Abstract
Skeletal muscle insulin resistance has been implicated in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) and atherogenic dyslipidemia associated with the metabolic syndrome by altering the distribution pattern of postprandial energy storage. We conducted a study to examine this hypothesis by reversing muscle insulin resistance with a single bout of exercise and measuring hepatic de novo lipogenesis and hepatic triglyceride synthesis after a carbohydrate-rich meal. We studied 12 healthy, young, lean, insulin resistant individuals in an interventional, randomized cross-over trial. The response to the ingestion of a carbohydrate-rich meal was studied at rest and after one 45-min bout of exercise on an elliptical trainer. Hepatic de novo lipogenesis was assessed by using 2H2O, and changes in glycogen and fat content in liver and muscle were measured by 13C and 1H magnetic resonance spectroscopy, respectively. Exercise resulted in a greater than threefold increase in postprandial net muscle glycogen synthesis (P < 0.001), reflecting improved muscle insulin responsiveness, and a ≈40% reduction (P < 0.05) in net hepatic triglyceride synthesis. These changes in whole body energy storage were accompanied by a ≈30% decrease in hepatic de novo lipogenesis (P < 0.01) and were independent of changes in fasting or postprandial plasma glucose and insulin concentrations. These data demonstrate that skeletal muscle insulin resistance is an early therapeutic target for the treatment and prevention of atherogenic dyslipidemia and NAFLD in young insulin resistant individuals who are prone to develop the metabolic syndrome and type 2 diabetes.
Keywords: liver triglyceride, nuclear magnetic resonance spectroscopy
The metabolic syndrome is characterized by a clustering of risk factors for cardiovascular disease that include abdominal obesity, atherogenic dyslipidemia, hypertension, hyperuricemia, a prothrombotic state, a proinflammatory state, nonalcoholic fatty liver disease (NAFLD), and insulin resistance (1, 2). The metabolic syndrome is estimated to afflict 50 million Americans, and approximately half of all Americans are predisposed to it (1). Individuals with the metabolic syndrome are at increased risk for the development of coronary heart disease and other diseases related to plaque buildup in artery walls, such as stroke and peripheral vascular disease, as well as type 2 diabetes. However, the underlying mechanism(s) responsible for these cardiovascular risk factors associated with the metabolic syndrome are not fully understood and appear to be complex.
In this regard, insulin resistance, localized to skeletal muscle, has been hypothesized to cause atherogenic dyslipidemia and NAFLD by changing the pattern of storage of ingested carbohydrate away from skeletal muscle glycogen synthesis into hepatic de novo lipogenesis, resulting in an increase in plasma triglyceride concentrations, reduction in plasma high-density lipoprotein concentrations and increased liver triglyceride synthesis in healthy, young, lean insulin resistant individuals (3). This hypothesis has important implications for the treatment of hyperlipidemia and NAFLD associated with the metabolic syndrome in that it implicates skeletal muscle insulin resistance as a primary therapeutic target.
To directly examine this hypothesis, we examined whether this altered pattern of postprandial energy storage could be reversed in a similar group of young lean insulin-resistant individuals after one bout of exercise. Previous studies have shown that a single bout of exercise can normalize insulin-stimulated muscle glycogen synthesis by increasing insulin-stimulated glucose transport activity in muscle (4).
We assessed liver and skeletal muscle triglyceride synthesis by 1H magnetic resonance spectroscopy (MRS) and liver and muscle glycogen synthesis by 13C MRS after ingestion of a carbohydrate-rich meal before and after one 45-min bout of exercise on an elliptical trainer. Additionally, we measured hepatic de novo lipogenesis by monitoring the incorporation of deuterium from deuterium-labeled water into plasma very-low-density lipoprotein (VLDL) triglycerides (5, 6). The advantage of studying this group of healthy, young, lean insulin resistant subjects is that they do not have the confounding factors typically associated with the metabolic syndrome (e.g., obesity, hypertension, and medications) and the earliest factors responsible for the pathogenesis of atherogenic dyslipidemia and NAFLD associated with the metabolic syndrome can be examined.
Results
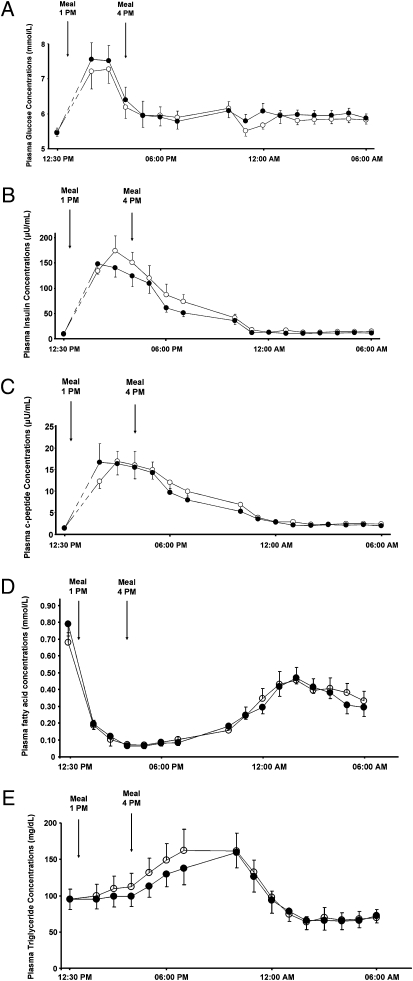
All subjects were young, lean, and insulin resistant as defined by an insulin sensitivity index (ISI) below 3.9 × 104 dL/min per microunit/mL, which is in the lower quartile of the ISI distribution for healthy, young, lean, sedentary individuals (7) (Table 1). Fasting plasma glucose (5.1 ± 0.1 vs. 5.0 ± 0.2 mmol/L) and fasting plasma insulin (9.2 ± 0.9 vs. 9.3 ± 1.0 microunits/mL) as well as postprandial plasma glucose, insulin, and C-peptide concentrations were similar at both visits (Fig. 1 A–C). Similarly, fasting and postprandial plasma triglyceride and plasma fatty acid concentration did not differ between study days (Fig. 1 D and E). Plasma triglyceride concentrations increased as a result of the meals and decreased during the night, whereas plasma fatty acid concentrations were suppressed by food intake and increased during the night (Fig. 1 D and E). Mean liver and muscle glycogen concentrations were 105 ± 9 mmol/L and 168 ± 12 mmol/L, respectively, on the morning of the resting studies and 78 ± 6 mmol/L and 152 ± 13 mmol/L, respectively, on the morning after the exercise studies. Mean intramyocellular lipid (IMCL) and intrahepatic lipid content (IHL) were 1.29 ± 0.11% and 0.81 ± 0.21%, respectively, on the morning of the resting studies and 1.10 ± 0.07% and 0.76 ± 0.21%, respectively, on the morning after the exercise studies.
Table 1.
Subject baseline characteristics
| Baseline characteristics | |
| No. of subjects | 12 |
| Age, yr | 24 ± 1 |
| Body weight, kg | 77.8 ± 2.5 |
| Body Mass Index, kg/m2 | 23.8 ± 0.7 |
| Body fat, % | 16.5 ± 1.6 |
| Lean body mass, kg | 64.8 ± 1.8 |
| Insulin Sensitivity Index, dL/min per microunits/mL | 2.7 ± 0.1 |
| Activity, miles per day | 2.3 ± 0.2 |
Fig. 1.
(A) Plasma concentrations of glucose in response to two carbohydrate-rich mixed meals ingested at 1 PM and 4 PM resting study (○) and exercise study (●). (B) Plasma concentrations of insulin in response to two carbohydrate-rich mixed meals ingested at 1 PM and 4 PM resting study (○) and exercise study (●). (C) Plasma concentrations of c-peptide in response to two carbohydrate-rich mixed meals ingested at 1 PM and 4 PM resting study (○) and exercise study (●). (D) Plasma concentrations of fatty acids in response to two carbohydrate-rich mixed ingested served at 1 PM and 4 PM resting study (○) and exercise study (●). (E) Plasma concentrations of triglycerides in response to two carbohydrate-rich mixed meals ingested at 1 PM and 4 PM resting study (○) and exercise study (●).
The respiratory quotient (RQ) measured by indirect calorimetry increased similarly after the meals during the resting visit (from 0.81 ± 0.02 to 0.93 ± 0.01; P < 0.001) and during the exercise visit (from 0.82 ± 0.01 to 0.92 ± 0.01; P < 0.001). There were no differences in the change in RQ between the baseline visit and the exercise visit (0.11 ± 0.02 vs. 0.10 ± 0.02; P = not significant). Basal and postprandial rates of oxygen consumption (VO2) and rates of carbon dioxide expiration (VCO2) did not differ between study days.
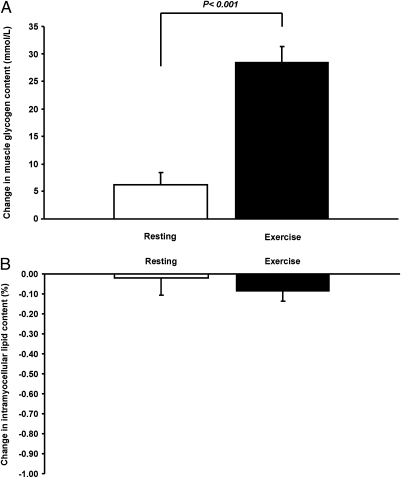
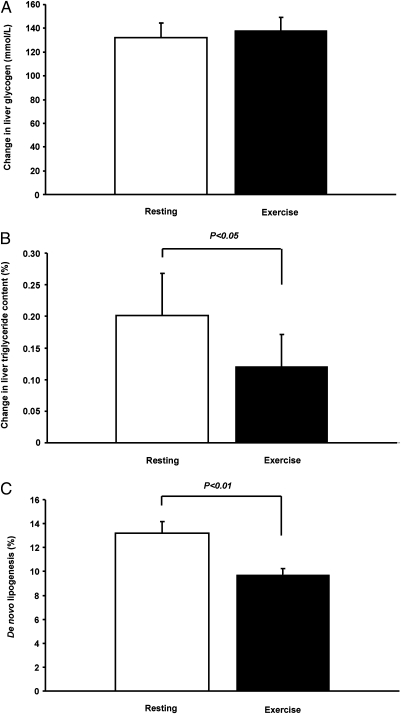
The single bout of exercise resulted in a more than threefold increase in postprandial muscle glycogen synthesis (from 6.2 ± 2.3 to 28.5 ± 2.9 mmol/L muscle; P < 0.001) (Fig. 2) and a 40% reduction in hepatic triglyceride synthesis (Fig. 3). Postprandial hepatic glycogen synthesis did not differ between the resting and the exercise study days (Fig. 3). The single bout of exercise also resulted in a 27% reduction in hepatic de novo lipogenesis after carbohydrate ingestion (from 13.2 ± 1.0 to 9.7 ± 0.5%; P < 0.01) (Fig. 3). Intramyocellular lipid content did not differ between baseline and exercise measurements, and did not change as a result of the meals (Fig. 2).
Fig. 2.
(A) 13C MRS measurements of changes in muscle glycogen concentrations for the resting and exercise study. (B) 1H MRS measurements of changes in intramyocellular lipid concentration for the resting and exercise study.
Fig. 3.
(A) 13C MRS measurements of changes in liver glycogen concentrations for the resting and exercise study. (B) 1H MRS measurements of changes in liver triglyceride content for the resting and exercise study. (C) Hepatic de novo lipogenesis for the resting and exercise study.
Discussion
We found that reversal of muscle insulin resistance, with one bout of exercise, increased postprandial muscle glycogen synthesis and decreased postprandial hepatic de novo lipogenesis and hepatic triglyceride synthesis in young, lean, insulin resistant individuals. Taken together, these data demonstrate that a single bout of exercise can have a profound effect on the distribution and metabolic fate of ingested carbohydrate by diverting ingested carbohydrate away from the liver and into the muscle, thereby reducing hepatic de novo lipogenesis and net hepatic triglyceride synthesis. This study offers strong evidence in support of the hypothesis that muscle insulin resistance promotes the early development of atherogenic dyslipidemia and NAFLD and that it is an early factor in the pathogenesis of the metabolic syndrome (3).
The advantage of examining this question in healthy, young, lean, insulin-resistant individuals is that they have none of the other confounding factors that are typically associated with obesity and type 2 diabetes, which have been postulated to play a major role in causing the metabolic syndrome. Therefore, the role of skeletal muscle insulin resistance per se in the pathogenesis of atherogenic dyslipidemia can be examined at its earliest stages. Furthermore, previous studies have demonstrated that insulin resistance in these subjects can mostly be attributed to defects in insulin-stimulated muscle glycogen synthesis caused by defects in insulin-stimulated glucose transport activity in skeletal muscle (8). We found that one bout of exercise increased postprandial muscle glycogen synthesis more than threefold. These results are consistent with our prior studies that found that one bout of exercise increased insulin-stimulated muscle glycogen synthesis by ≈70% in young, lean, insulin resistant individuals during a hyperinsulinemic-hyperglycemic clamp (4). Using 31P NMR to monitor intramyocellular glucose-6-phosphate concentrations, it could be demonstrated that this improvement in insulin-stimulated muscle glycogen synthesis could be attributed to reversal of defects in insulin-stimulated muscle glucose transport/phosphorylation activity (9).
Exercise did not induce changes in postprandial hepatic glycogen synthesis in our young, insulin resistant subjects. Taken together these findings support the hypothesis that skeletal muscle insulin resistance precedes hepatic insulin resistance in young, lean, insulin resistant individuals and that muscle insulin resistance leads to postprandial increases in net hepatic triglyceride synthesis, potentially leading to NAFLD. NAFLD is now the most common liver disease in both adults and children, and hepatic insulin resistance is strongly linked to NAFLD, which is a major factor responsible for the transition from normoglycemia to impaired glucose tolerance and type 2 diabetes (10–14).
It is also noteworthy that we, despite observing major differences in intra- and interorgan postprandial transfer of metabolic energy in the form of glucose and lipid after exercise, did not find any detectable differences in fasting or postprandial plasma glucose, fatty acids, triglycerides, insulin, and C-peptide concentrations. These observations demonstrate the importance of monitoring intracellular processes in an organ-dependent fashion for delineating the pathophysiology of the metabolic syndrome.
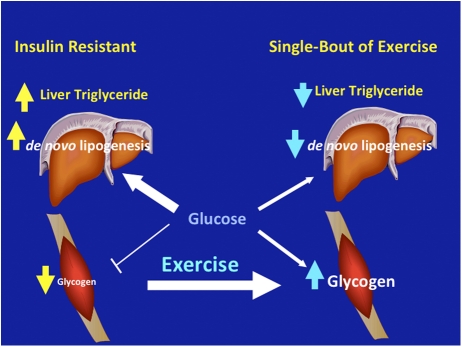
In conclusion, we have shown that one bout of exercise improved postprandial skeletal muscle glycogen synthesis and decreased postprandial de novo lipogenesis and hepatic triglyceride synthesis in young, lean, insulin resistant individuals (Fig. 4). These data demonstrate that muscle specific insulin resistance, due to reduced insulin-stimulated muscle glycogen synthesis, alters the distribution pattern of postprandial energy storage resulting in increased hepatic de novo lipogenesis and increased net hepatic trigylceride synthesis. Chronically these processes will lead to hypertriglyceridemia, reductions in plasma HDL concentrations, and NAFLD. Taken together these data suggest muscle insulin resistance is one of the earliest factors in the pathogenesis of the metabolic syndrome and reversing muscle insulin resistance, by exercise or pharmacological intervention, may be an excellent therapeutic strategy for the treatment and prevention of atherogenic dyslipidemia and NAFLD in young insulin resistance individuals who are prone to develop the metabolic syndrome and type 2 diabetes.
Fig. 4.
Schematic of whole-body energy distribution after a carbohydrate-rich meal at rest and after one bout of exercise in young, lean, insulin resistant subjects.
Methods
Twelve young (age: 24 ± 1 y), lean (body mass index: 23.8 ± 0.7 kg/m2), insulin resistant volunteers (10 Caucasian/2 African-American) were identified from a group of n = 88 healthy, lean, nonsmoking, male volunteers with normal birth weight, normal glucose tolerance, and a sedentary lifestyle. Insulin sensitivity was assessed by the ISI (15), and subjects were selected from the lower quartile of the ISI distribution (3).
All subjects completed a diet history and physical activity questionnaires (16), and their sedentary lifestyle was confirmed by activity monitoring for three consecutive days by using pedometers. Body composition was measured by bioelectrical impedance (Tanita BC-418; Tanita). All meals and snacks were provided for 3 d before each study to ensure that all subjects consumed a eucaloric diet containing 55% carbohydrate, 10% protein, 35% fat. These meals consisted of regular food and were prepared by the Metabolic Kitchen of the Yale Center for Clinical Investigation Hospital Research Unit (HRU) (17).
On study day 1, the subjects were admitted to the HRU at 5 PM, served dinner at 6 PM (33% of their daily caloric intake), and remained fasting until the first meal the following day (Day 2). On Day 2 at 8:30 AM, the subjects were transported in a wheelchair to the Yale Magnetic Resonance Research Center (MRRC) for measurements of baseline muscle and liver lipid and glycogen content by using 1H/13C MRS. These measurements were begun at 9 AM and completed by 11:30 AM. The subjects were then returned to the HRU where an i.v. line was inserted into an antecubital vein and baseline blood samples were collected. The liquid high carbohydrate meal was served in two portions of equal size at 1 PM and 4 PM. The meals were prepared by the HRU Metabolic Kitchen and contained all of the required daily energy (35 kcal/kg body weight, 55% carbohydrate, 10% protein, 35% fat) with an additional 25% of the daily energy requirements added in the form of sucrose (3). At 2 PM and 2:30 PM, loading doses of deuterium-labeled water (2H2O) were given orally (3 mL 2H2O per kg of body water; 99.8%, Cambridge Isotopes). Plasma deuterium enrichment was maintained by providing the subjects with deuterium-labeled water (0.45% 2H2O) for ad lib drinking during the remaining part of the study.
At 7:30 PM, the subjects were returned to the MRRC and, at 8 PM, postprandial muscle and liver lipid and glycogen contents were measured by using 1H/13C MRS. The subjects stayed in bed throughout the study (except for bathroom breaks), and all transport to and from the MRRC was done by wheelchair.
The exercise study included a total of 45 min of exercise on an elliptical trainer from 7 AM to 8 AM on Day 2 before the baseline measurements of liver and muscle glycogen and lipid content. The subjects performed three sets of 15 min of exercise on a Nautilus NE 2000 elliptical trainer (Nautilus) at 75–85% of their calculated maximal heart rate. The subjects were allowed 5 min of rest between sets. The order of the exercise study was randomized to control for a potential effect of adaptation to the study protocol.
On both the resting and the exercise study day, indirect calorimetry (Deltatrac; Datex-Ohmeda) was performed in the fasted state (at 6 AM on Day 2) and in the postprandial state (at 6 PM on Day 2).
Hepatic de Novo Lipogenesis.
The incorporation of deuterium into plasma VLDL during administration of deuterium-labeled water was used to determine the fractional synthetic rate of fatty acids as described (5, 6). Blood was collected to assess de novo lipogenesis before the first dose of deuterium-labeled water and hourly from 10 PM until 6 AM (18). After centrifugation, plasma was split into two parts, one part was stored at −20 °C until analysis, and the other part was processed immediately by ultracentrifugation for purification and removal of chylomicrons and plasma triglyceride extraction (5, 6). Deuterium enrichment in palmitate and plasma water was measured by gas chromatography–mass spectrometry (GC-MS) (5971A Mass Selective Detector; Hewlett–Packard) as described (5, 6), and fractional rates of de novo lipogenesis were calculated as described (19).
1H/13C MRS Measurements of Triglyceride and Glycogen Content in Liver and Muscle.
All MRS measurements were performed on a whole-body, 4.0-T Medspec (Bruker) system. Muscle lipid content was measured in the soleus muscle by using an 8.5-cm diameter, circular 13C surface coil with twin, orthogonal circular 13-cm 1H coils arranged in quadrature as previously described (7, 20–22). Then after repositioning the subject, 13C MRS spectra were acquired in a 40 × 80 × 80 mm3 volume placed within the vastus lateralis to measure glycogen content, using a coil assembly composed of a 8-cm, circular 13C coil and twin 11 × 14 cm elliptical 1H coils arranged in quadrature. Protons were decoupled during the 25-ms acquisition time with a WALTZ-4, and localization of the volume was performed by using 3D adiabatic outer-volume suppression. Glycogen content was calculated by comparison to a glycogen solution of known concentration.
Intrahepatic lipid content was measured with a coil assembly composed of a 12-cm, circular 13C coil and twin 13 × 19 cm elliptical 1H coils arranged in quadrature as described (7). The liver triglyceride content was measured by 1H respiratory-gated stimulated echo acquisition mode sequence spectroscopy in a 15 × 15 × 15 mm3 voxel (21, 22). Acquisition was synchronized to the respiratory cycle and triggered at the end of expiration. A water-suppressed lipid spectrum and a lipid-suppressed water spectrum were acquired and carried out in three different locations of the liver. The total lipid content was expressed as a percentage of the water content, averaged for the three locations and corrected for T1 and T2 relaxation. Liver glycogen content was acquired with the same coil assembly and method as the one used for the muscle glycogen content.
Analytical Methods.
Plasma glucose concentrations were measured by using a YSI STAT 2700 Analyzer (Yellow Springs Instrument). Plasma concentrations of insulin and C-peptide were measured with double-antibody RIA kits (Linco). Plasma fatty acid and triglyceride concentrations were determined by using a microfluorimetric method (24).
Statistical Analysis.
Statistical analyses were performed by using the StatView package (Abacus Concepts). Paired Student's t tests were performed where appropriate to detect statistical differences. All data are expressed as mean ± SEM.
Acknowledgments
These studies were supported by Public Health Service Grants R01 AG-23686, R01 DK-49230, R24 DK-085836, UL1 RR-024139, and P30 DK-45735, and Distinguished Clinical Scientist Awards from the American Diabetes Association (to K.F.P. and G.I.S.). R.R. was supported by a grant from the Danish Research Council.
Footnotes
The authors declare no conflict of interest.
References
- 1.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association; National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Petersen KF, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perseghin G, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. doi: 10.1056/NEJM199610313351804. [DOI] [PubMed] [Google Scholar]
- 5.Previs SF, et al. Assay of the deuterium enrichment of water via acetylene. J Mass Spectrom. 1996;31:639–642. doi: 10.1002/(SICI)1096-9888(199606)31:6<639::AID-JMS336>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Diraison F, Pachiaudi C, Beylot M. Measuring lipogenesis and cholesterol synthesis in humans with deuterated water: Use of simple gas chromatographic/mass spectrometric techniques. J Mass Spectrom. 1997;32:81–86. doi: 10.1002/(SICI)1096-9888(199701)32:1<81::AID-JMS454>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Petersen KF, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothman DL, et al. Decreased muscle glucose transport/phosphorylation is an early defect in the pathogenesis of non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci USA. 1995;92:983–987. doi: 10.1073/pnas.92.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose AJ, Richter EA. Skeletal muscle glucose uptake during exercise: how is it regulated? Physiology (Bethesda) 2005;20:260–270. doi: 10.1152/physiol.00012.2005. [DOI] [PubMed] [Google Scholar]
- 10.Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen KF, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuel VT, et al. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- 13.Yki-Järvinen H. Liver fat in the pathogenesis of insulin resistance and type 2 diabetes. Dig Dis. 2010;28:203–209. doi: 10.1159/000282087. [DOI] [PubMed] [Google Scholar]
- 14.Petersen KF, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 16.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 17.Krssak M, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: A 1H NMR spectroscopy study. Diabetologia. 1999;42:113–116. doi: 10.1007/s001250051123. [DOI] [PubMed] [Google Scholar]
- 18.Kreisberg RA. Diabetic dyslipidemia. Am J Cardiol. 1998;82(12A):67U–73U. doi: 10.1016/s0002-9149(98)00848-0. discussion 85U–86U. [DOI] [PubMed] [Google Scholar]
- 19.Diraison F, Pachiaudi C, Beylot M. In vivo measurement of plasma cholesterol and fatty acid synthesis with deuterated water: Determination of the average number of deuterium atoms incorporated. Metabolism. 1996;45:817–821. doi: 10.1016/s0026-0495(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 20.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 21.Frahm J, et al. Localized high-resolution proton NMR spectroscopy using stimulated echoes: Initial applications to human brain in vivo. Magn Reson Med. 1989;9:79–93. doi: 10.1002/mrm.1910090110. [DOI] [PubMed] [Google Scholar]
- 22.Pauly J, Le Roux P, Nishimura D, Macovski A. Parameter relations for the Shinnar-Le Roux selective excitation pulse design algorithm [NMR imaging] IEEE Trans Med Imaging. 1991;10:53–65. doi: 10.1109/42.75611. [DOI] [PubMed] [Google Scholar]
- 23.Mayerson AB, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miles J, Glasscock R, Aikens J, Gerich J, Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983;24:96–99. [PubMed] [Google Scholar]