Abstract
The majority of outer membrane proteins (OMPs) from Gram-negative bacteria and many of mitochondria and chloroplasts are β-barrels. Insertion and assembly of these proteins are catalyzed by the Omp85 protein family in a seemingly conserved process. All members of this family exhibit a characteristic N-terminal polypeptide-transport–associated (POTRA) and a C-terminal 16-stranded β-barrel domain. In plants, two phylogenetically distinct and essential Omp85's exist in the chloroplast outer membrane, namely Toc75-III and Toc75-V. Whereas Toc75-V, similar to the mitochondrial Sam50, is thought to possess the original bacterial function, its homolog, Toc75-III, evolved to the pore-forming unit of the TOC translocon for preprotein import. In all current models of OMP biogenesis and preprotein translocation, a topology of Omp85 with the POTRA domain in the periplasm or intermembrane space is assumed. Using self-assembly GFP-based in vivo experiments and in situ topology studies by electron cryotomography, we show that the POTRA domains of both Toc75-III and Toc75-V are exposed to the cytoplasm. This unexpected finding explains many experimental observations and requires a reevaluation of current models of OMP biogenesis and TOC complex function.
The majority of outer membrane proteins (OMPs) from Gram-negative bacteria and many proteins of mitochondria and chloroplast outer membranes are β-barrels. Insertion and assembly of these proteins is catalyzed by the highly conserved Omp85 protein family (outer membrane protein of 85 kDa) (1, 2) in a seemingly conserved process. Omp85's consist of a C-terminal 16-stranded pore-forming β-barrel and an N-terminal hydrophilic domain, with up to six polypeptide-transport–associated (POTRA) repeats (3–6). The bacterial Omp85—also known as YaeT, D15, Oma87, or BamA (β-barrel assembly machinery protein A)—is part of a lipoprotein complex that facilitates the folding and insertion of the OMPs from the periplasm into the outer membrane (7, 8). In current models of bacterial OMP biogenesis, the N-terminal POTRA domains (five) of BamA play a central role in the recognition of periplasmic chaperones and folding OMPs and in the assembly of OMPs into functional complexes (4). On the basis of topology modeling, the comparison with the related two-partner secretion protein FhaC and its conserved function (9, 10), the N-terminal domain has been predicted to be exposed to the periplasm (11). Apart from their essential role in OMP biogenesis, bacterial Omp85's have been shown to be excellent targets for antibacterial drugs (12), which makes their detailed study worthwhile.
Three eukaryotic Omp85 proteins have been described so far, the mitochondrial protein Sam50/Tob55 (e.g., ref. 13) and the chloroplast proteins Toc75-III and Toc75-V/Oep80 (14). Remarkably, all three are essential as mutations such as sam50 in yeast and toc75-III or toc75-V are lethal (15–17).
Like its bacterial counterpart, the mitochondrial Omp85 homolog Sam50 is involved in the biogenesis of mitochondrial OMPs, e.g., the mitochondrial porin-like voltage-dependent anion channel (VDAC) (13). Together with the peripheral proteins Sam37/Mas37 and Sam35/Tob38, Sam50 recognizes mitochondrial OMPs in the intermembrane space (IMS) after they have passed the outer envelope membrane via the TOM complex (translocator of the outer envelope membrane of mitochondria) and catalyzes their insertion and assembly in the outer membrane (14, 18). In contrast to BamA, Sam50 has only a single POTRA domain. Using controlled proteolysis of isolated mitochondria from yeast cells expressing recombinant Sam50, it was shown that the POTRA domain is exposed to the IMS (19). Similar to BamA, Sam50 is also capable of binding β-barrel proteins (19). This contrasts with the finding that the cytoplasmically exposed Sam35 is the major OMP receptor in yeast (20). Remarkably, Sam35 is absent in plants (21), indicating that the complex involved in mitochondrial OMP biogenesis is less conserved than anticipated.
As mentioned, chloroplasts have two families of Omp85 homologs (1, 22). Toc75-V (for translocon of the outer envelope membrane of chloroplast protein of 75 kDa encoded on the fifth chromosome in Arabidopsis thaliana; also termed Oep66 or Oep80 for outer envelope protein of 66/80 kDa in Pisum sativum/A. thaliana) has been suggested to possess the genuine bacterial Omp85 function (23). In contrast to Toc75-III, Toc75-V does not contain a cleavable transit peptide (24). It was therefore concluded that the protein uses a different import mechanism than Toc75-III, which might be similar to the one used by all other chloroplast OMPs. In analogy to mitochondrial OMP biogenesis, it might be speculated that chloroplast OMPs have to be imported into the IMS via the TOC complex first, before they get assembled into the outer envelope membrane by Toc75-V (14). How the protein crosses the outer envelope membrane (OEM) is currently unknown but similar to Sam50, the N-terminal POTRA domains (three) of Toc75-V are expected to be exposed to the IMS.
Toc75-III (for translocon of the outer envelope membrane of chloroplast protein of 75 kDa encoded on the third chromosome in A. thaliana) is involved in preprotein translocation across the chloroplast outer envelope membrane. Together with the two cytoplasmically exposed receptor GTPases Toc159 and Toc33, Toc75-III forms the TOC core complex (14). Therefore, Toc75-III constitutes the barrel-shaped translocation pore of the complex. Toc75-III is nuclear-encoded as preprotein (p75) targeted to the chloroplast outer envelope by an N-terminal bipartide transit peptide (25, 26), where the N-terminal half displays the canonical signal for nuclear-encoded chloroplast proteins, and which is capable of directing the small subunit of Rubisco into the chloroplast stroma. The C-terminal half is responsible for outer envelope targeting and thought to function as “stop-transfer” sequence to prevent the complete translocation of the protein. After processing by the stromal processing peptidase (SPP) (27), the protein is thought to remain in the intermembrane space (i75). Here it is processed further by an IMS localized type I signal peptidase (28) to its mature form (m75) before it is integrated into the OEM (26, 29). The import pathway of Toc75-III parallels that of mitochondrial OMPs, which would require a topology similar to that of Sam50 with the POTRA domains facing the IMS (14).
Interestingly, an interaction between the POTRA domains of the pea Toc75-III and the in vitro translated pea Toc33 G domain was observed (30). This could indicate either a Toc33-dependent import pathway of Toc75-III or an interaction within the TOC complex, which would then require a reversed topology, contrary to what would be predicted. More recently, data on an Omp85-like protein in the diatom Phaeodactylum tricornutum suggested that at least in complex plastids, both termini might face the outside of the plastid envelope (31, 32). Thus, the available data are contradictory concerning the topology of the chloroplast Omp85 proteins. Because the POTRA domains provide a specific binding site for chloroplast preproteins (30) and display a flexible interface for protein–protein interaction as shown by MD simulations based on the crystal structure of the POTRA domains of alr2269, the cyanobacterial ancestor of Toc75-III (6), both possible topologies are reasonable. The importance of solving this question for the understanding of TOC function becomes apparent when the potential function of the POTRA domains during the translocation event is considered. In the IMS, they could be involved in the handing over of substrate proteins to the IMS or TIC complex. Exposed on the chloroplast surface, the POTRA domains could participate in TOC receptor regulation (33). Here we show that contrary to the current models, the POTRA domains of both Toc75's are exposed to the cytoplasm and discuss the mechanistic consequences on protein function.
Results
GFP-Based in Vivo Topology Assessment of Membrane Proteins.
Because of the paucity of data concerning the topology of chloroplast Omp85 proteins and the importance of the POTRA domain as a central functional element in the models of chloroplast OMP integration/assembly and preprotein import, we decided to investigate the topology of the atToc75-III (AT3G46740) and atToc75-V (AT5G19620). We applied the recently established self-assembling split GFP (saGFP) (34), which has been used to determine membrane protein topology in vivo before (35). Here, the 11-stranded β-barrel of GFP was split into saGFP1–10, comprising β-strands 1–10 (short 1–10), and a saGFP11 (short 11) fragment, containing β-strand 11. Neither fragment was fluorescent by itself, but when located in the same cellular subcompartment, the two fragments assembled into fluorescent GFP (35).
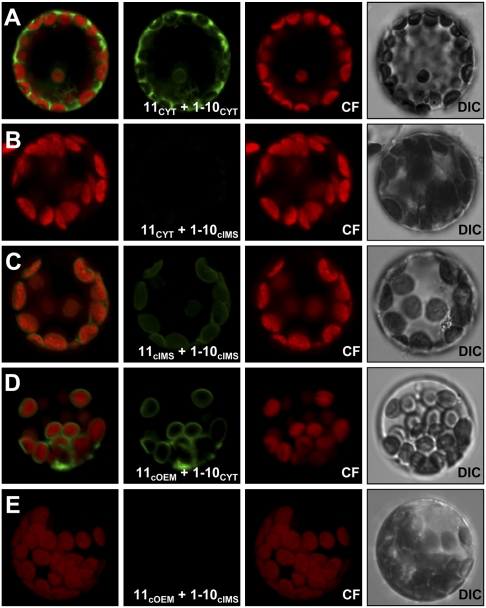
First, we proved that this approach is applicable to the study of membrane protein topology in plant cells. We fused the two fragments N/C terminally to appropriate marker proteins and coexpressed them constitutively in A. thaliana mesophyll protoplasts (Fig. 1). The marker proteins are listed in Table 1. As expected, when both fragments were cytoplasmically expressed (11CYT with 1–10CYT), we saw the typical GFP pattern in the periphery of the cell surrounding the vacuole (Fig. 1A). Consistently, we observed no fluorescence when the 1–10 fragment was targeted to the chloroplast intermembrane space (11CYT with 1–10cIMS; Fig. 1B). Both fragments targeted to the cIMS (11cIMS with 1–10cIMS) again resulted in the expected ring-like fluorescence surrounding the chlorophyll autofluorescence (Fig. 1C).
Fig. 1.
saGFP-based in vivo membrane protein topology assessment in A. thaliana mesophyll protoplasts. (A and C) The two saGFP fragments targeted to the same compartment, either the cytoplasm (11CYT and 1–10CYT) or the intermembrane space (11cIMS and 1–10cIMS), showed the expected ring-like fluorescence surrounding the chloroplast autofluorescence, whereas (B) both fragments expressed in different compartments (11CYT and 1–10cIMS) expectedly showed no GFP signal at all. (D and E) The approved COUT-NIN orientation of Oep7 could be confirmed when the protein C-terminally fused to saGFP 11 (11OEM) was coexpressed with 1–10CYT. (Left to Right) Overlay, GFP fluorescence, chlorophyll fluorescence (CF), differential interference contrast (DIC) images.
Table 1.
List of constructs used in this study
| Name | Gene | Gene no. | Tag | Protein function and localization |
| 11CYT | atHsp18.5 | AT2G19310 | C | Small cytoplasmic heat shock protein of 18.5 kDa (atHsp18.5); cytoplasm |
| 1–10CYT | saGFP1-10 | Self-assembling GFP 1–10 fragment (saGFP); cytoplasm | ||
| 11cOEM | atOep7 | AT3G52420 | C | Outer envelope protein of 7 kDa (atOep7); chloroplast outer envelope membrane |
| 11cIMS | psOep21 | N | Outer envelope protein of 21 kDa (psOep21); intermembrane space | |
| 1–10cIMS | atMgd1 | AT4G31780 | C | Monogalactosyldiacylglycerol synthase 1 (Mgd1); outer leaflet of the inner chloroplast envelope membrane |
| III-11N | atToc75-III | AT3G46740 | N | Translocator of the outer chloroplast membrane protein of 75 kDa III (Toc75III); outer envelope membrane |
| III-11C | atToc75-III | AT3G46740 | C | |
| V-11N | atToc75-V | AT5G19620 | N | Translocator of the outer chloroplast membrane protein of 75 kDa V (Toc75V); outer envelope membrane |
| V-11C | atToc75-V | AT5G19620 | C |
The saGFP11 or 1–10 fragments were either fused C-terminally (C), N-terminally, or internally, between the transit peptide and mature domain of the protein, respectively (N) to the test and reporter proteins. at, Arabidopsis thaliana; ps, Pisum sativum.
Next we confirmed the topology of the well-known monotopic chloroplast outer envelope protein Oep7 by this technique. Oep7 has a single helical transmembrane segment (36–38). When C-terminally fused to the 11 fragment (11cOEM), Oep7 was able to recruit 1–10CYT to the outer envelope membrane, resulting in ring-like fluorescence around the circumference of the chloroplasts (11cOEM with 1–10CYT Fig. 1D), whereas no fluorescence was observed when 1–10 was present in the IMS (11cOEM with 1–10cIMS; Fig. 1E). This confirmed the established topology of Oep7 with its C terminus being exposed to the cytoplasm (36, 37) and proved the validity of this technique for further studies.
POTRA Domains of Both Toc75 Isoforms Are Facing Outward.
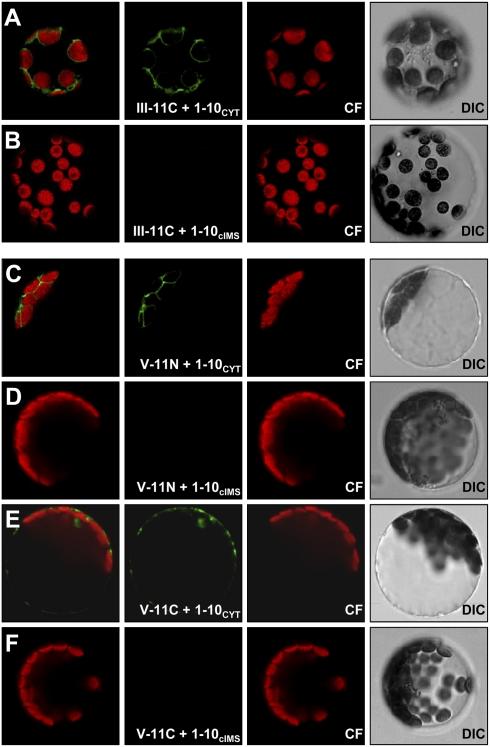
Having shown that the method works for plant membranes, we next investigated the topology of atToc75-III and atToc75-V. Coexpression of 1–10CYT with III-11C, where the 11-fragment was C-terminally fused to atToc75-III, resulted in the same ring-like GFP fluorescence around the circumference of the chloroplasts as for Oep7 (Fig. 2A). Again, no fluorescence was observed when III-11C was coexpressed with 1–10cIMS, the chloroplast IMS marker (Fig. 2B and Table 1). Analogously, coexpression of 1–10CYT with III-11N (where the 11-fragment was inserted between the transit peptide and the mature domain of atToc75-III) showed similar albeit less pronounced fluorescence around the chloroplasts (Fig. S1 A and B). Note that we observed the same fluorescence pattern as for atToc75-III, when the 11-fragment was fused to the N terminus of atToc75V (V-11N) and coexpressed with 1–10CYT (Fig. 2 C vs. D). This clearly demonstrates that the POTRA domains of atToc75-III and atToc75-V are exposed to the cytoplasm. In contrast to atToc75-III, C-terminal fusion of the 11-fragment to atToc75-V (V-11C) resulted in mistargeting and aggregation of the protein in the cytoplasm (Fig. 2 E and F). This implies that the topogenic signal of atToc75-V is located close to its C terminus, as previously described for bacterial and mitochondrial OMPs (20, 39).
Fig. 2.
Topology of the chloroplast Omp85's atToc75-III and atToc75-V. (A and B) atToc75-III and (C–F) atToc75V were C- (III-11C) or N- and C-terminally (V-11N/V-11C) fused to the saGFP11 fragment and coexpressed with the saGFP1–10 fragment in Arabidopsis mesophyll protoplasts targeted either to the cytoplasm (1–10CYT; A, C, and E) or the intermembrane space (1–10cIMS; B, D, and F). (Left to Right) Overlay, GFP fluorescence, chlorophyll fluorescence (CF), differential interference contrast (DIC) images.
Isolated Chloroplast Outer Envelope Vesicles (OEVs) Possess a “Right-Side-Out” Orientation.
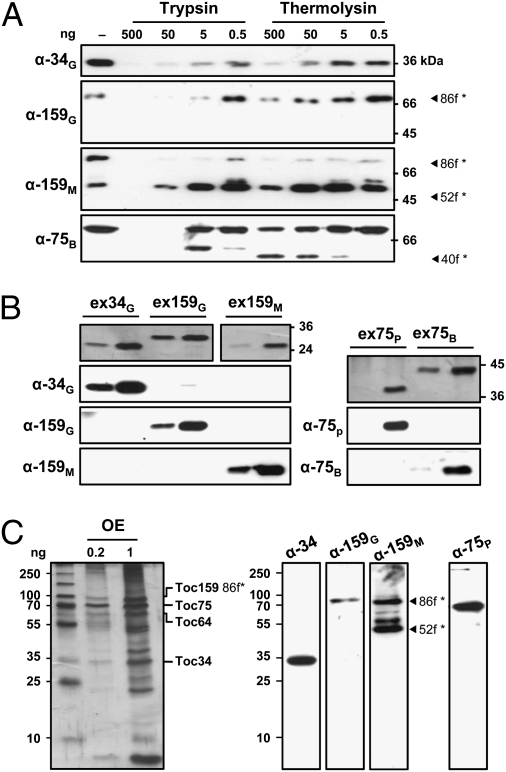
Our in vivo analysis suggested a cytoplasmic exposure of the POTRA domains of the two chloroplast OMP85's. To substantiate this observation we used electron cryotomography (cryo-ET) to determine the topology of psToc75 (the pea atToc75-III homolog) in the context of the TOC complex directly. Pea right-side-out OEVs (38) were isolated and their orientation confirmed by protease treatment (Fig. 3). To this end, OEVs (40) equivalent to ∼10 μg of protein were treated with the (non)membrane-permeable proteases, thermolysin or trypsin (Fig. 3A). After digestion and repurification, the remaining fragment sizes were estimated by immunoblotting with domain-specific antibodies against the different TOC core components psToc34, psToc159 (the latter represented by its 86 kDa fragment (*86f; see ref. 41) lacking its A(cid) domain and psToc75 (Fig. 3 B and C).
Fig. 3.
Isolated pea outer envelope vesicles possess a “right-side-out” orientation. (A) Outer envelope vesicles were treated with the (non)membrane-permeable proteases thermolysin and trypsin and the remaining fragment sizes estimated by immunoblotting. The specificity of the antibodies used was tested against recombinant protein (B) or isolated outer envelope (C). For details see text.
Antibody specificity was tested against recombinant protein or isolated OEVs (Fig. 3 C and D). As expected, all proteins are protease sensitive (Fig. 3A); the amount of detectable G-domain or full-length protein rapidly decreases upon increased protease concentration (lanes α-34G and α-159G). Whereas Toc34 is totally degraded, a protease-resistant 52 kDa (*52f; see ref. 42) fragment displaying the large M(embrane) domain of Toc159 (amino acids 1123–1469) is stabilized (lane α-159M). Control OEVs (−) were not subjected to proteolysis. Note that we only observed a slight reduction of Toc75 when treated with thermolysin (lane α-75B). This phenomenon was observed before and ascribed to the high abundance of the protein (43, 44). The protease sensitivity of the receptors clearly showed the right-side-out orientation of the isolated OEVs.
High Molecular Weight Complexes in Isolated Chloroplast Outer Envelopes.
These right-side-out OEVs were subsequently vitrified for cryo-ET (Figs. 4 and 5). Tomographic data were recorded with a 300 keV FEI Polara electron microscope at liquid nitrogen temperature. We observed protein particles spanning the membrane, which indicated a common, roughly dome-shaped appearance, and occurred at a density of up to 80 particles per μm2. This is in the range expected for the TOC complex (Materials and Methods). The particles had a diameter of 15 ± 1 nm perpendicular to the membrane plane and projected about 9 ± 1 nm from the cytoplasmic face of the outer envelope vesicles (Fig. 4). Taking into account the membrane thickness (∼4 nm), the total height of the complex was estimated as 13 nm, in good agreement with the dimensions of a 3D map previously obtained by single-particle EM (40). Interestingly, additional densities were regularly observed on the side of the complexes facing the IMS. Conceivably these additional densities are proteins bound on the IMS side of the TOC machinery (14).
Fig. 4.
High molecular weight complexes in chloroplast outer envelopes. EM tomograms from plunge-frozen isolated pea chloroplast outer envelope vesicles. (A) Tomographic slice, (B) section (in close-up) through a 3D reconstruction and of a single outer envelope vesicle showing a dome-shaped high molecular weight complex spanning the membrane (red box). (C–F) Sections and corresponding surface representation of two observed complexes. (Scale bars, 15 nm.)
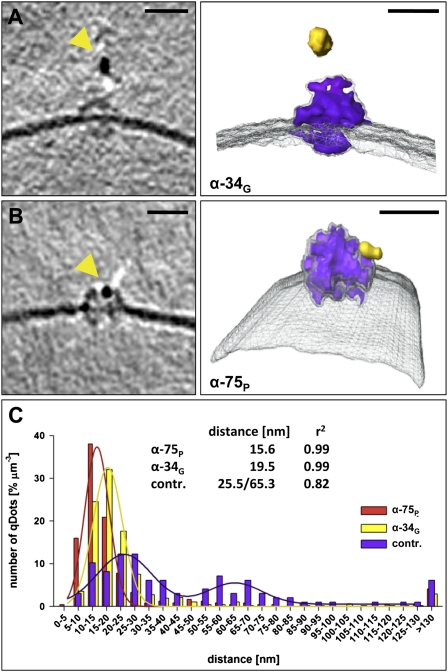
Fig. 5.
Qdot labeling of TOC complexes in outer envelope vesicles. (A and B) Tomographic slices and surface representations of single TOC complexes (purple), specifically labeled by Qdots (yellow) in the presence of domain-specific anti-Toc34 G(TPase) domain (α-34G; A) or anti-Toc75 P(OTRA) (α-75P; B) antibodies. Outer envelope vesicles without primary antibodies were used as control. (C) Quantification of the measured distances between the observed Qdots and the membrane. (Scale bars, 15 nm.)
Cryo-EM Tomography of Membrane Embedded TOC Complexes.
To identify the complexes as TOC, and the topology of Toc75 within them, we incubated OEVs with domain-specific antibodies against the POTRA domain of psToc75 (α-75P) or the cytoplasmic G(TPase) domain of the TOC receptor psToc34 (the pea homolog of Toc33; α-34G; Fig. 3B) and detected them with Qdot conjugated secondary antibodies (negative control is without primary antibody). In both experiments more than 70% of the visible outer membrane complexes were close to a dark, elongated ∼0.5 × 10 nm density of a size and shape characteristic for Qdots (Fig. 5 A and B). In all cases, the Qdots were associated with the cytoplasmic protrusions of the complexes. The center-to-center distance between the Qdots and the complexes was less than 25 nm and therefore well within the expected binding range (Fig. 4C), which can be extrapolated from the dimension of secondary antibody with ∼15 nm, the radius of a Qdot of ∼7.5–10 nm and the radius of the TOC complex of ∼6.5 nm. The specificity of the immunolabeling was confirmed by measuring the distance between the Qdots and the closest membrane (Fig. 4C). Whereas most Qdots where found within 30 nm of the membrane in the presence of a primary antibody, only a small fraction of Qdots were found near a membrane in the negative control. Moreover, in the control, none of the membrane protein complexes appeared to be labeled. Thus, we conclude that the dome-shaped densities in the outer membrane are indeed single TOC complexes. Because both α-34G and α-75P antibodies labeled the cytoplasmic side of the particles efficiently, our data confirm the cytoplasmic exposure of the POTRA domains of atToc75-III. The dimensions measured for the TOC complex by cryo-ET are consistent with structural models placing the POTRA domains on the cytoplasmic face of the complex (Fig. S2). On the basis of our self-assembly split GFP experiments, we propose that the topology of Oep80/Toc75-V has the same topology as Toc75/TOC75-III, with cytoplasmically exposed POTRA domains.
Discussion
The Evolutionary Twist of Omp85 Proteins in Chloroplast Membranes.
Proteins of the Omp85 family have essential functions in the biogenesis of outer membrane proteins and preprotein translocation in Gram-negative bacteria and the organelles descended from them. To understand the exact mechanism of these processes, it is clearly important to assess the topology of these proteins. Due to the paucity of data, especially for chloroplast Omp85's, we combined in vivo and in situ topology approaches to address this question. Self-assembling GFP revealed the differences in the transport paths for Toc75-III and Toc75-V. In contrast to Toc75-III, where saGFP could be fused N- and C terminally to the mature domain, only the N-terminal fusion of saGFP to Toc75-V was found in chloroplast envelopes. The C-terminal fusion of saGFP to Toc75-V resulted in cytoplasmic aggregation (Fig. 2). This observation underlines the different targeting mechanisms by which the two proteins are inserted into the OEM (24). It furthermore might indicate a conservation of the C-terminal signal for proper targeting and membrane insertion, as recently identified in barrel-shaped OMPs from bacteria and mitochondria (e.g., ref. 20). Although yet unidentified, such a signal has been proposed for chloroplast OMPs as well, on the basis of the absence of transit peptides with cytosolic precursors of the protein type (14). Thus, we provide evidence for the existence of this signal, and it can now be identified by the established methods.
The saGFP data further point to an inversion of the topology of Toc75-V with respect to the orientation of the POTRA domains of BamA in bacteria or Sam50 in mitochondria (11, 19). This is unexpected, as chloroplast OMP biogenesis is proposed to occur from the IMS (14), similar to the biogenesis of mitochondrial OMPs. Considering that chloroplast OMPs do not possess canonical targeting signals for passing the OEM (14) and that chloroplast OMPs are synthesized in the cytoplasm, a topological flip of Toc75-V would account for the different translocation site. Thus, chloroplast OMPs—if they use Toc75-V—could be inserted posttranslationally directly into the OEM without the need for a translocation signal.
Equally remarkable was the observation that Toc75-III fused to saGFP shows the same topology as Toc75-V (Fig. 2). This result is supported by the analysis of the topology of Toc75-III within the TOC complex by cryo-ET in situ using right-side-out vesicles as confirmed e.g., by the thermolysin sensitivity of the TOC receptors (Fig. 3). We observed characteristic dome-shaped protein densities (Fig. 4) corresponding to the expected size and abundance of the TOC complex (40, 45). Moreover, these densities reacted with TOC-specific antibodies against both Toc33 and Toc75-III, confirming their assignment as TOC complexes and cytoplasmic exposure of the POTRA domains (Figs. 3 and 5). The latter is in line with the observed structure of the TOC complex by single particle analysis (40), as the dimension of a hypothetical model placing the GTPase and the POTRA domains on the same side of the membrane shows a remarkable similarity to the structure observed, whereas the converse topology is not explained by the available structural information (Fig. S2). The evolutionary flip of Toc75-III is conceivable, given that Toc75-III has changed its evolutionarily conserved function to become the translocation pore of the TOC complex, whose functional direction is opposite to the genuine Omp85 function. The easiest way to achieve such a drastically different function is by an inversion of the topology.
It remains unknown whether the topological flip occurred before or after diversification of Toc75 in plants, but most likely the duplication and relocation of the gene from the plastome to the genome preceeded the change in topology. The flip would have allowed for the import of other nuclear-encoded proteins into the chloroplast by taking advantage of the ability of the POTRA domain to interact with transit peptides (30), and hence to serve as the first receptor of this ancestral “TOC” complex. In line with this idea, Toc75 and Toc159 constitute the essential functional unit of the TOC in vitro (46), whereas the cytoplasmic G domain of Toc159 discussed as part of the preprotein recognizing receptor units is dispensable in vivo (47).
Impact of the Cytosolic POTRA Domain on the Model of Protein Translocation.
No current model of TOC complex function considers the POTRA domain of Toc75-III (e.g., refs. 14, 48), even though this domain is thought to play a central role in all Omp85 proteins. Current functional models consider that incoming precursor proteins are recognized by the GTPases, Toc33 and Toc159, and are subsequently threaded into Toc75-III, a process requiring at least Toc75-III and Toc159 (46). The Toc33/Toc159 receptors belong to the group of dimerizing GTPases (ref. 33 and references therein and ref. 49), which are regulated by homo- or heterodimerization. Thus, it is discussed that dimerization serves as a regulatory circuit of the TOC complex. Recently it has been demonstrated that the homodimeric state of Toc33 displays the inactive ground state of the receptor, which opens after preprotein binding (50). This is believed to be a prerequisite for Toc33/Toc159 heterodimer formation, leading to an activation of the GTPases and a passing over of the preprotein to Toc75-III (33). However, the crystal structures of the Toc33 proteins in their monomeric or dimeric form in complex with the GTP analog GMPPNP or GDP (51, 52) did not reveal the expected typical molecular switch thought to be required for G proteins (reviewed in ref. 53). This led to the assumption that a further factor, a so called co-GAP, is required (51).
The cytoplasmic exposure of the POTRA domains of Toc75-III introduces an additional functional domain on the cytoplasmic side of the TOC complex, where it is in close proximity to Toc33. Thus, the POTRA domain might regulate the GTPase activity of the TOC receptors, because this domain provides a Toc33 binding site (30). This would also explain why Toc33 has to be released from the complex after phosphorylation, which is thought to inactivate the receptor (54). Alternatively or in addition, the POTRA domains with their affinity for precursor proteins (30) could interact with the two known receptors in the perception of the targeting signal in general. The molecular interlink of Toc33 with the POTRA domains within the TOC complex can be seen as a prerequisite for TOC function either by receptor activation or by corecognition of the preprotein. Thus, after our discovery of a third cytoplasmic domain of the TOC complex, the mechanisms of precursor recognition and TOC function have to be revisited.
Materials and Methods
saGFP Construct Generation.
All required genes or gene fragments for self-assembling GFP construct generation were amplified from A. thaliana Col0 or P. sativum var. avica cDNA and cloned by conventional cloning techniques using intermediate vectors (primers are listed in Table S1) into vectors pAVA (55). Templates for the saGFP1–10 or -11 fragments were obtained from G. S. Waldo (Los Alamos National Laboratory, Los Alamos, NM).
Protoplast Isolation/Transfection and saGFP Analyses.
For isolation of mesophyll protoplasts, leaves of 4-wk-old A. thaliana plants were rubbed on K240 sandpaper before incubation with 25 mL of 1% (wt/vol) cellulase R10, 0.3% (wt/vol) macerozyme in MCP (29 mM Mes-KOH pH 5.6, 500 mM sorbitol, 1 mM CaCl2,) for 2 h at 30 °C. Released protoplasts were filtered through a 75-μm nylon mesh, underlayed with 2.5 mL of 100% (vol/vol) Percoll MCP (pH 5.6 containing 5 mM Mes, 500 mM sorbitol, 1 mM CaCl2,) and pelleted at 405 × g for 8 min. Approximately 20 mL of the clear supernatant was removed. The remaining (protoplast containing) fraction was gently mixed with the Percoll cushion to yield approximately 40% (vol/vol) Percoll (final) and was subsequently overlayed with 7.5 mL 25% (vol/vol) Percoll in MCP and 5 mL MCP. After centrifugation at 270 × g for 8 min, the protoplasts were collected [green band between MCP and 25% (vol/vol) Percoll], pelleted at 100 × g for 5 min and diluted in MMg (5 mM Mes-KOH pH 5.6, 400 mM sorbitol, 15 mM MgCl2) to a cell number of 106 cells per mL. For transfection, 100 μL protoplasts were mixed with 10 μg pDNA per construct and 100 μL 40% (wt/vol) PEG-4000, 100 mM Ca(NO3)2, 400 mM sorbitol and incubated for 20 min at RT, before the reaction was stopped using 1 mL of K3 (20 mM Mes-KOH pH 5.6, 400 mM sucrose, 1 mM CaCl2, MS salts). GFP and chloroplast autofluorescence was monitored by confocal laser scanning microscopy using a TCS SP5 microscope (Leica) with a HCX PL APO CS 40× 1.25 NA 1.25 oil objective. Fluorescence was excited and detected as follows: GFP 488 nm/505–525 nm, chlorophyll fluorescence 514 nm/650–750 nm. Imaris x64 6.2.1 (Bitplane) software was used for image processing.
Isolation of Pea OEVs and Protease Treatment.
Isolation and protease treatment of pea outer chloroplast envelope vesicles was performed (according to refs. 38 and 40). Domain-specific antisera were raised and purified against recombinant His-tagged pea TOC proteins (ex34G aa M1-G266, ex159G aa M740-N1048, ex159M aa F1123-Y1469, ex75P aa T149-L439 or ex75B aa E440-Y809) at Pineda Antibody Service.
Electron Cryotomography and Immunodecoration of TOC Complexes.
Electron cryotomography of pea outer envelope vesicles was carried out as described before (56). For in situ labeling of TOC complexes before cryo-ET, outer envelope equivalent to approximately 50 nM of TOC complex was labeled with 125 nM (final) purified primary antibody (rabbit α-34G, α-75P, or buffer as control) in 200 μL PBS overnight at 4 °C under gentle agitation, before 175 nM (final) of secondary antibody (Qdot 525 coupled goat α-rabbit F(ab′)2 IgG, Invitrogen) was added. After an additional 1 h of incubation at 4 °C in darkness, unbound antibody was removed by pelleting the membranes for 5 min at 25,000 × g, 4 °C and repeated washes with fresh ice-cold PBS. The final pellet was resuspended in 20 μL of PBS and directly mixed 1:1 with 6 nm colloidal gold fiducials (Aurion). A total of 3 μL of the solution was applied to previously glow-discharged carbon-coated Quantifoil grids (R2/2). The grids were vitrified in liquid ethane using a home-made gravity-driven plunge-freezing device. Tomograms were collected at 300 kV in a FEI Polara electron microscope equipped with a 2k × 2k CCD camera (Gatan) and a postcolumn energy filter (GIF Tridiem 863; Gatan), operating at a slit width of 20 eV for zero loss energy filtering. Single axis tomograms were collected between ±60°, with a step size of 1.5° and a defocus of 7 μm at a magnification of 41,000. Tomograms were collected under low-dose conditions using the FEI automatic tomogram collection software. Tomographic volumes were reconstructed with IMOD software (57). Contrast was enhanced by nonlinear anisotropic diffusion (58) and segmentation was performed using Amira software (Mercury Systems).
Estimation of Number of TOC Complexes in a Given Membrane Area.
The number of TOC complexes we would expect in a defined membrane area of a chloroplast/outer envelope vesicle was estimated as follows. Given that the chloroplast is 5 μm in length (a) and 1–2 μm in width (b) (59), the surface of a chloroplast using an ellipsoid for approximation (E1) is 1,000 μm2. By immunodecoration 20,000 TOC complexes were identified on the surface of a single chloroplast (45), of which 10% are synchronously active (60). Accordingly, we would expect 20 (averaged) complexes per μm2. Considering that the isolation procedure is designed to enrich the TOC complex (40), up to 100 complexes per μm2 are estimated.
Supplementary Material
Acknowledgments
We thank G. S. Waldo (Los Alamos National Laboratory, Los Alamos, NM) for providing templates for the self-assembling GFP and D. Mills for maintenance of the EM facility. This study was supported by the Deutsche Forschungsgemeinschaft (SFB-807 and Cluster of Excellence Macromolecular Complexes to W.K. and E.S.), Graduate School 1216 (to U.-G.M.), the Center of Membrane Proteomics Frankfurt (E.S.), and the Max Planck Foundation (W.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108626108/-/DCSupplemental.
References
- 1.Bredemeier R, et al. Functional and phylogenetic properties of the pore-forming beta-barrel transporters of the Omp85 family. J Biol Chem. 2007;282:1882–1890. doi: 10.1074/jbc.M609598200. [DOI] [PubMed] [Google Scholar]
- 2.Gentle I, Gabriel K, Beech P, Waller R, Lithgow T. The Omp85 family of proteins is essential for outer membrane biogenesis in mitochondria and bacteria. J Cell Biol. 2004;164:19–24. doi: 10.1083/jcb.200310092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim S, et al. Structure and function of an essential component of the outer membrane protein assembly machine. Science. 2007;317:961–964. doi: 10.1126/science.1143993. [DOI] [PubMed] [Google Scholar]
- 4.Knowles TJ, Scott-Tucker A, Overduin M, Henderson IR. Membrane protein architects: The role of the BAM complex in outer membrane protein assembly. Nat Rev Microbiol. 2009;7:206–214. doi: 10.1038/nrmicro2069. [DOI] [PubMed] [Google Scholar]
- 5.Sánchez-Pulido L, Devos D, Genevrois S, Vicente M, Valencia A. POTRA: A conserved domain in the FtsQ family and a class of beta-barrel outer membrane proteins. Trends Biochem Sci. 2003;28:523–526. doi: 10.1016/j.tibs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Koenig P, et al. Conserved properties of polypeptide transport-associated (POTRA) domains derived from cyanobacterial Omp85. J Biol Chem. 2010;285:18016–18024. doi: 10.1074/jbc.M110.112649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sklar JG, et al. Lipoprotein SmpA is a component of the YaeT complex that assembles outer membrane proteins in Escherichia coli. Proc Natl Acad Sci USA. 2007;104:6400–6405. doi: 10.1073/pnas.0701579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu T, et al. Identification of a multicomponent complex required for outer membrane biogenesis in Escherichia coli. Cell. 2005;121:235–245. doi: 10.1016/j.cell.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Clantin B, et al. Structure of the membrane protein FhaC: A member of the Omp85-TpsB transporter superfamily. Science. 2007;317:957–961. doi: 10.1126/science.1143860. [DOI] [PubMed] [Google Scholar]
- 10.Gatzeva-Topalova PZ, Warner LR, Pardi A, Sousa MC. Structure and flexibility of the complete periplasmic domain of BamA: The protein insertion machine of the outer membrane. Structure. 2010;18:1492–1501. doi: 10.1016/j.str.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 12.Su YC, Wan KL, Mohamed R, Nathan S. Immunization with the recombinant Burkholderia pseudomallei outer membrane protein Omp85 induces protective immunity in mice. Vaccine. 2010;28:5005–5011. doi: 10.1016/j.vaccine.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Paschen SA, et al. Evolutionary conservation of biogenesis of beta-barrel membrane proteins. Nature. 2003;426:862–866. doi: 10.1038/nature02208. [DOI] [PubMed] [Google Scholar]
- 14.Schleiff E, Becker T. Common ground for protein translocation: Access control for mitochondria and chloroplasts. Nat Rev Mol Cell Biol. 2011;12:48–59. doi: 10.1038/nrm3027. [DOI] [PubMed] [Google Scholar]
- 15.Baldwin A, et al. A molecular-genetic study of the Arabidopsis Toc75 gene family. Plant Physiol. 2005;138:715–733. doi: 10.1104/pp.105.063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel R, Hsu SC, Bédard J, Inoue K, Jarvis P. The Omp85-related chloroplast outer envelope protein OEP80 is essential for viability in Arabidopsis. Plant Physiol. 2008;148:235–245. doi: 10.1104/pp.108.122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozjak V, et al. An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem. 2003;278:48520–48523. doi: 10.1074/jbc.C300442200. [DOI] [PubMed] [Google Scholar]
- 18.Becker T, Gebert M, Pfanner N, van der Laan M. Biogenesis of mitochondrial membrane proteins. Curr Opin Cell Biol. 2009;21:484–493. doi: 10.1016/j.ceb.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Habib SJ, et al. The N-terminal domain of Tob55 has a receptor-like function in the biogenesis of mitochondrial beta-barrel proteins. J Cell Biol. 2007;176:77–88. doi: 10.1083/jcb.200602050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kutik S, et al. Dissecting membrane insertion of mitochondrial beta-barrel proteins. Cell. 2008;132:1011–1024. doi: 10.1016/j.cell.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Carrie C, Murcha MW, Whelan J. An in silico analysis of the mitochondrial protein import apparatus of plants. BMC Plant Biol. 2010;10:249. doi: 10.1186/1471-2229-10-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moslavac S, et al. Conserved pore-forming regions in polypeptide-transporting proteins. FEBS J. 2005;272:1367–1378. doi: 10.1111/j.1742-4658.2005.04569.x. [DOI] [PubMed] [Google Scholar]
- 23.Soll J, Schleiff E. Protein import into chloroplasts. Nat Rev Mol Cell Biol. 2004;5:198–208. doi: 10.1038/nrm1333. [DOI] [PubMed] [Google Scholar]
- 24.Inoue K, Potter D. The chloroplastic protein translocation channel Toc75 and its paralog OEP80 represent two distinct protein families and are targeted to the chloroplastic outer envelope by different mechanisms. Plant J. 2004;39:354–365. doi: 10.1111/j.1365-313X.2004.02135.x. [DOI] [PubMed] [Google Scholar]
- 25.Tranel PJ, Froehlich J, Goyal A, Keegstra K. A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO J. 1995;14:2436–2446. doi: 10.1002/j.1460-2075.1995.tb07241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tranel PJ, Keegstra K. A novel, bipartite transit peptide targets OEP75 to the outer membrane of the chloroplastic envelope. Plant Cell. 1996;8:2093–2104. doi: 10.1105/tpc.8.11.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanderVere PS, Bennett TM, Oblong JE, Lamppa GK. A chloroplast processing enzyme involved in precursor maturation shares a zinc-binding motif with a recently recognized family of metalloendopeptidases. Proc Natl Acad Sci USA. 1995;92:7177–7181. doi: 10.1073/pnas.92.16.7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue K, et al. Complete maturation of the plastid protein translocation channel requires a type I signal peptidase. J Cell Biol. 2005;171:425–430. doi: 10.1083/jcb.200506171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue K, Demel R, de Kruijff B, Keegstra K. The N-terminal portion of the preToc75 transit peptide interacts with membrane lipids and inhibits binding and import of precursor proteins into isolated chloroplasts. Eur J Biochem. 2001;268:4036–4043. doi: 10.1046/j.1432-1327.2001.02316.x. [DOI] [PubMed] [Google Scholar]
- 30.Ertel F, et al. The evolutionarily related beta-barrel polypeptide transporters from Pisum sativum and Nostoc PCC7120 contain two distinct functional domains. J Biol Chem. 2005;280:28281–28289. doi: 10.1074/jbc.M503035200. [DOI] [PubMed] [Google Scholar]
- 31.Bullmann L, et al. Filling the gap, evolutionarily conserved Omp85 in plastids of chromalveolates. J Biol Chem. 2010;285:6848–6856. doi: 10.1074/jbc.M109.074807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wunder T, Martin R, Löffelhardt W, Schleiff E, Steiner JM. The invariant phenylalanine of precursor proteins discloses the importance of Omp85 for protein translocation into cyanelles. BMC Evol Biol. 2007;7:236. doi: 10.1186/1471-2148-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sommer MS, Schleiff E. Molecular interactions within the plant TOC complex. Biol Chem. 2009;390:739–744. doi: 10.1515/BC.2009.051. [DOI] [PubMed] [Google Scholar]
- 34.Cabantous S, Terwilliger TC, Waldo GS. Protein tagging and detection with engineered self-assembling fragments of green fluorescent protein. Nat Biotechnol. 2005;23:102–107. doi: 10.1038/nbt1044. [DOI] [PubMed] [Google Scholar]
- 35.van Dooren GG, Tomova C, Agrawal S, Humbel BM, Striepen B. Toxoplasma gondii Tic20 is essential for apicoplast protein import. Proc Natl Acad Sci USA. 2008;105:13574–13579. doi: 10.1073/pnas.0803862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schleiff E, Tien R, Salomon M, Soll J. Lipid composition of outer leaflet of chloroplast outer envelope determines topology of OEP7. Mol Biol Cell. 2001;12:4090–4102. doi: 10.1091/mbc.12.12.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salomon M, Fischer K, Flügge UI, Soll J. Sequence analysis and protein import studies of an outer chloroplast envelope polypeptide. Proc Natl Acad Sci USA. 1990;87:5778–5782. doi: 10.1073/pnas.87.15.5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waegemann K, Eichacker S, Soll J. Outer envelope membranes from chloroplasts are isolated as right-side-out vesicles. Planta. 1992;187:89–94. doi: 10.1007/BF00201628. [DOI] [PubMed] [Google Scholar]
- 39.Robert V, et al. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006;4:e377. doi: 10.1371/journal.pbio.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schleiff E, Soll J, Küchler M, Kühlbrandt W, Harrer R. Characterization of the translocon of the outer envelope of chloroplasts. J Cell Biol. 2003;160:541–551. doi: 10.1083/jcb.200210060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bölter B, May T, Soll J. A protein import receptor in pea chloroplasts, Toc86, is only a proteolytic fragment of a larger polypeptide. FEBS Lett. 1998;441:59–62. doi: 10.1016/s0014-5793(98)01525-7. [DOI] [PubMed] [Google Scholar]
- 42.Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. A receptor component of the chloroplast protein translocation machinery. Science. 1994;266:1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- 43.Becker T, et al. Preprotein recognition by the Toc complex. EMBO J. 2004;23:520–530. doi: 10.1038/sj.emboj.7600089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sveshnikova N, Grimm R, Soll J, Schleiff E. Topology studies of the chloroplast protein import channel Toc75. Biol Chem. 2000;381:687–693. doi: 10.1515/BC.2000.089. [DOI] [PubMed] [Google Scholar]
- 45.Morin XK, Soll J. Immunogold labelling of cryosectioned pea chloroplasts and initial localization of the proteins associated with the protein import machinery. Planta. 1997;201:119–127. [Google Scholar]
- 46.Schleiff E, Jelic M, Soll J. A GTP-driven motor moves proteins across the outer envelope of chloroplasts. Proc Natl Acad Sci USA. 2003;100:4604–4609. doi: 10.1073/pnas.0730860100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee KH, Kim SJ, Lee YJ, Jin JB, Hwang I. The M domain of atToc159 plays an essential role in the import of proteins into chloroplasts and chloroplast biogenesis. J Biol Chem. 2003;278:36794–36805. doi: 10.1074/jbc.M304457200. [DOI] [PubMed] [Google Scholar]
- 48.Jarvis P. Targeting of nucleus-encoded proteins to chloroplasts in plants. New Phytol. 2008;179:257–285. doi: 10.1111/j.1469-8137.2008.02452.x. [DOI] [PubMed] [Google Scholar]
- 49.Oreb M, Tews I, Schleiff E. Policing Tic ‘n’ Toc, the doorway to chloroplasts. Trends Cell Biol. 2008;18:19–27. doi: 10.1016/j.tcb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Oreb M, et al. Substrate binding disrupts dimerization and induces nucleotide exchange of the chloroplast GTPase Toc33. Biochem J. 2011;436:313–319. doi: 10.1042/BJ20110246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koenig P, et al. The GTPase cycle of the chloroplast import receptors Toc33/Toc34: Implications from monomeric and dimeric structures. Structure. 2008;16:585–596. doi: 10.1016/j.str.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Sun YJ, et al. Crystal structure of pea Toc34, a novel GTPase of the chloroplast protein translocon. Nat Struct Biol. 2002;9:95–100. doi: 10.1038/nsb744. [DOI] [PubMed] [Google Scholar]
- 53.Gasper R, Meyer S, Gotthardt K, Sirajuddin M, Wittinghofer A. It takes two to tango: Regulation of G proteins by dimerization. Nat Rev Mol Cell Biol. 2009;10:423–429. doi: 10.1038/nrm2689. [DOI] [PubMed] [Google Scholar]
- 54.Oreb M, Höfle A, Mirus O, Schleiff E. Phosphorylation regulates the assembly of chloroplast import machinery. J Exp Bot. 2008;59:2309–2316. doi: 10.1093/jxb/ern095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.von Arnim AG, Deng XW, Stacey MG. Cloning vectors for the expression of green fluorescent protein fusion proteins in transgenic plants. Gene. 1998;221:35–43. doi: 10.1016/s0378-1119(98)00433-8. [DOI] [PubMed] [Google Scholar]
- 56.Daum B, Nicastro D, Austin J, 2nd, McIntosh JR, Kühlbrandt W. Arrangement of photosystem II and ATP synthase in chloroplast membranes of spinach and pea. Plant Cell. 2010;22:1299–1312. doi: 10.1105/tpc.109.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 58.Frangakis AS, Hegerl R. Noise reduction in electron tomographic reconstructions using nonlinear anisotropic diffusion. J Struct Biol. 2001;135:239–250. doi: 10.1006/jsbi.2001.4406. [DOI] [PubMed] [Google Scholar]
- 59.Buchanan BB, Gruissem W, Jones RL, editors. Biochemistry and Molecular Biology of Plants. 6th Ed. Rockville, MD: American Society of Plant Biologists; 2006. [Google Scholar]
- 60.Friedman AL, Keegstra K. Chloroplast protein import : Quantitative analysis of precursor binding. Plant Physiol. 1989;89:993–999. doi: 10.1104/pp.89.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.