Abstract
Context
Statins are widely prescribed for primary and secondary prevention of ischemic cardiac and cerebrovascular disease. Although serious adverse effects are uncommon, results from a recent clinical trial suggested increased risk of intracerebral hemorrhage (ICH) associated with statin use. For patients with baseline elevated risk of ICH, it is not known whether this potential adverse effect offsets the cardiovascular and cerebrovascular benefits.
Methods
We used Markov decision modeling to address the following clinical question: Given a history of prior ICH, should statin therapy be avoided? We investigated how a range of clinical parameters affects this decision, including hemorrhage location (deep vs lobar), ischemic cardiac and cerebrovascular risks, and magnitude of ICH risk associated with statins.
Findings
Avoiding statins was favored over a wide range of values for many clinical parameters, particularly in survivors of lobar ICH who are at highest risk of ICH recurrence. In survivors of lobar ICH without prior cardiovascular events, avoiding statins yielded a life expectancy gain of 2.2 quality-adjusted life years compared with statin use. This net benefit persisted even at the lower 95% confidence interval of the relative risk of statin-associated ICH. In lobar ICH patients with prior cardiovascular events, the annual recurrence risk of myocardial infarction would have to exceed 90% to favor statin therapy. Avoiding statin therapy was also favored, although by a smaller margin, in both primary and secondary prevention settings for survivors of deep ICH.
Conclusions
Avoiding statins should be considered for patients with a history of ICH, particularly those of lobar location.
Introduction
While the benefits of HMG coenzyme A reductase inhibitors (statins) for reducing cardiac and cerebrovascular disease risk are well established1,2, more widespread use of statin therapy remains controversial. A particular subgroup where the advisability of statin use is unclear involves patients at high risk for intracerebral hemorrhage (ICH)3. The reason for added concern is the increased incidence of ICH observed among subjects randomized to statin therapy in a clinical trial of secondary stroke prevention2,4. This risk amplification might have greatest relevance to patients at high risk for hemorrhage by virtue of prior ICH, particularly hemorrhages in lobar brain regions characteristic of the degenerative vascular condition cerebral amyloid angiopathy5,6. Since ICH survivors commonly have co-morbid cardiovascular risk factors that would otherwise warrant cholesterol-lowering medication, it is important to weigh the risks and benefits of statin therapy in this population.
Given the uncertainty surrounding this clinical decision, we developed a decision analytic model7. Decision analytic models have been applied to the clinical issue of anticoagulation in patients with high fall risk8 or history of ICH9, and to statin cost effectiveness in coronary and cerebrovascular disease1,10,11. To provide guidance for the frequently encountered question of whether statin use is safe after ICH, we used a decision analytic model incorporating published data regarding the beneficial effects of statins1, the risk of recurrent deep versus lobar ICH9, and the reported impact of statin use on ICH risk2,4.
Methods
Simulated clinical trials were conducted with a Markov state transition model7 implemented in Matlab™ (The Mathworks, Natick, MA). The base case for these analyses is a 65 year-old male ICH survivor. The impact of statin therapy versus no statin therapy was considered under three basic scenarios involving differing risk for future cerebro-cardio-vascular events: 1) Primary prevention: no prior cerebral ischemic event (transient ischemic attack (TIA) or ischemic stroke), and no prior cardiac ischemic event (angina or MI); 2) Prior stroke: prior ischemic stroke, at least one year in the past; 3) Prior MI: prior myocardial infarction (MI), at least one year in the past. For each scenario we computed the expected total quality-adjusted life years (QALYs) on versus off statin therapy. We separately considered hemorrhages occurring in the two brain locations that jointly account for >80% of hemorrhagic strokes12: “deep” ICH (thalamus or basal ganglia) and “lobar” ICH (frontal, parietal, temporal, or occipital), because of different recurrence risks, reflecting distinct underlying pathophysiologies. Non-traumatic lobar ICH in the age range considered here is mainly due to cerebral amyloid angiopathy (CAA)17 and carries a higher risk of recurrent ICH compared with deep ICH9, which is primarily related to chronic hypertension14.
Details of the model structure and the associated assumptions can be found in the Online Supplemental Materials. In brief, the model consists of states that correspond to disease risk, in which simulated patients can experience any combination of “events” (for example, ischemic stroke, MI, ICH), which may lead to increased risk of future events, change in quality of life, or death. Values for risk, outcomes, and quality of life are adopted from a recent systematic review of statin therapy1 and a previously reported decision analysis9. Quality of life adjustment factors (Q) were used to account for the relative decrease in quality of life following cardiac and cerebral ischemic events1. Risks for recurrent ICH after prior deep or lobar ICH were taken from previously reported studies5,6. The relative risk (RR) of ICH on statin therapy from the Stroke Prevention by Aggressive Reduction in Cholesterol Levels(SPARCL) trial analysis was 1.68 (95% confidence interval 1.09 to 2.59); this risk was assumed to apply to both deep and lobar ICH. Event probabilities, relative risks, and quality of life adjustment factors pertaining to the base case are summarized in Table 1. A model schematic, as well as further details, can be found in the Online Supplemental Material.
Table 1.
Base-case event risks, relative risks, and quality-of-life adjustment factors.
| Risk in PP | Risk in SP | RR on statin | Q | |
|---|---|---|---|---|
| Stable angina | 4.8 | * | 0.59 | 0.81 |
| Unstable angina | 1.8 | * | 0.72 | 0.77 |
| MI, nonlethal | 3.8 | 18.5 | 0.66 | 0.76 |
| MI, lethal | 2.1 | 15.2 | 0.74 | 0 |
| TIA | 2.2 | 2.2 | 0.79 | 1 |
| Stroke, nonlethal | 6.0 | 2.2 | 0.77 | 0.63 |
| Stroke, lethal | 1.4 | 1.4 | 1 | 0 |
| Lobar ICH, nonlethal | 113.4 | 1.68 | 0.47 | |
| Lobar ICH, lethal | 26.6 | 0 | ||
| Deep ICH, nonlethal | 16.7 | 0.45 | ||
| Deep ICH, lethal | 4.3 | 0 | ||
PP, primary prevention. SP, secondary prevention, i.e. >1 year post-MI. Q, quality of life adjustment factor. Risks are expressed as number of events per 1000 patients per year.
Asterisks * for the risk of stable and unstable angina post-MI signify that, in the model, these lower risk health states are not accessible after an MI.
Results
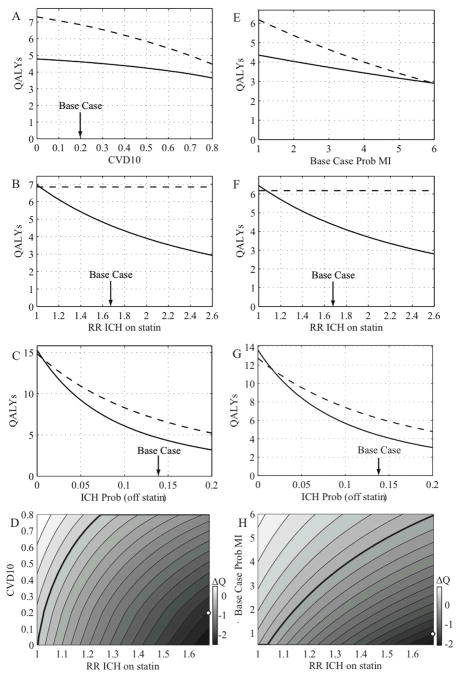
We simulated the impact of statins on quality-adjusted life expectancy(QALYs) for patients with a history of lobar ICH separately from those with prior deep ICH. Table 1 lists the base-case values used for these analyses and Supplemental Table 1 the transition probabilities and quality of life values for the individual states. The increased risk of ICH associated with statin therapy (1.68) was taken from the SPARCL trial15.
Lobar ICH
Our base case patient is a 65 year-old man with a 10-year CVD risk of 20% at the time of a lobar ICH. In the primary prevention setting (i.e. no prior history of ischemic cerebrovascular or cardiac events), avoiding statins was the preferred course. This option resulted in accrual of 6.8 QALYs, whereas statin therapy resulted in 4.6 QALYs, a net loss for statin use of 2.2 QALYs. Modeling of statin therapy under various secondary prevention scenarios also suggested net benefit for avoiding statins. Predicted outcomes in the setting of a history of prior MI were 4.4 QALYs on statin vs 6.2 QALYS off; for history of prior ischemic stroke, the figures were 4.2 vs 6.0 QALYs, respectively.
In sensitivity analyses, avoiding statins remained the preferred option following lobar ICH over a wide range of values for the statin-associated RR of ICH (Table 3, Figure 1). Even at the lower limit of the 95% confidence interval of the RR for ICH reported in the SPARCL study (1.09)15, avoiding statins remained the preferred option by a small margin (net loss for statin use of 0.3 QALYs). For statin therapy to be favored, the RR of ICH would need to be less than or equal to 1.03 for primary prevention, 1.07 for secondary prevention after MI, and 1.06 for secondary prevention after ischemic stroke.
Table 3.
Sensitivity Analyses
| Lobar ICH | Deep ICH | |
|---|---|---|
| RR of ICH while receiving statins | Threshold (basecase 1.68; range: 1–2.6) | |
|
| ||
| Primary prevention | 1.03 | 1.20 |
| Prior MI | 1.07 | 1.50 |
| Prior ischemic stroke | 1.06 | 1.41 |
|
| ||
| ICH Annual Recurrence probability | Threshold (range: 0–15%)† | |
|
| ||
| Primary prevention | 0.6 | 0.6 |
| Prior MI | 1.6 | 1.5 |
| Prior ischemic stroke | 1.3 | 1.3 |
|
| ||
| 10-Year CVD Probability | Threshold (range: 0–80%) | |
|
| ||
| PP | * | 0.46 |
|
| ||
| MI Annual Recurrence probability | Threshold (range: 0–80%) | |
|
| ||
| Prior MI | 20 (5.9 × base case) | 4.6 (1.4 × base case) |
Basecase annual recurrence rate for lobar ICH – 14%/year; deep ICH – 1.7%/year.
Statin never preferred. For RR of ICH thresholds, 10-year CVD probability was set to 20%. For 10-year CVD probability thresholds, RR of ICH was set to 1.68.
Figure 1.
In complementary sensitivity analyses, we found the strategy of avoiding statins following lobar ICH to be robust to other values used in the base-case model as well. Avoiding statins remained the preferred strategy at (off-statin) annual ICH recurrence probabilities well below the base-case value of 14% per year (Table 3). Avoiding statins was also preferred over the entire range of 10-year CVD risk values considered (0–80%) for primary prevention, and for risks of MI recurrence up to 6-fold greater than the base-case assumption for secondary prevention (Fig. 1E). These results indicate that the risk of ICH on statin therapy is not offset by the secondary prevention benefits, even if the cardiovascular risks are artificially forced to be extremely high. Two-way sensitivity analyses varying both the RR of ICH and the 10-year CVD risk (for primary prevention; Figure 1D) or MI recurrence probability (for secondary prevention; Figure 1H) again demonstrate that avoiding statins is preferred through a wide range of values around the base-case assumptions.
Deep ICH
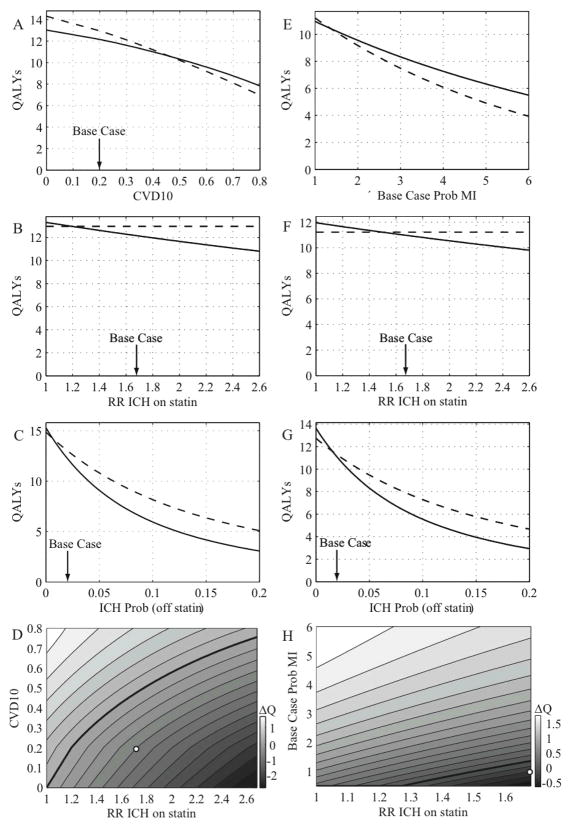
We performed similar analyses for our other base case patient, a 65 year-old man with a 10-year CVD risk of 20% at the time of a deep ICH. In this scenario, the risk of recurrent ICH is substantially lower9,5. Assuming the 1.68 RR of ICH from the SPARCL data, in the primary prevention setting, statin therapy confers a net loss of 0.8 QALYs (13.0 vs 12.2 QALYs, Table 2). In the secondary prevention settings, statin therapy also produced net QALY losses, although they were smaller (0.2 for the post-MI and 0.3 for the post-ischemic stroke settings).
Table 2.
Results of Base Case Decision Analysis
| Prior ICH Location | Setting Effectiveness (QALYs) | No Statin | Statin RR 1.68 |
|---|---|---|---|
| Lobar ICH | Primary prevention | 6.8 | 4.6 |
| Prior MI | 6.2 | 4.4 | |
| Prior ischemic stroke | 6.0 | 4.2 | |
| Deep ICH | Primary prevention | 13.0 | 12.2 |
| Prior MI | 11.2 | 11.0 | |
| Prior ischemic stroke | 10.6 | 10.3 |
Thresholds for the statin-associated RR of ICH below which statin therapy was preferred were 1.20 for primary prevention, 1.50 for secondary prevention after MI, and 1.41 for secondary prevention after ischemic stroke (Table 3 and Figure 2B, F). The off-drug probabilities for ICH recurrence at which statin therapy was preferred also fell relatively close to the base-case value (2.1%), including the secondary prevention settings of prior MI (1.5%) or ischemic stroke (1.3%; Table 3). In the post-MI scenario, statin therapy was preferred if the probability of MI increased to 1.4 times the base case probability (Table 3). Decision boundaries from a two-way sensitivity analysis of RR ICH and 10-year CVD risk in the primary prevention setting, and of RR ICH vs MI recurrence probability in the post-MI setting for deep ICH, are shown in Figure 2D and Figure 2H.
Figure 2.
The differences in QALYs for the various scenarios discussed above emerge from interactions among multiple competing factors over a patient’s lifespan. It is also instructive to consider the impact of individual factors over shorter time spans. Using the base-case assumptions and measuring over a single year of follow-up, primary prevention with statin therapy is projected to prevent fewer than 2 deaths from either MI or ischemic stroke per 1000 patients, at the expense of causing 18 lobar ICHs (in patients with prior lobar ICH) or 3 deep ICHs (in patients with prior deep ICH) per 1000. From the perspective of disability and resulting loss of quality of life, each year primary prevention with statin treatment saves 2.6 QALYs from MI or 2.2 from ischemic stroke per 1000 patients, at the expense of 58.6 QALYs for lobar ICH or 9 QALYs for deep-ICH.
DISCUSSION
We used a decision analytic model to evaluate a common dilemma facing physicians of patients with history of prior ICH and indications for statin therapy: under what clinical circumstances should statin therapy be avoided due to risk of recurrent ICH2,4,14. Our analysis indicates that in settings of high recurrent ICH risk, avoiding statin therapy may be preferred. For lobar ICH in particular, which has a substantially higher recurrence rate than deep ICH, statin therapy is predicted to raise the baseline annual probability of recurrence from ~14% to ~22%, offsetting the cardiovascular benefits for both primary and secondary cardiovascular prevention9. Our results were robust over a wide range of CVD event rates in both primary and secondary prevention settings, and of estimates for the statin-associated RR of ICH. In the case of deep ICH, the substantially lower baseline annual recurrence rate translates into a much closer balance between statins’ risks and benefits, and consequently the optimal treatment option may vary with specific circumstances. For our base-case deep ICH patient, we found that statin therapy was worse than no treatment, but this conclusion is sensitive to variations in the assumed baseline recurrence rates of ischemic events and ICH, and the statin-associated RR of ICH, such that in some realistic secondary prevention settings statin therapy may be preferred.
The different results for statin use after lobar versus deep ICH arise primarily from the different annual ICH recurrence probabilities in these two groups5,6. These two ICH locations appear to reflect different underlying pathophysiology14,17: non-traumatic lobar ICH in the age range under consideration is mostly due to cerebral amyloid angiopathy (CAA)13, whereas hypertensive vascular disease is primarily responsible for deep ICH15. The risk of recurrent deep ICH can be lowered by appropriate antihypertensive medical therapy18–20, whereas CAA currently lacks an established preventive treatment. Patients with CAA are at risk for both symptomatic ICH and for accumulation of clinically silent microhemorrhages, which may serve as the substrate for subsequent larger ICH events, explaining the observed continued risk of lobar ICH over the lifetime21.
The mechanism by which statins might amplify the risk of hemorrhagic stroke remains unclear. Historically, concerns about increased ICH risk with lipid-lowering drug therapy have centered on epidemiological studies linking low cholesterol levels with an increased rate of hemorrhagic stroke. This apparent association may be weaker than originally thought22, and the increased risk of ICH with statin therapy found in SPARCL was independent of LDL levels2,4. Statins are known to have pleiotropic effects, independent of their impact on cholesterol levels23–25, and some of these have been proposed as possible mechanisms for increasing ICH risk26. For example, there is evidence that statins may have antithrombotic27–32 and fibrinolytic33–38 effects, and may enhance the activity of other fibrinolytic agents26,39. The dose and statin-type dependency of these effects is not yet well understood.
There are important limitations to this analysis. The data driving the statin-related RR of ICH derive from post-hoc analysis of a single clinical trial, performed in ischemic and hemorrhagic stroke patients randomized to a single dose and statin agent (atorvastatin 80 mg). It is therefore uncertain whether these results generalize across multiple populations, agents, and doses. A further potential limitation of the present study is that we restricted analysis to simple all-or-none strategies of either treatment or non-treatment whereas “switchover” strategies could be hypothetically superior. That is, given that the risk of recurrent ischemic cardiac and cerebrovascular events are higher nearer to the time of the index events, it might conceivably prove beneficial to provide statin treatment for a short period and then discontinue when the recurrence risk has decayed sufficiently. Strategies involving short-term statin treatment might be preferable if future data were to show that statin-related ICH risk is not constant over time, e.g. if the risk is smaller soon after ICH. Despite these limitations, it is notable that the finding of net loss of QALYs in statin treatment of lobar ICH patients persisted even at the lower 95% confidence limit observed in SPARCL, supporting its validity. A second major limitation that applies to all decision analyses is their reliance on parameters extracted from existing literature, which may contain uncertainties. Although only a randomized trial could definitively answer the questions raised in the current study, we note that the sensitivity analyses employed to address some of this uncertainty support our fundamental findings. Another important caveat is the possibility (identified in some, but not all observational studies40–44) that patients on statin therapy at the time of ICH have better outcomes than those not on statins. Although such statin-associated improvements in ICH outcome would partially mitigate the loss of QALYs associated with increased ICH incidence, they appear insufficient to significantly offset this effect in lobar ICH patients in our model (data not shown).
In summary, mathematical decision analysis of the available data suggests that, because of the high risk of recurrent ICH in survivors of prior hemorrhagic stroke, even a small amplification of this risk by statins suffices to recommend that they should be avoided after ICH. In the absence of data from a randomized clinical trial (ideally comparing various agents and doses), the current model provides some guidance for clinicians approaching this difficult decision.
Supplementary Material
Acknowledgments
We thank Sherry Chou, MD for critical review and discussions of the manuscript, and Alessandro Biffi, MD and Jonathon Rosand, MD for critical review and for sharing unpublished data regarding the impact of statin use on ICH outcomes.
Funding
This work was supported by a grant from the National Institutes of Health R01 AG026484.
References
- 1.Ward S, Lloyd Jones M, Pandor A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technol Assess. 2007;11(14):1–160. iii–iv. doi: 10.3310/hta11140. [DOI] [PubMed] [Google Scholar]
- 2.Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355(6):549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs BS, Greenberg SM. Statins, low cholesterol, and hemorrhagic stroke: An uncertain triangle. Neurology. 2008;70(24_Part_2):2355–2356. doi: 10.1212/01.wnl.0000314696.61422.12. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein MR, Mascitelli L, Pezzetta F. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2009;72(16):1448. doi: 10.1212/01.wnl.0000346751.39886.01. author reply 1448–1449. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RD, Hart RG, Benavente O, Pearce LA. Recurrent brain hemorrhage is more frequent than ischemic stroke after intracranial hemorrhage. Neurology. 2001;56(6):773–777. doi: 10.1212/wnl.56.6.773. [DOI] [PubMed] [Google Scholar]
- 6.O’Donnell HC, Rosand J, Knudsen KA, et al. Apolipoprotein E Genotype and the Risk of Recurrent Lobar Intracerebral Hemorrhage. N Engl J Med. 2000;342(4):240–245. doi: 10.1056/NEJM200001273420403. [DOI] [PubMed] [Google Scholar]
- 7.Detsky AS, Naglie G, Krahn MD, Naimark D, Redelmeier DA. Primer on medical decision analysis: Part 1--Getting started. Med Decis Making. 1997;17(2):123–125. doi: 10.1177/0272989X9701700201. [DOI] [PubMed] [Google Scholar]
- 8.Man-Son-Hing M, Nichol G, Lau A, Laupacis A. Choosing antithrombotic therapy for elderly patients with atrial fibrillation who are at risk for falls. Arch Intern Med. 1999;159(7):677–685. doi: 10.1001/archinte.159.7.677. [DOI] [PubMed] [Google Scholar]
- 9.Eckman MH, Rosand J, Knudsen KA, Singer DE, Greenberg SM. Can patients be anticoagulated after intracerebral hemorrhage? A decision analysis. Stroke. 2003;34(7):1710–1716. doi: 10.1161/01.STR.0000078311.18928.16. [DOI] [PubMed] [Google Scholar]
- 10.Plosker GL, Lyseng-Williamson KA. Atorvastatin: a pharmacoeconomic review of its use in the primary and secondary prevention of cardiovascular events. Pharmacoeconomics. 2007;25(12):1031–1053. doi: 10.2165/00019053-200725120-00005. [DOI] [PubMed] [Google Scholar]
- 11.Pletcher MJ, Lazar L, Bibbins-Domingo K, et al. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Ann Intern Med. 2009;150(4):243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The Effect of Warfarin and Intensity of Anticoagulation on Outcome of Intracerebral Hemorrhage. Arch Intern Med. 2004;164(8):880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 13.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5(4):260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 14.Woo D, Sauerbeck LR, Kissela BM, et al. Genetic and Environmental Risk Factors for Intracerebral Hemorrhage: Preliminary Results of a Population-Based Study * Editorial Comment: Preliminary Results of a Population-Based Study. Stroke. 2002;33(5):1190–1196. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LB, Amarenco P, Szarek M, et al. Hemorrhagic stroke in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels study. Neurology. 2007:2364–2370. doi: 10.1212/01.wnl.0000296277.63350.77. [DOI] [PubMed] [Google Scholar]
- 16.Mascitelli L, Pezzetta F, Goldstein MR. Low cholesterol, statin therapy, and intracerebral haemorrhage. Hong Kong Med J. 2009;15(5):403. [PubMed] [Google Scholar]
- 17.Rosand J, Hylek EM, O’Donnell HC, Greenberg SM. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology. 2000;55(7):947–951. doi: 10.1212/wnl.55.7.947. [DOI] [PubMed] [Google Scholar]
- 18.Tzourio C, Arima H, Harrap S, et al. APOE genotype, ethnicity, and the risk of cerebral hemorrhage. Neurology. 2008;70(16):1322–1328. doi: 10.1212/01.wnl.0000308819.43401.87. [DOI] [PubMed] [Google Scholar]
- 19.Bae H, Jeong D, Doh J, et al. Recurrence of Bleeding in Patients with Hypertensive Intracerebral Hemorrhage. Cerebrovascular Diseases. 1999;9(2):102–108. doi: 10.1159/000015906. [DOI] [PubMed] [Google Scholar]
- 20.Arakawa S, Saku Y, Ibayashi S, Nagao T, Fujishima M. Blood pressure control and recurrence of hypertensive brain hemorrhage. Stroke. 1998;29(9):1806–1809. doi: 10.1161/01.str.29.9.1806. [DOI] [PubMed] [Google Scholar]
- 21.Hart RG, Boop BS, Anderson DC. Oral Anticoagulants and Intracranial Hemorrhage: Facts and Hypotheses. Stroke. 1995;26(8):1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 22.Ariesen MJ, Claus SP, Rinkel GJE, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 23.Reiss AB, Wirkowski E. Role of HMG-CoA reductase inhibitors in neurological disorders: progress to date. Drugs. 2007;67(15):2111–2120. doi: 10.2165/00003495-200767150-00001. [DOI] [PubMed] [Google Scholar]
- 24.Takemoto M, Liao JK. Pleiotropic Effects of 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors. Arterioscler Thromb Vasc Biol. 2001;21(11):1712–1719. doi: 10.1161/hq1101.098486. [DOI] [PubMed] [Google Scholar]
- 25.Paciaroni M, Hennerici M, Agnelli G, Bogousslavsky J. Statins and Stroke Prevention. Cerebrovasc Dis. 2007;24(2–3):170–182. doi: 10.1159/000104474. [DOI] [PubMed] [Google Scholar]
- 26.Meier N, Nedeltchev K, Brekenfeld C, et al. Prior Statin Use, Intracranial Hemorrhage, and Outcome After Intra-Arterial Thrombolysis for Acute Ischemic Stroke. Stroke. 2009:1729–1737. doi: 10.1161/STROKEAHA.108.532473. [DOI] [PubMed] [Google Scholar]
- 27.Undas A, Brummel KE, Musial J, Mann KG, Szczeklik A. Simvastatin Depresses Blood Clotting by Inhibiting Activation of Prothrombin, Factor V, and Factor XIII and by Enhancing Factor Va Inactivation. Circulation. 2001;103(18):2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- 28.Notarbartolo A, Davì G, Averna M, et al. Inhibition of thromboxane biosynthesis and platelet function by simvastatin in type IIa hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15(2):247–251. doi: 10.1161/01.atv.15.2.247. [DOI] [PubMed] [Google Scholar]
- 29.Mayer J, Eller T, Brauer P, et al. Effects of long-term treatment with lovastatin on the clotting system and blood platelets. Ann Hematol. 1992;64(4):196–201. doi: 10.1007/BF01696223. [DOI] [PubMed] [Google Scholar]
- 30.Huhle G, Abletshauser C, Mayer N, et al. Reduction of Platelet Activity Markers in Type II Hypercholesterolemic Patients by a HMG-CoA-Reductase Inhibitor. Thrombosis Research. 1999;95(5):229–234. doi: 10.1016/s0049-3848(99)00037-7. [DOI] [PubMed] [Google Scholar]
- 31.Szapary L, Horvath B, Marton Z, et al. Short-term effect of low-dose atorvastatin on haemorrheological parameters, platelet aggregation and endothelial function in patients with cerebrovascular disease and hyperlipidaemia. CNS Drugs. 2004;18(3):165–172. doi: 10.2165/00023210-200418030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Ural A, Yilmaz M, Avcu F, Yalcin A. Treatment with Cerivastatin in Primary Mixed Hyperlipidemia Induces Changes in Platelet Aggregation and Coagulation System Components. International Journal of Hematology. 2002;76(3):279–283. doi: 10.1007/BF02982799. [DOI] [PubMed] [Google Scholar]
- 33.Essig M, Nguyen G, Prie D, et al. 3-Hydroxy-3-Methylglutaryl Coenzyme A Reductase Inhibitors Increase Fibrinolytic Activity in Rat Aortic Endothelial Cells: Role of Geranylgeranylation and Rho Proteins. Circ Res. 1998;83(7):683–690. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- 34.Essig M, Vrtovsnik F, Nguyen G, Sraer J, Friedlander G. Lovastatin modulates in vivo and in vitro the plasminogen activator/plasmin system of rat proximal tubular cells: role of geranylgeranylation and Rho proteins. J Am Soc Nephrol. 1998;9(8):1377–1388. doi: 10.1681/ASN.V981377. [DOI] [PubMed] [Google Scholar]
- 35.Aarons CB, Cohen PA, Gower A, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg. 2007;245(2):176–184. doi: 10.1097/01.sla.0000236627.07927.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai W, Lee K, Chu C, et al. Influence of withdrawal of statin treatment on proinflammatory response and fibrinolytic activity in humans: an effect independent on cholesterol elevation. Int J Cardiol. 2005;98(3):459–464. doi: 10.1016/j.ijcard.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Fogari R, Derosa G, Lazzari P, et al. Effect of amlodipine-atorvastatin combination on fibrinolysis in hypertensive hypercholesterolemic patients with insulin resistance. Am J Hypertens. 2004;17(9):823–827. doi: 10.1016/j.amjhyper.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Orem C, Uydu HA, Yilmaz R, et al. The effects of atorvastatin treatment on the fibrinolytic system in dyslipidemic patients. Jpn Heart J. 2004;45(6):977–987. doi: 10.1536/jhj.45.977. [DOI] [PubMed] [Google Scholar]
- 39.Undas A, Celinska-Löwenhoff M, Löwenhoff T, Szczeklik A. Statins, fenofibrate, and quinapril increase clot permeability and enhance fibrinolysis in patients with coronary artery disease. J Thromb Haemost. 2006;4(5):1029–1036. doi: 10.1111/j.1538-7836.2006.01882.x. [DOI] [PubMed] [Google Scholar]
- 40.Eichel R, Khoury ST, Ben-Hur T, et al. Prior use of statins and outcome in patients with intracerebral haemorrhage. Eur J Neurol. 2010;17(1):78–83. doi: 10.1111/j.1468-1331.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 41.Leker RR, Khoury ST, Rafaeli G, et al. Prior Use of Statins Improves Outcome in Patients With Intracerebral Hemorrhage: Prospective Data from the National Acute Stroke Israeli Surveys (NASIS) Stroke. 2009;40(7):2581–2584. doi: 10.1161/STROKEAHA.108.546259. [DOI] [PubMed] [Google Scholar]
- 42.Gomis M, Ois A, Rodríguez-Campello A, et al. Outcome of intracerebral haemorrhage patients pre-treated with statins. Eur J Neurol. 2010;17(3):443–448. doi: 10.1111/j.1468-1331.2009.02838.x. [DOI] [PubMed] [Google Scholar]
- 43.Naval NS, Abdelhak TA, Zeballos P, et al. Prior statin use reduces mortality in intracerebral hemorrhage. Neurocrit Care. 2008;8(1):6–12. doi: 10.1007/s12028-007-0080-2. [DOI] [PubMed] [Google Scholar]
- 44.FitzMaurice E, Wendell L, Snider R, et al. Effect of statins on intracerebral hemorrhage outcome and recurrence. Stroke. 2008;39(7):2151–2154. doi: 10.1161/STROKEAHA.107.508861. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.