivatives (Figure 2), it is tantalizing to suggest that ClaY/AbeY catalyzes the hydrolysis of an amide bond in the N-methylsuccinimide ring, which is followed by oxidative decarboxylation. In fact, there is no other candidate in the genes sequenced to date that could catalyze these reactions: likely roles for all enzymes in the pathways are now assigned, and heterologous expression of the BE-54017 gene cluster shows production of both ‘closed’ and ‘opened’ BE-54017 derivatives, showing that all enzymatic machinery is present in the heterologously expressed gene cluster.
An alternative explanation for the production of cladoniamides D–G and the ‘opened’ BE-54017 derivatives (Figure 2), which are minor compounds in the two series, is that hydrolysis and oxidative decarboxylation of the N-methylsuccinimide is spontaneous. However, no instability of either cladoniamide A or BE-54017 has been reported, and none of the related indolocarbazole compounds demonstrate spontaneous opening of the N-methylsuccinimide ring. Further biochemical studies will reveal if the α/β hydrolase ClaY plays a role in this reaction, in another reaction, or is entirely dispensable to the pathway.
Likely biosynthetic route to the cladoniamides
An overall biosynthetic scheme to account for the construction of the indenotryptoline cores is shown in Figure 4. Although the order of chlorinations and methylations relative to other biosynthetic steps is currently unknown, this scheme is drawn to be consistent with the known pathway to rebeccamycin, where the chlorination occurs first [37] and the methylation occurs at the end of the biosynthesis [38], [39]. First, l-tryptophan is chlorinated at the 5′ position by the action of ClaH and a partner flavin reductase ClaF [28]. Next, 5-chloro-l-tryptophan reacts with ClaO, an amino acid oxidase [37], generating an indole-3-pyruvate imine. ClaD dimerizes two of these molecules to generate a chromopyrrolic acid molecule [40]. This molecule is in turn the substrate for ClaP, a cytochrome P450 enzyme, and ClaC, a flavin monooxygenase, which react in tandem to generate 1 [14]. Next, ClaX1 installs an epoxide over a double bond, followed by a spontaneous or enzyme-mediated epoxide opening to generate 2. ClaX2 reacts to install a hydroxyl group or an epoxide, followed by spontaneous or enzyme-mediated chemistry to generate 3. Methyltranferase reactions install methyl groups, with ClaM1 installing the methyl group on the nitrogen of the succinimide ring and ClaM3 installing a methyl group on the appended hydroxyl group. In the BE-54017 pathway, a methyl group would additionally be installed on the indolic nitrogen. Finally, the minor compounds cladoniamides D–G are generated through the opening of the N-methylsuccinimide ring and oxidative decarboxylation.
Conclusion
This report of the cladoniamide gene cluster, together with the BE-54017 gene cluster and its genetic analysis [13], provides insight into the biosynthesis of the indenotryptoline scaffold from two molecules of l-tryptophan. Collectively, these results suggest that indenotryptolines are generated from the oxidative rearrangement of an indolopyrrolocarbazole scaffold via the action of two flavin-dependent enzymes, together with spontaneous chemistry. This work sets the stage for future biochemical, structural, and combinatorial biosynthetic studies of these new bis-indole biosynthetic pathways.
Materials and Methods
Materials
The cladoniamide producer Streptomyces uncialis was kindly provided by Julian E. Davis. Chemicals were purchased from Fisher Scientific Canada, except where noted otherwise below.
Purification of genomic DNA
A mycelium glycerol stock of Streptomyces uncialis was streaked on ISP4 media (Difco) and grown at 30°C. A single colony was used to inoculate 2 mL of Tryptic Soy Broth medium (Difco) and grown at 30°C at 200 rpm for 5 days. This starter culture (1 mL) was used to inoculate 50 mL of YEME media (3 g/L yeast extract, 5 g/L bacto-peptone, 3 g/L malt extract, 10 g/L glucose, 340 g/L sucrose, 5 mM MgCl2), which was grown at 30°C at 200 rpm for 2 weeks. DNA was extracted via standard methods [41]. Specifically, cells were pelleted at 8000 rpm and washed in 10 mL of TE25S buffer (25 mM Tris-HCl pH 8, 25 mM EDTA, 0.3 M sucrose) three times. The pellet was resuspended in lysis buffer (TES25S supplemented with 3 mg/mL lysozyme from Sigma and 100 ng/mL RNAse from BioBasic Inc.) and incubated with frequent inversions at 37°C for 2 h. Proteinase K (100 µL of 20 mg/mL) and 1 mL of 10% SDS were added and the mixture was incubated at 55°C for 1 h. The mixture was placed on ice for 10 min, and 2.5 mL of 5 M potassium acetate was added and mixed by inversion. Phenol/chloroform/isoamyl alcohol (25∶24∶1) pH 8.05 (Invitrogen) (8 mL) was added and mixed by inversion for 6 min. This mixture was centrifuged for 15 min at 5000 x g at 4°C and the supernatant was transferred to a new tube using wide bore tips. The phenol/chloroform/isoamyl alcohol extraction step was repeated. Chloroform (8 mL) was added, followed by gentle inversion for 8 min and centrifugation. The supernatant was transferred to a new tube. Isopropanol (0.6 V) was added and mixed by inversion. Purified DNA was spooled using a sterile, flame-sealed Pasteur glass pipette. DNA was rinsed with 1 mL of 70% ethanol, air dried for 10 s, and dissolved overnight in TE (10 mM Tris pH 8.0, 1 mM EDTA) at 4°C.
Design of primers for PCR-based library screening
A series of degenerate primers were designed to amplify indolocarbazole biosynthetic genes. Primers were purchased from Integrated DNA Technologies, Inc. The successfully used degenerate primer set, specific to the indolocarbazole biosynthetic gene rebC and its homologs (Figure S1), is RebC-degen-FP (5′-TCGGBCCSCGSTCSATGGA-3′) and RebC-degen-RP (5′-GCRCGGAASAGSAYGTTSCGRAA-3′) where R = A,G; Y = C,T; S = C,G; and B = C,G,T. This primer set was used to amplify genomic DNA from Streptomyces uncialis via PCR with a reaction mixture consisting of 1x EconoTaq buffer with MgCl2 (Lucigen), 5% DMSO, 250 µM dNTPs, 2.5 µM of each RebC-degen-FP and RebC-degen-RP, 0.8 ng/µL genomic DNA, and 2 U Taq polymerase in a temperature cycle of 95°C for 2 min; 30 cycles of 95°C for 30 s, gradient of 50–70°C for 45 s, 72°C for 2 min; 72°C for 10 min; 4°C hold. Successful amplification of a >400 basepair fragment was observed, as determined via ethidium bromide stained agarose gel electrophoresis, from reactions with extension temperatures between 53°C and 61°C. This fragment was purified from the PCR reactions according to the manufacturer's instructions using the GenElute PCR Clean-up kit (Sigma) and submitted for sequencing (Nucleic Acid Protein Service Unit, Michael Smith Laboratories, University of British Columbia) using the primer RebC-degen-FP. The resulting sequencing data (440 base pairs) was used for design of non-degenerate PCR primers specific to this sequencing data. These primers are RebC-uncial-FP (5′-GGACTGCGTCTGGGTCAC-3′) and RebC-uncial-RP (5′-CTCGATACCGGCGTCCTT-3′).
Library construction and screening
Purified genomic DNA from Streptomyces uncialis (70 µg) was serially digested with Sau3A1 in 1x NEBuffer 4 (New England Biolabs) and 100 µg/mL bovine serum albumin (New England Biolabs). Appropriately digested genomic DNA fractions, as assayed by ethidium bromide staining of an agarose gel, were pooled and purified by phenol/chloroform/isoamyl alcohol extraction, chloroform extraction, and ethanol precipitation. FastAP (4 µL) (Fermentas) and 1x FastAP buffer (Fermentas) were added and the mixture was incubated at 37°C for 2 h. DNA was purified again by phenol/chloroform/isoamyl alcohol extraction, chloroform extraction, and ethanol precipitation. Separately, 16 µg of Supercos1 (Stratagene) was digested with 100 U of XbaI (New England Biolabs) in 1x NEBuffer 3 (New England Biolabs) and 100 µg/mL bovine serum albumin (New England Biolabs) for 3 h at 37°C. Enzyme was deactivated at 65°C for 20 min. FastAP (4 µL) (Fermentas) was added at 37°C for 3 h followed by enzyme deactivation at 70°C for 20 min. Finally, BamHI (125 U) (New England Biolabs) was added, and the mixture was incubated at 37°C overnight. DNA was purified by phenol/chloroform/isoamyl alcohol extraction, chloroform extraction, and ethanol precipitation.
Digested genomic DNA (2.5 µg), 1 µg of digested Supercos1, 2.0 µL of 10x T4 DNA ligase buffer (New England Biolabs) and 1 µL of T4 DNA ligase (400,000 cohesive end units/ml; New England Biolabs) were incubated in a 20 µL volume at 4°C for 2 d. Ligations were titered using Max Planx Lambda Packaging Extract (Epicentre). Packaging extract (25 µL) was incubated with 10 µL of ligation mixture at 30°C for 90 min, 25 µL of additional packaging extract was added, followed by an additional incubation at 30°C for 90 min. Phage dilution buffer (10 mM Tris HCl, 100 mM NaCl, 10 mM MgCl2) (500 µL) was added, followed by gentle vortexing, and 25 µL of chloroform was added followed by gentle vortexing. Mixtures were again diluted 1∶1 in phage dilution buffer. XL1-MRF′ Blue Cells (Stratagene) were separately grown in LB + 10 mM MgSO4 + 0.2 % maltose from single colonies to an OD600 of ∼0.7. Cells were centrifuged at 500 g for 10 min and resuspended in an equal volume of 10 mM MgSO4 and incubated on ice. Cells (384 µL) were incubated with 16 µL of the diluted phage mixture and incubated at room temperature for 30 min. LB (800 µL) was added and the tubes were incubated at 37°C for 1 hour with gentle mixing every 15 min. 150 µL of this mixture was plated on each of six LB-agar-kanamycin sulfate (50 µg/mL) plates, and plates were incubated overnight at 37°C. Single colonies were picked from plates using toothpicks and used to inoculate individual wells of sixteen 96-well plates containing 150 µL of LB-kanamycin sulfate (50 µg/mL) per well and incubated overnight at 37°C.
Colonies from individual plates, individual rows, and individual columns were each pooled, glycerol was added, and plates were frozen at −80°C. Pools of colonies from each plate were individually analyzed via PCR using a reaction mixture consisting of 1x Lucigen EconoTaq buffer with MgCl2, 5% DMSO, 250 µM dNTPs, 250 nM of each RebC-uncial-FP and RebC-uncial-RP, 2 U Taq polymerase, and 2 µL of the pooled plates in a reaction volume of 25 µL. Reactions were cycled at 95°C for 2 min; 30 cycles of 95°C for 30 s, 66°C for 45 s, 72°C for 2 min; 72°C for 10 min; 4°C hold. Reactions were analyzed via ethidium bromide stained agarose gel electrophoresis. Plates that contained a rebC homolog by this PCR-based screening were subjected to further PCR-based screening to identify individual cosmids containing the rebC homolog. Five individual cosmids were identified that contained the rebC homolog via PCR-based screening. These cosmids were purified via standard methods and subjected to end sequencing using T3 and T7 primers (Nucleic Acid Protein Service Unit, Michael Smith Laboratories, University of British Columbia). One cosmid was chosen for total sequencing.
Cosmid sequencing and annotation
The cosmid containing the cladoniamide gene cluster was sequenced via primer walking with at least double-stranded sequencing coverage at the Nucleic Acid Protein Service Unit at the Michael Smith Laboratories at the University of British Columbia. Primers were purchased from Integrated DNA Technologies, Inc. The DNA sequence was assembled and annotated using Sequencher (www.genecodes.com), FramePlot (http://www.nih.go.jp/fjun/cgi-bin/frameplot.pl) [42], and NCBI Blast (blast.ncbi.nlm.nih.gov/Blast.cgi) [43]. Transmembrane helices in ClaT were predicted using the TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM/) [44].
This sequence is available through GenBank (accession code JN165773).
Supporting Information
Partial sequence alignment of DNA encoding select rebC homologs. staC-1 is the staC gene from Streptomyces sp. TP-A0274 (GenBank accession AB088119.1) [9], staC-2 is the staC gene from Streptomyces longisporoflavus (accession DQ861905.1) [14], staC-3 is the putative staC gene from Salinispora arenicola (accession CP000850.1; Sare_2333) [15], inkE is from Nonomuraea longicatena (accession DQ399653.1) [17], rebC is from Lechevalieria aerocolonigenes (accession AJ414559.1) [10], and atC is from Actinomadura melliaura (accession DQ297453.1) [16]. Only DNA from 1 to ∼640 basepairs is shown. Highlighted in yellow are the DNA sequences targeted by the degenerate primers.
(PDF)
Reaction of ClaX1. (A) The closest characterized homologs to ClaX1 and AbeX1 are SsfO2 and OxyL. SsfO2 and OxyL react with 6-methyl-pretetramid through two rounds of flavin-based hydroxylation chemistry to give 4-keto-anhydrotetracycline [20], [21]. (B) The overall mechanism for the best-studied flavin-dependent hydroxylase, para-hydroxybenzoate hydroxylase (pHBH) is shown. After flavin-based chemistry occurs, the non-aromatic intermediate undergoes a rapid keto-enol tautomerization to restore aromaticity to the ring [45]. (C) If intermediate 1 underwent a single round of flavin-based hydroxylation chemistry to install a first hydroxyl group as shown, restoration of aromaticity following the reaction is not possible because the hydroxy group is on a carbon (starred) that lacks hydrogens. Without hydrogens, a tautomerization analogous to that shown for pHBH is not possible. (D) An epoxidation of 1, followed by epoxide hydrolysis, would give rise to the proposed product 2, containing two tertiary alcohols, and is consistent with the better established roles of MtmOII and TcmG.
(TIF)
Reaction of ClaX2. (A) One possible reaction mechanism for ClaX2 catalysis is epoxidation of 2 followed by sigma-bond cleavage and rearrangment of the indolocarbazole to give the indenotryptoline core structure 3. (B) The closest characterized homolog to ClaX2/AbeX2 is RemO, which catalyzes a single hydroxylation of a multi-ringed aromatic substrate on the re face [24]. (C) Similar flavin-based hydroxylation chemistry on 2 could lead to an intermediate that undergoes sigma-bond cleavage and rearrangment of the indolocarbazole to give the indenotryptoline core 3.
(TIF)
Acknowledgments
I thank Julian Davies, Vivian Miao, and David Ballou for helpful discussions and feedback on the manuscript. I thank Nima Mazinani for assistance with annotation.
Footnotes
Competing Interests: The author has declared that no competing interests exist.
Funding: This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Davies J, Wang H, Taylor T, Warabi K, Huang XH, et al. Uncialamycin, a new enediyne antibiotic. Org Lett. 2005;7:5233–5236. doi: 10.1021/ol052081f. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Zhang H, Chen JS, Crawford JJ, Pasunoori L. Total synthesis and stereochemistry of uncialamycin. Angew Chem Int Ed Engl. 2007;46:4704–4707. doi: 10.1002/anie.200700917. [DOI] [PubMed] [Google Scholar]
- 3.Williams DE, Davies J, Patrick BO, Bottriell H, Tarling T, et al. Cladoniamides A-G, tryptophan-derived alkaloids produced in culture by Streptomyces uncialis. Org Lett. 2008;10:3501–3504. doi: 10.1021/ol801274c. [DOI] [PubMed] [Google Scholar]
- 4.Sánchez C, Méndez C, Salas JA. Indolocarbazole natural products: occurrence, biosynthesis, and biological activity. Nat Prod Rep. 2006;23:1007–1045. doi: 10.1039/b601930g. [DOI] [PubMed] [Google Scholar]
- 5.Bush JA, Long BH, Catino JJ, Bradner WT, Tomita K. Production and biological activity of rebeccamycin, a novel antitumor agent. J Antibiot (Tokyo) 1987;40:668–678. doi: 10.7164/antibiotics.40.668. [DOI] [PubMed] [Google Scholar]
- 6.Prudhomme M. Rebeccamycin analogues as anti-cancer agents. Eur J Med Chem. 2003;38:123–140. doi: 10.1016/s0223-5234(03)00011-4. [DOI] [PubMed] [Google Scholar]
- 7.Omura S, Iwai Y, Hirano A, Nakagawa A, Awaya J, et al. A new alkaloid AM-2282 of Streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J Antibiot (Tokyo) 1977;30:275–282. doi: 10.7164/antibiotics.30.275. [DOI] [PubMed] [Google Scholar]
- 8.Ruegg UT, Burgess GM. Staurosporine, K-252 and UCN-01: potent but nonspecific inhibitors of protein kinases. Trends Pharmacol Sci. 1989;10:218–220. doi: 10.1016/0165-6147(89)90263-0. [DOI] [PubMed] [Google Scholar]
- 9.Onaka H, Taniguchi S, Igarashi Y, Furumai T. Cloning of the staurosporine biosynthetic gene cluster from Streptomyces sp. TP-A0274 and its heterologous expression in Streptomyces lividans. J Antibiot (Tokyo) 2002;55:1063–1071. doi: 10.7164/antibiotics.55.1063. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez C, Butovich IA, Braña AF, Rohr J, Méndez C, et al. The biosynthetic gene cluster for the antitumor rebeccamycin: characterization and generation of indolocarbazole derivatives. Chem Biol. 2002;9:519–531. doi: 10.1016/s1074-5521(02)00126-6. [DOI] [PubMed] [Google Scholar]
- 11.Onaka H, Taniguchi S, Igarashi Y, Furumai T. Characterization of the biosynthetic gene cluster of rebeccamycin from Lechevalieria aerocolonigenes ATCC 39243. Biosci Biotechnol Biochem. 2003;67:127–138. doi: 10.1271/bbb.67.127. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez C, Zhu L, Braña AF, Salas AP, Rohr J, et al. Combinatorial biosynthesis of antitumor indolocarbazole compounds. Proc Natl Acad Sci U S A. 2005;102:461–466. doi: 10.1073/pnas.0407809102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang FY, Brady SF. Cloning and characterization of an environmental DNA-derived gene cluster that encodes the biosynthesis of the antitumor substance BE-54017. J Am Chem Soc. 2011;133:9996–9999. doi: 10.1021/ja2022653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard-Jones AR, Walsh CT. Staurosporine and rebeccamycin aglycones are assembled by the oxidative action of StaP, StaC, and RebC on chromopyrrolic acid. J Am Chem Soc. 2006;128:12289–12298. doi: 10.1021/ja063898m. [DOI] [PubMed] [Google Scholar]
- 15.Penn K, Jenkins C, Nett M, Udwary DW, Gontang EA, et al. Genomic islands link secondary metabolism to functional adaptation in marine Actinobacteria. ISME J. 2009;3:1193–1203. doi: 10.1038/ismej.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Q, Zhang C, Blanchard S, Thorson JS. Deciphering indolocarbazole and enediyne aminodideoxypentose biosynthesis through comparative genomics: insights from the AT2433 biosynthetic locus. Chem Biol. 2006;13:733–743. doi: 10.1016/j.chembiol.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Park JS, Chae CS, Hyun CG, Choi BW, et al. Genetic organization of the biosynthetic gene cluster for the indolocarbazole K-252a in Nonomuraea longicatena JCM 11136. Appl Microbiol Biotechnol. 2007;75:1119–1126. doi: 10.1007/s00253-007-0924-x. [DOI] [PubMed] [Google Scholar]
- 18.Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, et al. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol Mol Biol Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medema MH, Trefzer A, Kovalchuk A, van den Berg M, Müller U, et al. The sequence of a 1.8-mb bacterial linear plasmid reveals a rich evolutionary reservoir of secondary metabolic pathways. Genome Biol Evol. 2010;2:212–224. doi: 10.1093/gbe/evq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Watanabe K, Cai X, Jung ME, Tang Y, et al. Identifying the minimal enzymes required for anhydrotetracycline biosynthesis. J Am Chem Soc. 2008;130:6068–6069. doi: 10.1021/ja800951e. [DOI] [PubMed] [Google Scholar]
- 21.Pickens LB, Kim W, Wang P, Zhou H, Watanabe K, et al. Biochemical analysis of the biosynthetic pathway of an anticancer tetracycline SF2575. J Am Chem Soc. 2009;131:17677–17689. doi: 10.1021/ja907852c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelfattah MS, Rohr J. Premithramycinone G, an early shunt product of the mithramycin biosynthetic pathway accumulated upon inactivation of oxygenase MtmOII. Angew Chem Int Ed Engl. 2006;45:5685–5689. doi: 10.1002/anie.200600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafanan ER, Jr, Hutchinson CR, Shen B. Triple hydroxylation of tetracenomycin A2 to tetracenomycin C involving two molecules of O(2) and one molecule of H(2)O. Org Lett. 2000;2:3225–3227. doi: 10.1021/ol0002267. [DOI] [PubMed] [Google Scholar]
- 24.Ishida K, Maksimenka K, Fritzsche K, Scherlach K, Bringmann G, et al. The boat-shaped polyketide resistoflavin results from re-facial central hydroxylation of the discoid metabolite resistomycin. J Am Chem Soc. 2006;128:14619–14624. doi: 10.1021/ja064550u. [DOI] [PubMed] [Google Scholar]
- 25.Fujimori DG, Hrvatin S, Neumann CS, Strieker M, Marahiel MA, et al. Cloning and characterization of the biosynthetic gene cluster for kutznerides. Proc Natl Acad Sci U S A. 2007;104:16498–16503. doi: 10.1073/pnas.0708242104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heemstra JR, Jr, Walsh CT. Tandem action of the O2- and FADH2-dependent halogenases KtzQ and KtzR produce 6,7-dichlorotryptophan for kutzneride assembly. J Am Chem Soc. 2008;130:14024–14025. doi: 10.1021/ja806467a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zehner S, Kotzsch A, Bister B, Süssmuth RD, Méndez C, et al. A regioselective tryptophan 5-halogenase is involved in pyrroindomycin biosynthesis in Streptomyces rugosporus LL-42D005. Chem Biol. 2005;12:445–452. doi: 10.1016/j.chembiol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Yeh E, Garneau S, Walsh CT. Robust in vitro activity of RebF and RebH, a two-component reductase/halogenase, generating 7-chlorotryptophan during rebeccamycin biosynthesis. Proc Natl Acad Sci U S A. 2005;102:3960–3965. doi: 10.1073/pnas.0500755102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammer PE, Hill DS, Lam ST, Van Pée KH, Ligon JM. Four genes from Pseudomonas fluorescens that encode the biosynthesis of pyrrolnitrin. Appl Environ Microbiol. 1997;63:2147–2154. doi: 10.1128/aem.63.6.2147-2154.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keller S, Wage T, Hohaus K, Hölzer M, Eichhorn E, et al. Purification and partial characterization of tryptophan 7-halogenase (PrnA) from Pseudomonas fluorescens. Angew Chem Int Ed Engl. 2000;39:2300–2302. doi: 10.1002/1521-3773(20000703)39:13<2300::aid-anie2300>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 31.Daum M, Peintner I, Linnenbrink A, Frerich A, Weber M, et al. Organisation of the biosynthetic gene cluster and tailoring enzymes in the biosynthesis of the tetracyclic quinone glycoside antibiotic polyketomycin. Chembiochem. 2009;10:1073–1083. doi: 10.1002/cbic.200800823. [DOI] [PubMed] [Google Scholar]
- 32.Ramos A, Lombó F, Braña AF, Rohr J, Méndez C, et al. Biosynthesis of elloramycin in Streptomyces olivaceus requires glycosylation by enzymes encoded outside the aglycon cluster. Microbiology. 2008;154:781–788. doi: 10.1099/mic.0.2007/014035-0. [DOI] [PubMed] [Google Scholar]
- 33.Wendt-Pienkowski E, Huang Y, Zhang J, Li B, Jiang H, et al. Cloning, sequencing, analysis, and heterologous expression of the fredericamycin biosynthetic gene cluster from Streptomyces griseus. J Am Chem Soc. 2005;127:16442–16452. doi: 10.1021/ja054376u. [DOI] [PubMed] [Google Scholar]
- 34.De Schrijver A, De Mot R. A subfamily of MalT-related ATP-dependent regulators in the LuxR family. Microbiology 145 ( Pt. 1999;6):1287–1288. doi: 10.1099/13500872-145-6-1287. [DOI] [PubMed] [Google Scholar]
- 35.Jochens H, Hesseler M, Stiba K, Padhi SK, Kazlauskas RJ, et al. Protein engineering of α/β-hydrolase fold enzymes. Chembiochem. 2011;12:1508–1517. doi: 10.1002/cbic.201000771. [DOI] [PubMed] [Google Scholar]
- 36.Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, et al. The alpha/beta hydrolase fold. Protein Eng. 1992;5:197–211. doi: 10.1093/protein/5.3.197. [DOI] [PubMed] [Google Scholar]
- 37.Nishizawa T, Aldrich CC, Sherman DH. Molecular analysis of the rebeccamycin L-amino acid oxidase from Lechevalieria aerocolonigenes ATCC 39243. J Bacteriol. 2005;187:2084–2092. doi: 10.1128/JB.187.6.2084-2092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh S, McCoy JG, Zhang C, Bingman CA, Phillips GN, Jr, et al. Structure and mechanism of the rebeccamycin sugar 4′-O-methyltransferase RebM. J Biol Chem. 2008;283:22628–22636. doi: 10.1074/jbc.M800503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, Albermann C, Fu X, Peters NR, Chisholm JD, et al. RebG- and RebM-catalyzed indolocarbazole diversification. Chembiochem. 2006;7:795–804. doi: 10.1002/cbic.200500504. [DOI] [PubMed] [Google Scholar]
- 40.Howard-Jones AR, Walsh CT. Enzymatic generation of the chromopyrrolic acid scaffold of rebeccamycin by the tandem action of RebO and RebD. Biochemistry. 2005;44:15652–15663. doi: 10.1021/bi051706e. [DOI] [PubMed] [Google Scholar]
- 41.Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DF. Norwich, England: The John Innes Foundation; 2000. Practical Streptomyces Genetics. [Google Scholar]
- 42.Ishikawa J, Hotta K. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol Lett. 1999;174:251–253. doi: 10.1111/j.1574-6968.1999.tb13576.x. [DOI] [PubMed] [Google Scholar]
- 43.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 44.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 45.Palfey BA, Murthy YV, Massey V. Altered balance of half-reactions in p-hydroxybenzoate hydroxylase caused by substituting the 2′-carbon of FAD with fluorine. J Biol Chem. 2003;278:22210–22216. doi: 10.1074/jbc.M301830200. [DOI] [PubMed] [Google Scholar]
- 46.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, et al. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Partial sequence alignment of DNA encoding select rebC homologs. staC-1 is the staC gene from Streptomyces sp. TP-A0274 (GenBank accession AB088119.1) [9], staC-2 is the staC gene from Streptomyces longisporoflavus (accession DQ861905.1) [14], staC-3 is the putative staC gene from Salinispora arenicola (accession CP000850.1; Sare_2333) [15], inkE is from Nonomuraea longicatena (accession DQ399653.1) [17], rebC is from Lechevalieria aerocolonigenes (accession AJ414559.1) [10], and atC is from Actinomadura melliaura (accession DQ297453.1) [16]. Only DNA from 1 to ∼640 basepairs is shown. Highlighted in yellow are the DNA sequences targeted by the degenerate primers.
(PDF)
Reaction of ClaX1. (A) The closest characterized homologs to ClaX1 and AbeX1 are SsfO2 and OxyL. SsfO2 and OxyL react with 6-methyl-pretetramid through two rounds of flavin-based hydroxylation chemistry to give 4-keto-anhydrotetracycline [20], [21]. (B) The overall mechanism for the best-studied flavin-dependent hydroxylase, para-hydroxybenzoate hydroxylase (pHBH) is shown. After flavin-based chemistry occurs, the non-aromatic intermediate undergoes a rapid keto-enol tautomerization to restore aromaticity to the ring [45]. (C) If intermediate 1 underwent a single round of flavin-based hydroxylation chemistry to install a first hydroxyl group as shown, restoration of aromaticity following the reaction is not possible because the hydroxy group is on a carbon (starred) that lacks hydrogens. Without hydrogens, a tautomerization analogous to that shown for pHBH is not possible. (D) An epoxidation of 1, followed by epoxide hydrolysis, would give rise to the proposed product 2, containing two tertiary alcohols, and is consistent with the better established roles of MtmOII and TcmG.
(TIF)
Reaction of ClaX2. (A) One possible reaction mechanism for ClaX2 catalysis is epoxidation of 2 followed by sigma-bond cleavage and rearrangment of the indolocarbazole to give the indenotryptoline core structure 3. (B) The closest characterized homolog to ClaX2/AbeX2 is RemO, which catalyzes a single hydroxylation of a multi-ringed aromatic substrate on the re face [24]. (C) Similar flavin-based hydroxylation chemistry on 2 could lead to an intermediate that undergoes sigma-bond cleavage and rearrangment of the indolocarbazole to give the indenotryptoline core 3.
(TIF)