Abstract
In the majority of cases, advanced prostate cancer responds initially to androgen deprivation therapy by depletion of gonadal testosterone. The response is usually transient, and metastatic tumors almost invariably eventually progress as castration-resistant prostate cancer (CRPC). The development of CRPC is dependent upon the intratumoral generation of the potent androgen, dihydrotestosterone (DHT), from adrenal precursor steroids. Progression to CRPC is accompanied by increased expression of steroid-5α-reductase isoenzyme-1 (SRD5A1) over SRD5A2, which is otherwise the dominant isoenzyme expressed in the prostate. DHT synthesis in CRPC is widely assumed to require 5α-reduction of testosterone as the obligate precursor, and the increased expression of SRD5A1 is thought to reflect its role in converting testosterone to DHT. Here, we show that the dominant route of DHT synthesis in CRPC bypasses testosterone, and instead requires 5α-reduction of androstenedione by SRD5A1 to 5α-androstanedione, which is then converted to DHT. This alternative pathway is operational and dominant in both human CRPC cell lines and fresh tissue obtained from human tumor metastases. Moreover, CRPC growth in mouse xenograft models is dependent upon this pathway, as well as expression of SRD5A1. These findings reframe the fundamental metabolic pathway that drives CRPC progression, and shed light on the development of new therapeutic strategies.
Keywords: hormonal therapy, 5-alpha-androstanedione, tumor metabolism, abiraterone acetate, hormone resistance
Androgen deprivation therapy with depletion of gonadal testosterone (T) is the frontline treatment for advanced prostate cancer and is usually initially effective (1). Metastatic disease almost always eventually acquires resistance to gonadal T depletion and is termed “castration-resistant prostate cancer” (CRPC). A critical mechanism in driving CRPC tumor progression is a gain-of-function in the androgen receptor (AR) (2). Multiple clinical studies have shown that intratumoral concentrations of T and 5α-dihydrotestosterone (DHT) sufficient to activate AR-dependent transcription are maintained in CRPC despite suppression of serum T (3–6). The requirement for the intratumoral generation of androgen ligand is exemplified by frequent and often sustained responses to AR antagonists (7, 8), as well as an unprecedented prolongation of survival in chemotherapy-refractory metastatic CRPC conferred by abiraterone acetate, a potent inhibitor of CYP17A1, which is required for the synthesis of adrenal 19-carbon steroid precursors of T and DHT (9).
Although T is a modest AR agonist, DHT is more potent (10) and is the principal androgen bound to AR in the prostate cell nucleus (11). In the setting of CRPC and the absence of gonadal T, dehydroepiandrosterone (DHEA) and its sulfate, the chief adrenal 19-carbon steroid in circulation (12), undergoes conversion to Δ4-androstenedione (AD) in prostate cancer (Fig. 1) by 3β-hydroxysteroid dehydrogenase/isomerase (13–15). Intratumoral synthesis of DHT in CRPC is generally thought to require 17-keto reduction of AD to T, followed by 5α-reduction of T to DHT (2, 6, 16), thereby following the synthesis and processing dictated by gonadal T physiology. This “conventional” pathway (AD→T→DHT) in CRPC has been generally surmised by genetic deficiencies in enzymes required to convert AD to T in the testes (17) and T to DHT in the normal prostate (18), and furthermore implies the requirement for T as an obligate precursor of DHT. The existence of an alternative pathway to DHT synthesis in peripheral tissue cell lines has been described (19). However, the role and requirement for this pathway in driving CRPC has never been directly defined.
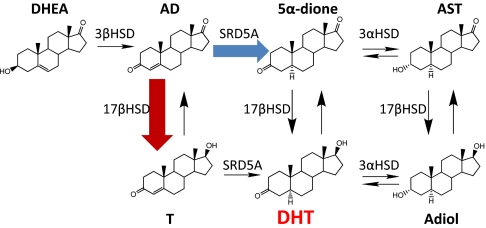
Fig. 1.
Pathways of DHT synthesis from adrenal DHEA. DHEA is converted by 3β-hydroxysteroid dehydrogenase/isomerase (3βHSD) to AD. In the conventional pathway, AD is first transformed to T (red arrow), which is then converted by SRD5A to DHT. In the alternative pathway, AD is the principal substrate for SRD5A and is converted to 5α-dione (blue arrow), a necessary precursor to DHT. Both 5α-dione and DHT are reversibly interconvertible by 3α-hydroxysteroid dehydrogenases (3αHSD) to the other 5α-reduced steroids, androsterone (AST) and 5α-androstane-3α,17β-diol (Adiol), respectively.
Here, we show that the dominant pathway to DHT synthesis from adrenal precursors in CRPC follows an alternative route that bypasses T and requires steroid 5α-reductase isoenzyme-1 (SRD5A1). The Δ4, 3-keto structure of T makes it susceptible 5α-reduction by SRD5A isoenzymes. Similar to T, AD also has a Δ4, 3-keto structure, permitting it to be 5α-reduced to 5α-androstanedione (5α-dione) (6, 20), which suggests an alternative pathway to DHT synthesis (Fig. 1). This alternative pathway, therefore, circumvents T and instead requires 5α-dione as a necessary precursor to DHT.
Results
Alternative Pathway to DHT Synthesis in CRPC.
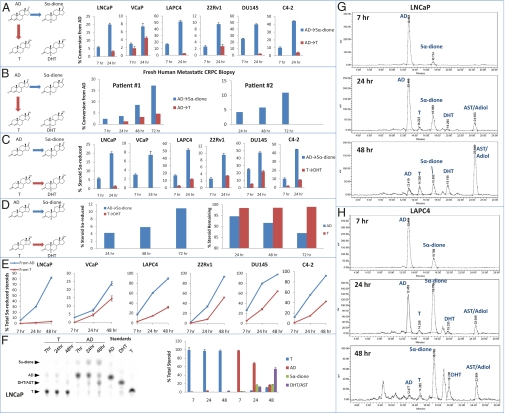
AD may either be 17-keto–reduced to T or alternatively 5α-reduced to 5α-dione. To establish the dominant pathway to DHT synthesis, six established human prostate cancer cell lines originally obtained and derived from patients with CRPC were treated with [3H]-AD (Fig. 2A). In all six cell lines, flux from AD→5α-dione occurs earlier and more rapidly than flux from AD→T, as evidenced by 5α-dione and T detection by HPLC. To establish proof-of-principle for the preferred route of AD metabolism in clinical samples, metastatic tumors were biopsied under radiologic-guidance from two men with CRPC under an institutional review board-approved protocol (treatment history in Methods). Fresh tumor collected by 18-G core biopsy was incubated with [3H]-AD. In patient #2, from whom tissue obtained was sufficient for two incubations, an equal portion of tumor was incubated with [3H]-T. As in the six CRPC cell lines, the dominant route of AD metabolism is 5α-reduction to 5α-dione (Fig. 2B). In patient #2, there was no conversion from AD→T. To determine the preferred Δ4, 3-keto–steroid substrate for endogenously expressed SRD5A isoenzymes, flux from AD→5α-dione was compared with flux from T→DHT (Fig. 2C). In all models, flux from AD→5α-dione is uniformly dominant. In patient #2, no DHT synthesis was observed from the incubation with [3H]-T (Fig. 2D). The four 5α-reduced 19-carbon steroids [5α-dione, DHT, androsterone, and Adiol] are interconvertible through reversible reactions (Fig. 1). Comparison of the accumulation of total 5α-reduced steroids with [3H]-AD and [3H]-T treatments further shows that AD→5α-dione is the dominant entryway to 5α-reduced steroids (Fig. 2E). In the VCaP model, which has the lowest difference between [3H]-AD and [3H]-T treatments, this finding was attributable in part because of the conversion of T to AD, which then more readily undergoes 5α-reduction. Analysis by TLC in LNCaP confirms that AD is rapidly consumed to 5α-reduced steroids, whereas T is not readily metabolized under similar conditions (Fig. 2F). The complete time course and HPLC tracings of DHT synthesis from AD in LNCaP and LAPC4 is shown in Fig. 2 G and H. Similar analyses with LNCaP and LAPC4 cells cocultured with bone marrow stromal cells to mimic the bone metastatic microenvironment yield similar results as metabolism by all six cell-line models alone, as well as the freshly collected human tumors (Fig. S1).
Fig. 2.
An alternative pathway to the synthesis of DHT bypasses T. (A) All six human CRPC cell lines uniformly transform AD preferentially to 5α-dione over T by HPLC. (B) Freshly collected metastatic CRPC tissue exhibits similar metabolism from AD→5α-dione. (C) AD is a preferred substrate over T for endogenously expressed SRD5A in CRPC cell lines. (D) In patient #2, AD is readily consumed by 5α-reduction to 5α-dione, whereas there is no detectable 5α-reduction of T to DHT and no depletion of T over time. (E) Accumulation of 5α-reduced steroids occurs more robustly in CRPC cell lines from AD compared with T. In A–E, blue arrows, bars, and lines represent flux through the alternative pathway and red indicates the conventional pathway. (F) Alternative analysis by TLC demonstrates the preference for 5α-reduction of AD. (G) HPLC tracings demonstrate the conversion of AD to 5α-dione in LNCaP at 7 h and subsequent conversion to the other 5α-reduced steroids (DHT, AST, and Adiol). (H) Similar HPLC analysis in LAPC4. Error bars in A, C, E and F represent the SD from experiments performed in triplicate.
Alternative Pathway Drives CRPC Progression.
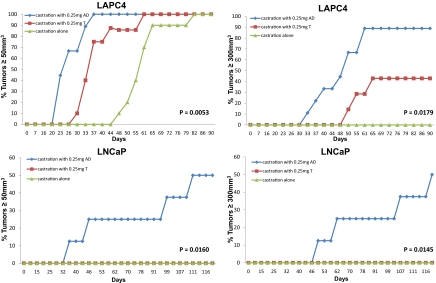
To test the requirement of the conventional pathway (AD→T→DHT) versus the alternative pathway (AD→5α-dione→DHT) for CRPC growth, we performed subcutaneous xenograft studies with LAPC4 and LNCaP models in surgically orchiectomized mice supplemented with T and AD. A priori, one would expect that the dominance of the alternative pathway in inducing CRPC growth might not be demonstrable given (i) two steps for the transformation of AD to DHT instead of one step for T to DHT, and (ii) modest AR agonism by T but not AD in the absence of further metabolism. Both LAPC4 and LNCaP tumors in mice supplemented with AD reached the predetermined endpoints of tumor volume ≥ 50 mm3 and ≥ 300 mm3 significantly more rapidly than T-supplemented mice (Fig. 3). In general, tumor take in LNCaP was less efficient than with LAPC4, as is consistent with the experience of others (21).
Fig. 3.
The alternative pathway to DHT synthesis drives CRPC progression. CRPC growth in the LAPC4 (Upper) and LNCaP (Lower) CRPC models have more robust growth in orchiectomized mice supplemented with AD versus T (0.25 mg sustained-release steroid pellets). Control mice underwent orchiectomy and no steroid supplementation. Time from injection of tumor cells to tumor volume ≥ 50 mm3 (Left) and tumor volume ≥ 300 mm3 (Right) is statistically significantly different for both models and both endpoints using a log rank test and a pairwise comparison of AD versus T cohorts.
SRD5A1 Is the Dominant Isoenzyme Required for the Alternative Pathway.
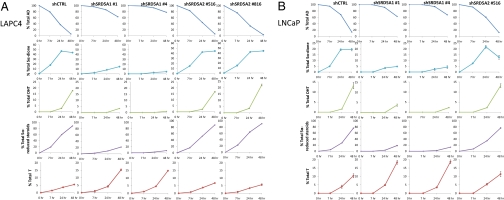
Several independent studies have shown that expression of SRD5A1 is increased and SRD5A2 is decreased in the transition from hormone-naive prostate cancer to CRPC (4, 22, 23). However, the role of any increase in SRD5A1 expression is thought to reflect a role in converting T to DHT. To test whether SRD5A1 might catalyze flux from AD→5α-dione in the alternative pathway to DHT to sustain CRPC, both SRD5A1 and SRD5A2 were stably knocked down in LNCaP and LAPC4 using lentiviral shRNAs (Fig. S2), and metabolism from [3H]-AD was assessed by HPLC (Fig. 4). In control cells, AD is almost completely consumed by 48 h, whereas the majority of AD is preserved when SRD5A1 is knocked down. Synthesis of 5α-dione, DHT, and total 5α-reduced steroids is largely blocked by effectively silencing SRD5A1 using two independent shRNAs. Notably, blocking flux from AD→5α-dione by silencing SRD5A1 diverts AD instead to increased T. Silencing SRD5A2 does not reduce flux from AD to 5α-dione, DHT, or other 5α-reduced steroids, suggesting little or no participation of this SRD5A isoenzyme in the alternative pathway. Representative HPLC plots are shown in Fig. S3. Similar experiments done with a SRD5A2 selective concentration of finasteride (24) and a clinically relevant concentration of dutasteride (25) corroborate these findings (Fig. S4).
Fig. 4.
SRD5A1 is required for the conversion of AD to 5α-dione in the alternative pathway to DHT synthesis. (A) LAPC4 and (B) LNCaP cells stably expressing nonsilencing (shCTRL), SRD5A1 silencing (shSRD5A1 #1 and #4) and SRD5A2 silencing (shSRD5A2 #516 and #816) lentiviral constructs were treated with [3H]-AD in triplicate, with HPLC quantitation of the indicated steroids at the designated time points. In both models, AD is depleted over time in control and SRD5A2 silenced cells, but AD is largely preserved and not metabolized in SRD5A1 silenced cells. Synthesis of 5α-dione, DHT and total 5α-reduced steroids are dependent on SRD5A1 but not SRD5A2 expression. Blocking AD→5α-dione by silencing SRD5A1 hastens conversion of AD→T, resulting in elevated T. Error bars represent the SD.
Blocking SRD5A1 Inhibits AR-Responsive Gene Expression and CRPC Progression.
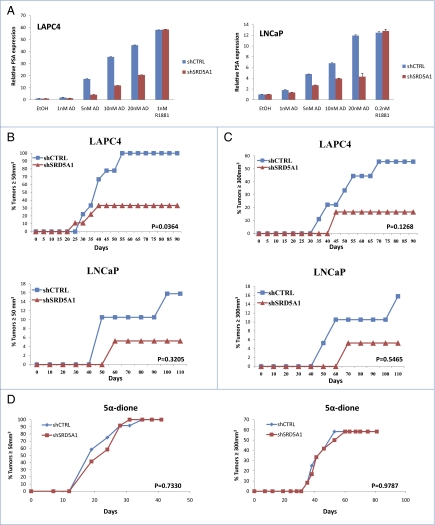
To determine the effect of blocking flux from AD→5α-dione by silencing SRD5A1 expression on AR-dependent transcription, cells with silenced SRD5A1 expression were treated with AD, and prostate-specific antigen (PSA) expression was assessed (Fig. 5A). AD-induced PSA expression is muted in the absence of SRD5A1. To determine if the role of SRD5A1 expression in conducting the alternative pathway is critical to drive CRPC growth, surgically orchiectomized mice supplemented with sustained-release AD pellets were injected subcutaneously with cells expressing an SRD5A1 shRNA or control nonsilencing shRNA (Fig. 5 B and C). Time-to-tumor volume ≥ 50 mm3 was significantly decreased for SRD5A1-silenced LAPC4 cells (P = 0.0364). Although not statistically significant, the trend for the LNCaP model and the volume ≥ 300-mm3 endpoint all consistently suggested disadvantaged growth for cells lacking SRD5A1 expression. SRD5A1 Western blot in tumors collected at the end of study showed that SRD5A1 expression is increased in the knockdown tumors compared with the original cells injected with silenced SRD5A1 expression (Fig. S5). This finding suggests there is selection for higher SRD5A1-expressing cells, which likely underestimates the true requirement for SRD5A1 expression in these xenograft experiments. On the other hand, xenograft growth in 5α-dione supplemented mice had nearly identical growth between shCTRL and shSRD5A1 groups (Fig. 5D), further demonstrating that the specific role of SRD5A1 in the alternative pathway and CRPC growth is the conversion of AD→5α-dione, and that the differences in tumor growth in Fig. 5 B and C are not the result of other effects of SRD5A1 on growth.
Fig. 5.
SRD5A1 is required for PSA expression and CRPC tumor progression in vivo. (A) Silencing SRD5A1 expression blunts PSA expression in response to AD, which is a substrate for SRD5A1, but not R1881, which does not require metabolism to bind AR. PSA expression is normalized to RPLP0 and vehicle (EtOH) control. Error bars represent the SD of experiments performed in triplicate. (B) Silencing SRD5A1 expression inhibits CRPC growth in orchiectomized mice supplemented with AD, as assessed by time for LNCaP and LAPC4 tumors to reach 50 mm3 and (C) 300 mm3. Only LAPC4 tumors reaching the 50-mm3 endpoint was statistically significantly different, although the trend for all models and time points had a disadvantage for the shSRD5A1 group. (D) Bypassing the requirement for SRD5A1 by supplementing orchiectomized mice with 5α-dione leads to nearly superimposable growth for control and shSRD5A1 LAPC4 cells. Control and shSRD5A1 groups for all xenograft studies were compared using a log rank test.
Discussion
DHT in CRPC tumors might derive either from de novo steroidogenesis starting with cholesterol (26) or by metabolism of highly abundant adrenal precursors, which requires only a few enzymes and appears to be the dominant component (27). In contradistinction to widely held assumptions about the major pathway that drives CRPC progression, our findings show that the major metabolic pathway from adrenal precursor steroids to DHT in CRPC circumvents T as an obligate precursor and that the transformation of AD→5α-dione by SRD5A1 is a required step for DHT synthesis and tumor progression. The specific requirement for SRD5A1 in this pathway suggests that SRD5A1 up-regulation, which occurs concurrently with SRD5A2 down-regulation clinically in the transition from hormone-naive prostate cancer to CRPC (4, 22, 23), reflects selection for tumor cells that efficiently synthesize DHT through the alternative pathway. These results are also in line with the initial studies of SRD5A1, which suggest that AD is a better substrate for this isoenzyme than T (28). Notably, this pathway of adrenal steroid metabolism is likely not unique to CRPC (19).
Strikingly, the consistent finding of the dominance of the alternative pathway across all six CRPC cell-line models tested suggests that this is the common pathway shared in CRPC. We took the further step to biopsy tumors from two patients, which yielded results that are exactly the same as the cell-line models, as well as the stromal cocultures. Together, these results confirm that the findings in the models tested are not merely attributable to an artifact in cell-line models and, furthermore, are of clinical import. To our knowledge, these ex vivo metabolic studies of metastatic CRPC are unprecedented. It is important to note that studies on “banked” tissue, which is enzymatically dead, give a snapshot picture of androgen concentrations at one point in time (3, 4). Our studies and approach require enzyme activity in freshly obtained tumors from patients to define the interconversion of these steroids and their origins.
The current findings must be reconciled with several observations that were interpreted to support the conventional pathway requiring T. First, although untreated prostate cancer and benign prostate have lower concentrations of T than DHT (T:DHT ratio = ∼1:10), indicating rapid and irreversible flux from T→DHT, clinical studies of CRPC indicate that this ratio is reversed and that concentrations of T are higher than DHT. These studies are generally interpreted to suggest that any DHT must arise from T. In contrast, an alternative explanation for the increase in precursor (T) to product (DHT) ratio is that flux to DHT may not occur readily through T but rather through the alternative route. In an analogous scenario, pharmacologically blocking flux from T→DHT in the prostates of eugonadal men yields similar increases in the T:DHT ratio as in CRPC (3, 4, 29). We would suggest that in CRPC a modicum of T synthesis is sufficient to increase the T:DHT ratio because T is not readily converted to DHT. With respect to the relative concentrations and contributions of T and DHT as AR agonists, it is important to note that intratumoral concentrations of androgens in clinical studies disproportionately reflect androgens found in the tumor interstitial space and cellular cytoplasm. On the other hand, intranuclear concentrations of androgens more accurately reflect the active androgen bound to AR (11). Nuclear DHT concentrations have not been determined in clinical CRPC tissue, and we would suggest this compartment is likely enriched with DHT beyond those observed in the sum of all tumor compartments (3, 4).
Second, clinical trials of the dual SRD5A inhibitor, dutasteride, that only showed modest clinical activity against CRPC (30) might be interpreted to signify a limited importance for DHT. However, our findings (Fig. S4) suggest that a pitfall of pharmacologically blocking SRD5A1 and the conversion of AD to 5α-dione is the diversion of AD to T, generating higher concentrations of T, which has modest AR agonist activity, and may thereby partially compensate for blocking DHT synthesis. This explanation is supported by the higher concentrations of T that occur with SRD5A1 knockdown, and the lack of complete inhibition of AR-dependent transcription and CRPC growth (Fig. 5). These findings suggest that the best points of pharmacologic intervention to treat CRPC should not shunt synthesis from one AR agonist (DHT) to another (T).
Our observation that DHT is synthesized through an alternative pathway involving conversion of AD to 5α-dione by SRD5A1 has broad implications for the development of new therapeutic agents and for determining mechanisms of resistance to hormonal therapies for CRPC. These data suggest that blocking the conversion of AD to T will not significantly inhibit DHT synthesis in CRPC. Furthermore, our findings suggest that T may not be the best marker for monitoring the intratumoral response or resistance to upstream inhibitors of adrenal steroid synthesis, such as abiraterone acetate (31). Future studies of intratumoral androgens should include previously unappreciated DHT intermediates.
Methods
Cells and Culture Conditions.
The 22Rv1, LNCaP, and DU145 prostate cancer cell lines and HS-27A bone marrow stromal cells were purchased from ATCC and maintained in RPMI 1640 with 10% FBS. VCaP cells were purchased from ATCC and maintained in DMEM with 10% FBS. The LAPC4 prostate cancer cell line was generously provided by Charles Sawyers (Memorial Sloan Kettering Cancer Center, New York, NY) and was grown in Iscove's modified Dulbecco's medium with 10% FBS. C4-2 prostate cancer cells were generously provided by J. T. Hsieh (University of Texas Southwestern, Dallas, TX) and maintained in RPMI 1640 with 10% FBS.
Steroid Metabolism.
Cells were seeded in 12-well dishes at 300,000 to 400,000 cells per well 24 h before the experiment in serum-free, phenol red-free medium. In coculture experiments, tumor and stromal cells were plated at a 1:1 ratio. [3H]-labeled steroids (100 nM, 300,000–600,000 cpm) were obtained from PerkinElmer, added in ethanol, and then cells were incubated at 37 °C for up to 48 h. Cells were concomitantly treated with finasteride (Sigma-Aldrich) and dutasteride (GlaxoSmithKline), where indicated. Aliquots of medium (0.25–0.5 mL) were treated with 1,000 units of β-glucuronidase (Helix Pomatia; Sigma-Aldrich) at 65 °C for 4 h to deconjugate glucuronidated steroids, extracted with 1 mL 1:1 ethyl acetate:isooctane, and concentrated under nitrogen. For HPLC analysis, the dried samples were dissolved in methanol and injected on a Breeze 1525 system equipped with model 717 plus autoinjector (Waters Corp.) and a Kinetix 100 × 2.1-mm, 2.6 μM C18 reverse-phase column (Phenomenex) and methanol/water gradients at 30 °C. The column effluent was analyzed using a β-RAM model 3 in-line radioactivity detector (IN/US Systems, Inc.) using Liquiscint scintillation mixture (National Diagnostics). Alternatively, dried samples were applied to plastic-backed silica gel plates (Whatman) and separated by TLC using a mobile phase of 3:1 chloroform:ethyl acetate, followed by exposure of the plates to a phosphorimager screen and quantitation with a Storm model 860 phosphorimager (Applied Biosystems). All HPLC and TLC studies were performed in triplicate and repeated in independent experiments.
Gene Expression and Immunoblot.
Briefly, total RNA was harvested using the RNeasy kit (Qiagen), and 1 μg RNA was used in a reverse-transcriptase reaction with the iScript cDNA synthesis Kit (Bio-Rad). Quantitative PCR (qPCR) analysis was performed in triplicate with the following primer set for SRD5A1 (Forward: 5′-CCTGTTGAATGCTTCATGACTTG-3′; Reverse: 5′-TAAGGCAAAGCAATGCCAGATG-3′), SRD5A2 (Forward: 5′-CTCTCTAAGGAAGGGGCCGAAC-3′; Reverse: 5′-GACAATGCATTCCGCAAACATA-3′), PSA (Forward: 5′-GCATGGGATGGGGATGAAGTAAG-3′; Reverse: 5′-CATCAAATCTGAGGGTTGTCTGGA -3′), and the housekeeping gene encoding large ribosomal protein P0 (RPLP0) (Forward: 5′-CGAGGGCACCTGGAAAAC-3′; Reverse: 5′-CACATTCCCCCGGATATGA-3′). The iTaq Fast SYBR Green Supermix with ROX kit (Bio-Rad) was used for the thermocycling reaction in an ABI-7500 Real-Time PCR machine (Applied Biosystems). Accurate quantitation of each mRNA was determined by normalizing the sample values to RPLP0 and to nonsilencing control cells (for knockdown) or to vehicle treated cells (for steroid treated cells).
For Western blot analysis, total protein was isolated using RIPA buffer (Sigma-Aldrich). Protein (40 μg) was resolved by 12% SDS/PAGE and incubated with a rabbit anti-SRD5A1 (Abnova), goat anti-SRD5A2, mouse anti-AR (Santa Cruz Biotechnology), and mouse anti–β-actin (Sigma-Aldrich) antibodies.
Human Tumor Studies.
Two patients with metastatic CRPC underwent 18-G CT-guided core biopsy under an institutional review board-approved protocol (STU-062010-212). Patient #1 is a 54 y old who underwent radical prostatectomy for a Gleason 8 T3b prostate cancer, subsequently was found to have liver metastases, and was treated with androgen deprivation therapy plus the AR antagonist bicalutamide. His disease progressed to CRPC after 10 mo, which was followed by treatment with the other AR antagonists, nilutamide and flutamide. Disease progression was marked by a pelvic mass, and treatment with two cycles of docetaxel chemotherapy followed. The pelvic mass (not present when androgen deprivation was initiated) was biopsied. Patient #2 is a 77 y old with Gleason 7 disease treated with radical prostatectomy and surgical orchiectomy, was found to have widely metastatic disease 10 y later, and subsequently progressed on bicalutamide and nilutamide. A left retroperitoneal lymph was biopsied. For each tumor, a portion of tumor tissue was confirmed as staining for PSA. Remaining tumor was minced and incubated in 1 mL serum-free DMEM with [3H]-AD (300 nM, 24,000,000 cpm). Aliquots of medium (0.25 mL) were collected at the indicated time points, steroids were treated with β-glucuronidase, extracted, and analyzed by HPLC as described above. For patient #2, there was also sufficient tissue for treatment with [3H]-T, done under the same conditions as the [3H]-AD treatment described above.
Supplementary Material
Acknowledgments
We thank Jean Wilson, David Russell, David Mangelsdorf, Mike Brown, and Mike McPhaul for helpful comments and the patients who made the studies of clinical tissues possible. This publication has been funded in part by a Howard Hughes Medical Institute Physician-Scientist Early Career Award, a Prostate Cancer Foundation Award, and from Grant PC080193 from the US Army Medical Research and Materiel Command (to N.S.); Clinical Scientist Award in Translational Research 1005954 from the Burroughs-Wellcome Fund (to R.J.A.); and the Charles A. and Elizabeth Ann Sanders Chair in Translational Research (R.J.A.).
Footnotes
Conflict of interest statement: N.S. and R.J.A. have been compensated as consultants for Ortho Biotech.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107898108/-/DCSupplemental.
References
- 1.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 3.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery RB, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geller J, et al. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978;46:440–444. doi: 10.1210/jcem-46-3-440. [DOI] [PubMed] [Google Scholar]
- 6.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–221. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Scher HI, et al. Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375:1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labrie F, et al. Benefits of combination therapy with flutamide in patients relapsing after castration. Br J Urol. 1988;61:341–346. doi: 10.1111/j.1464-410x.1988.tb13971.x. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, et al. COU-AA-301 Investigators Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deslypere JP, Young M, Wilson JD, McPhaul MJ. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88(1–3):15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 11.Bruchovsky N, Wilson JD. The intranuclear binding of testosterone and 5α-androstan-17-β-ol-3-one by rat prostate. J Biol Chem. 1968;243:5953–5960. [PubMed] [Google Scholar]
- 12.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. doi: 10.1210/er.2010-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3β-hydroxysteroid dehydrogenase is a possible pharmacological target in the treatment of castration-resistant prostate cancer. Endocrinology. 2010;151:3514–3520. doi: 10.1210/en.2010-0138. [DOI] [PubMed] [Google Scholar]
- 14.Simard J, et al. Molecular biology of the 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 15.Lorence MC, Murry BA, Trant JM, Mason JI. Human 3β-hydroxysteroid dehydrogenase/Δ5→4isomerase from placenta: Expression in nonsteroidogenic cells of a protein that catalyzes the dehydrogenation/isomerization of C21 and C19 steroids. Endocrinology. 1990;126:2493–2498. doi: 10.1210/endo-126-5-2493. [DOI] [PubMed] [Google Scholar]
- 16.Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008;281(1–2):1–8. doi: 10.1016/j.mce.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson S, Russell DW, Wilson JD. 17β-hydroxysteroid dehydrogenase 3 deficiency. Trends Endocrinol Metab. 1996;7(4):121–126. doi: 10.1016/1043-2760(96)00034-3. [DOI] [PubMed] [Google Scholar]
- 18.Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5α-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354(6349):159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luu-The V. Assessment of steroidogenic pathways that do not require testosterone as intermediate. Horm Mol Biol Clin Invest. 2011;5(3):161–165. doi: 10.1515/HMBCI.2011.007. [DOI] [PubMed] [Google Scholar]
- 20.Russell DW, Wilson JD. Steroid 5α-reductase: Two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 21.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 22.Titus MA, et al. Steroid 5α-reductase isozymes I and II in recurrent prostate cancer. Clin Cancer Res. 2005;11:4365–4371. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- 23.Stanbrough M, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 24.Guarna A, et al. 19-nor-10-azasteroids, a new class of steroid 5α-reductase inhibitors. 2. X-ray structure, molecular modeling, conformational analysis of 19-nor-10-azasteroids and comparison with 4-azasteroids and 6-azasteroids. J Med Chem. 1997;40:3466–3477. doi: 10.1021/jm970297k. [DOI] [PubMed] [Google Scholar]
- 25.Clark RV, et al. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. J Clin Endocrinol Metab. 2004;89:2179–2184. doi: 10.1210/jc.2003-030330. [DOI] [PubMed] [Google Scholar]
- 26.Locke JA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68:6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 27.Sharifi N, McPhaul MJ, Auchus RJ. “Getting from here to there”—Mechanisms and limitations to the activation of the androgen receptor in castration-resistant prostate cancer. J Investig Med. 2010;58:938–944. doi: 10.231/JIM.0b013e3181ff6bb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J Biol Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- 29.McConnell JD, et al. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1992;74:505–508. doi: 10.1210/jcem.74.3.1371291. [DOI] [PubMed] [Google Scholar]
- 30.Shah SK, et al. Phase II study of Dutasteride for recurrent prostate cancer during androgen deprivation therapy. J Urol. 2009;181:621–626. doi: 10.1016/j.juro.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Efstathiou E, et al. Use of “intracrine androgen signaling signature” to predict benefit from abiraterone acetate (AA) in patients with castrate-resistant prostate cancer (CRPC) 2010 ASCO Annual Meeting, June 4–8, 2010, Chicago, IL. 2010 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.