Abstract
Leukocyte telomere length (LTL) is a predictor of age-related disease onset and mortality. The association in adults of psychosocial stress or stress biomarkers with LTL suggests telomere biology may represent a possible underlying mechanism linking stress and health outcomes. It is, however, unknown whether stress exposure in intrauterine life can produce variations in LTL, thereby potentially setting up a long-term trajectory for disease susceptibility. We, therefore, as a first step, tested the hypothesis that stress exposure during intrauterine life is associated with shorter telomeres in adult life after accounting for the effects of other factors on LTL. LTL was assessed in 94 healthy young adults. Forty-five subjects were offspring of mothers who had experienced a severe stressor in the index pregnancy (prenatal stress group; PSG), and 49 subjects were offspring of mothers who had a healthy, uneventful index pregnancy (comparison group; CG). Prenatal stress exposure was a significant predictor of subsequent adult telomere length in the offspring (178-bp difference between prenatal stress and CG; d = 0.41 SD units; P < 0.05). The effect was substantially unchanged after adjusting for potential confounders (subject characteristics, birth weight percentile, and early-life and concurrent stress level), and was more pronounced in women (295-bp difference; d = 0.68 SD units; P < 0.01). To the best of our knowledge, this study provides the first evidence in humans of an association between prenatal stress exposure and subsequent shorter telomere length. This observation may help shed light on an important biological pathway underlying the developmental origins of adult health and disease risk.
Keywords: developmental programming, fetal origin
A rapidly growing body of empirical evidence suggests the origins of susceptibility for many common, complex age-related disorders that confer a major burden of disease can be traced back to the intrauterine period of life (i.e., the concept of fetal, or developmental, origins of health and disease risk). The developing fetus responds to, or is acted upon by, conditions in the internal or external environment during sensitive periods of cellular proliferation, differentiation, and maturation. These responses, in turn, may result in structural and/or functional changes in cells, tissues, and organ systems that have important long-term consequences for subsequent health and disease susceptibility (1–5). Exposure to psychosocial stress and/or biological stress mediators during gestation has been identified as one salient condition that may underlie the long-term programming effects of the intrauterine environment (2).
The link between psychosocial stress exposure and adverse health outcomes is well established. In particular, psychosocial stress appears to be an important risk factor for earlier onset of complex, common age-related diseases (6, 7). The elucidation of biological processes underlying this relationship is of considerable ongoing interest. In recent years, accumulating evidence supports the crucial role of telomere biology as a potential mechanism linking psychosocial stress exposure and disease risk (8, 9).
Telomeres are DNA–protein complexes that cap chromosomal ends, promoting chromosomal stability. When cells divide, the telomere is not fully replicated because of limitations of the DNA polymerases in completing the replication of the ends of the linear molecules, leading to telomere shortening with every replication (10). Telomeres that are shortened past a critical length cause the cell to enter a state of arrest (i.e., cell senescence) when cells can no longer divide. Telomeres shorten with age in all replicating somatic cells, including leukocytes (11). Telomerase, a cellular enzyme, provides maintenance of telomeres and can counteract shortening and its functional consequences by adding telomeric DNA to shortened telomeres. Telomere maintenance has relevance for long-term health. Shortened telomere length and/or reduced telomerase activity have been consistently associated with health risk and diseases (12–15). Declines in the telomere/telomerase maintenance system may play a causal role in aging, serve as a biomarker of aging, or both. A recent study in mice suggests that telomerase plays a causal role in aging and regeneration of cells, tissues, and physiological function (16).
Several cross-sectional studies in humans have reported associations between telomere biology and high levels of psychosocial stress exposure (8, 17, 18) or stress biomarkers (17, 19), suggesting that stress-related changes in telomere integrity may be one possible mechanism linking psychosocial stress and age-related disease (20). Experimentally, high levels of cortisol exposure (a potent stress hormone) have been shown to dampen telomerase activity in leukocytes (21). Behavioral interventions that reduce stress have also been linked to higher telomerase activity. For example, in one study, an intensive lifestyle change program consisting of dieting, counseling, and stress management was associated with increases in telomerase activity (22), and in a second study, intensive meditation was associated with higher postintervention telomerase (23).
Many, but not all, studies in humans have found an association between exposure to adverse conditions in early postnatal life (infancy and childhood) and subsequent telomere length (24–28). One important question that has yet to be addressed to our awareness is whether exposure to stress during intrauterine development can produce variations in telomere length, thereby potentially setting up a long-term trajectory at birth that defines or contributes to individual susceptibility for complex, common age-related diseases. Stress exposure during fetal development is an important factor shaping adult health and has been linked to adverse outcomes including, but not limited to, immune, endocrine, and metabolic dysregulation and related disorders (29–34). However, we are aware of no studies to date that have examined the relationship between prenatal stress exposure and telomere length. Evidence linking other adverse conditions during fetal development with subsequent telomere length provides biological plausibility for this relationship. Several studies in animals have reported that intrauterine adversity is associated with shorter telomeres in cells of different tissues. For instance, experimentally induced fetal growth restriction in rodents has been shown to produce significant telomere attrition in the kidneys (35), and manipulations of maternal diet during pregnancy have produced shortening of telomere length in aortic and pancreatic islet cells (36, 37). In humans, telomeres in placental trophoblasts are found to be shorter in pregnancies complicated with intrauterine growth restriction (38). Last, one study in preschool-age children found that children who were born low birth weight had shorter leukocyte telomere length (LTL) than age-matched children who had a normal birth weight (39).
The objective of the present study was to test the hypothesis that maternal psychosocial stress exposure during pregnancy is associated with shorter telomeres in their offspring in adult life. Because it is not possible to randomly assign exposure to stress during human pregnancy, we approximated experimental exposure by using a quasiexperimental design by enrolling young adults whose mothers happened to have experienced a high level of psychosocial stress during pregnancy (a major negative life event) and compared them with a group of subjects whose mothers had not been exposed to negative life events during pregnancy. Prenatal stress exposure was assessed retrospectively by using a semistructured interview of major life events in the index pregnancy. The potential confounding effects of other sociodemographic, obstetric, medical, and behavioral risk factors were addressed by using a stringent set of exclusionary criteria. Moreover, because prenatal stress exposure may be associated with subsequent conditions that may influence telomere length, such as presence of postnatal early-life adversity, inadequate parental care, or concurrent stress level, we assessed these constructs to statistically account for their possible residual confounding effects.
Results
Subject Characteristics.
Table 1 provides the subject characteristics for the two groups, including sociodemographic factors, birth outcomes, and the childhood and adult variables of interest. As depicted in the table, there were no differences between the two groups in any of the subject characteristics except body mass index (BMI), which was marginally higher (P = 0.07) in the prenatal stress group (PSG) than in the comparison group (CG).
Table 1.
Subject characteristics
| Variable | PSG (n = 45) | CG (n = 49) | P value |
| Current | |||
| Age, y | 25 ± 0.80 | 24 ± 0.60 | NS |
| Sex | |||
| Male | 7 | 14 | |
| Female | 38 | 35 | NS |
| BMI | 24.50 ± 4.58 | 23.05 ± 2.96 | 0.07 |
| Education | |||
| High school, % | 100% | 100% | NS |
| Perceived stress (PSS, mean item score) | 1.87 ± 0.54 | 1.75 ± 0.52 | NS |
| Depressive symptoms (CES-D) | 11.11 ± 7.62 | 9.86 ± 6.89 | NS |
| History | |||
| Birth characteristics | |||
| Birth weight (g) | 3331 ± 549 | 3272 ± 420 | NS |
| Birth weight percentile | 42.18 ± 26.84 | 41.13 ± 24.19 | NS |
| Length of gestation (wk) | 39.23 ± 1.88 | 39.53 ± 1.50 | NS |
| Maternal education | |||
| High school, % | 100 | 98 | NS |
| College graduate, % | 22 | 27 | NS |
| Presence of childhood traumatic events | 11 (24%) | 11 (22%) | NS |
| Perceived maternal care (PBI) | 24.80 ± 9.15 | 25.91 ± 8.04 | NS |
| Factors controlled for by study design | |||
| Current chronic diseases | None | None | — |
| Smoking | None | None | — |
| Obstetric risk condition during mother's pregnancy | None | None | — |
Values presented as means ± SD where applicable. CES-D, Center for Epidemiological Studies–Depression scale; PBI, Parental Bonding Instrument.
Telomere Length.
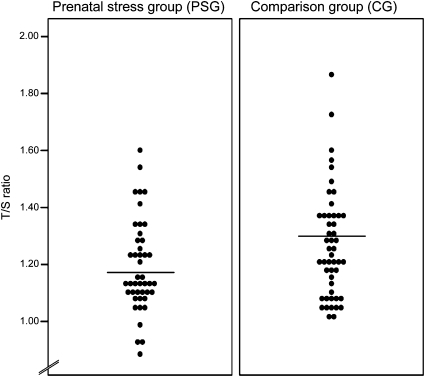
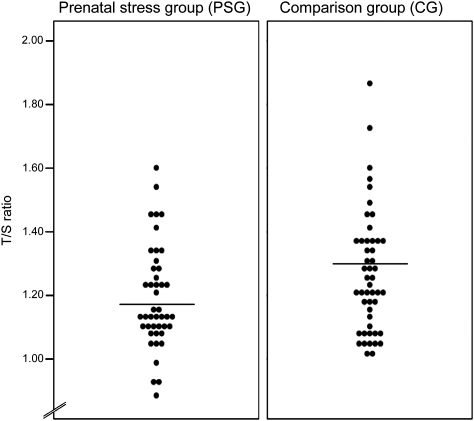
The mean LTL (±SD) was 1.23 ± 0.18 telomere repeat copy number-to-single gene copy number (T/S) ratio (which is equivalent to 6,234 ± 431 bp). LTL was normally distributed (Fig. 1).
Fig. 1.
Dot plot illustrating LTL (T/S ratio) in prenatally stressed individuals (PSG; n = 45) and CG subjects (n = 49). Lines indicate group means.
In the unadjusted univariate regression models, a crude relationship between prenatal stress and telomere length was found, with prenatal stress exposure predicting significantly shorter LTL [unstandardized β = −0.074; 95% confidence interval [CI], −0.146 to −0.001; P < 0.05) (Fig. 1). Birth weight percentile (birth weight adjusted for gestational age at delivery) also was significantly associated with LTL [β = 0.002; 95% CI = 0.000 to 0.003; P < 0.05), replicating an association that has been previously reported (39). Results from the fully adjusted multivariate regression model [that, in addition to prenatal stress exposure, included subject characteristics (e.g., age, BMI sex), birth weight percentile, postnatal early-life adversity (e.g., early trauma, maternal care), and exposure to concurrent life stress (e.g., chronic stress, depressive symptoms)] indicate this adjustment produced a small increase in the prenatal stress coefficient (β = −0.090; 95% CI = −0.179 to −0.001; P < 0.05). Birth weight percentile remained a significant predictor of LTL (β = 0.002; 95% CI = 0.001 to 0.004; P < 0.01), suggesting the effects of prenatal stress exposure and birth weight percentile were independent of one another, and that the effect of prenatal stress on LTL was not mediated via a reduction in birth weight.
The separate analyses stratified by sex (i.e., women-only and men-only groups) suggest that the effect of prenatal stress exposure was more pronounced in women: the unadjusted (β = −0.122; 95% CI, −0.205 to −0.039; P < 0.01) as well as fully adjusted (β = −0.141; 95% CI = −0.240 to −0.041; P < 0.01) prenatal stress coefficients were larger in the women-only models. The coefficients for all covariates for the unadjusted and fully adjusted regression models predicting LTL are depicted in Table S1. In the interest of presenting complete data, we include the regression coefficients for the men-only group; however, the relatively smaller number of men in the study sample and the larger variation in their estimates of LTL preclude inference regarding possible sex-specific effects.
On average, there was a 178-bp difference in LTL between PSG and CG in the whole group [i.e., unadjusted effect; effect size [Cohen's d] = 0.41 SD units], and a 295-bp difference in LTL between PSG and CG in the women-only group (d = 0.68 SD units).
Discussion
To the best of our knowledge, this is the first report in humans that demonstrates that exposure to maternal psychosocial stress during intrauterine life is associated subsequently with significantly shorter LTL in young adulthood. This effect persists after adjusting for a number of potential confounders, including age, sex, BMI, birth weight, postnatal/early-life adversity, and concurrent life stress. This finding suggests that cellular aging in humans may be influenced by prenatal stress, thereby potentially increasing the susceptibility of prenatally stressed individuals for complex, common age-related diseases. The magnitude of the observed difference in LTL between the prenatal stress exposure group and the CG is striking (0.41 SD units), and particularly so in the female offspring (0.68 SD units). The LTL of individuals in the prenatally stressed individuals was, on average, 178 bp shorter than that of individuals in the CG (and 295 bp shorter in female subjects). The most recent and comprehensive review of studies of age-related attrition in telomere length suggests that, in adults, telomere length attrition averages approximately 60 bp/y at 20 y of age, and the attrition rate appears to decrease to approximately 20 bp/y by age 80 y (40). Given that the participants in our study were approximately 25 y old, translating telomere shortening of this observed difference of 178 bp (295 bp for the women-only group) to years of aging indicates that the lymphocytes individuals in the PSG had aged the equivalent of approximately 3.5 additional years (5 additional years in the women-only group) relative to those in the CG.
One of the major paradigms to explain variation in susceptibility for complex, common adverse health disorders in adult life is the fetal or developmental origins model, which is believed to act through “biological embedding”—the ability of early life experience to change biology (41–44). Classic examples include fetal programming effects, such as maternal stress or undernutrition leading to fetal growth retardation, compromises in fetal cerebral development, catch-up growth, and early onset of insulin resistance and adult cardiometabolic diseases (1, 2, 45, 46). It has been suggested that telomere length may, in part, underlie this association (47–49). Telomere length differs widely at birth between babies. Okuda et al. (50), one of the few groups of investigators who have studied LTL in early life, state that “the variability in telomere length among newborns and synchrony in telomere length within organs of the newborn are consistent with the concept that variations in telomere length among adults are in large part attributed to determinants that start exerting their effect in utero.” Accordingly, it has been hypothesized that prenatal stress in utero would lead to shorter adult LTL (9). Our findings lend support for this concept.
There are several mutually nonexclusive pathways that may have led to the striking observation in the present study. There may be latency effects from early life that emerge at a later time point, or pathway effects wherein early stress leads to later vulnerability throughout the lifespan (4, 41). In terms of biological mechanisms, prenatal psychosocial stress exposure could affect cellular aging through several mechanisms: changes in immune function, changes in metabolic and oxidative stress-related pathways, and/or changes in telomerase activity. These changes, in turn, may be mediated by epigenetic modifications, thereby setting up long-term trajectories (27, 51). Stress is transduced from the pregnant mother to her fetus through various pathways, including transplacental transport of the stress hormone cortisol, maternal stress-induced release of placental hormones that enter the fetal circulation (e.g., placental corticotrophin-releasing hormone), and maternal stress-induced effects on placental physiology, including alterations in blood flow and changes in metabolism impacting oxygen and glucose availability and use (52, 53). Exposure to high levels of maternal stress hormones during pregnancy is known to produce deleterious effects on the offspring's developing immune system (32, 54, 55), and our previously published studies in this cohort have reported that the prenatally stressed individuals exhibited alterations in several immune parameters (33), including higher phytohemagglutinin-stimulated levels of IL-6, a proinflammatory cytokine that has been associated with shorter telomere length (56, 57). We also have found that the prenatally stressed individuals in this cohort exhibited insulin and leptin resistance, as well as a higher BMI (31). As insulin resistance is associated with chronological age, and longstanding insulin resistance can accelerate biological aging (58–60), it is possible that insulin resistance also may have contributed to more rapid cellular aging in the PSG individuals. Notably, we have described in this cohort alterations in the regulation of the hypothalamic–pituitary–adrenal axis in the PSG individuals (34). Finally, other studies found that blood levels of oxidative stress—a factor promoting telomere shortening (61)—may be elevated, in part, by stress hormones and insulin (62). Although we have previously reported these associations between prenatal stress exposure and immune, metabolic, and endocrine dysregulation in this study cohort, and the current report extends these findings to shortened telomere length, the cross-sectional design of this study precludes us from determining the temporal sequence and interrelationships between these different outcomes. Prospective, longitudinal studies are currently under way to address this important question.
The caveat that correlation cannot establish causality is well recognized. Although the use of animal models confers many benefits, such as the ability to perform experimental manipulations, one of their major limitations, particularly for research in the area of intrauterine development, is the considerable interspecies variation in the physiology of pregnancy and in the developmental timeline (63, 64). Because it is not possible to randomly assign humans to prenatal stress exposure, we approximated experimental exposure by using a quasiexperimental design [see, e.g., the considerations about association and causation first articulated by Bradford Hill in his seminal 1965 article (65), and elaborated subsequently by others (66–68)]. It is possible that the effects of prenatal stress exposure may be confounded or exacerbated or attenuated by postnatal experience. However, another strength of the present study was the ability to eliminate the potential confounding effects of many of these factors by study design (exclusionary criteria, e.g., obstetric risk, adverse birth outcomes, smoking, concurrent diseases). In addition, we assessed and accounted for the possible effects of several other key potential confounding factors, e.g., subject characteristics like age, BMI, sex, postnatal/early-life adversity, and exposure to concurrent life stress. The effect was substantially unchanged after adjusting for these potential confounders. Furthermore, the effect was not altered after entering birth weight into the model, suggesting that the association between prenatal stress and LTL was independent of alterations in birth weight. Last, it is established that stressful life events occur more frequently in individuals of lower socioeconomic status; however, we note that the SES range was narrow and did not differ across groups.
The present study had some limitations. First, prenatal stress exposure was assessed retrospectively. Although retrospective assessments of psychosocial factors such as stress are prone to biases such as “after-the-fact” reporting (i.e., individuals who develop health disorders are more prone to retrospectively report higher levels of adverse exposures before the development of the disorder) and those produced by memory and current psychological state (affect/mood), we believe it is unlikely these biases significantly impacted our assessment of prenatal stress in the present study. All subjects were healthy young adults (i.e., with no underlying disease); they received identical information before and upon entering the study; they were not provided any information about the study hypotheses; and they (as well as the experimenters) were blinded to and had no a priori knowledge about the expected direction of study findings. Subjects in the two groups did not differ in their current baseline psychological state (e.g., depressive symptoms, perceived stress) or memory performance scores. Moreover, our use of major negative life events to retrospectively assess psychosocial stress exposure provides greater confidence for construct validity than would have been the case for retrospective assessments of other components of stress, such as perceived severity of stress appraisals or stress symptoms. We note, however, that the occurrence of a major negative life event in the index pregnancy does not constitute a single, discrete programming event; it is well established that acute circumstances such as stressful life events produce chronic psychological distress and a concomitant cascade of progressive alterations in stress-related immunological, endocrine, and cellular physiological processes. Second, men were underrepresented in the study. We speculate that the difference in the numbers of men and women who volunteered to participate may relate to the observation that women are likely to be more interested in issues and questions concerning pregnancy. Some studies suggest there are sex-specific effects of prenatal stress exposures on various outcomes. However, given the smaller number of men who participated in the study and the larger variation in the distribution of their LTL, we, unfortunately, did not have the statistical power to examine and draw any inferences about possible sex-specific programming effects.
To summarize, prenatal stress exposure was a significant predictor of subsequent LTL, a marker of cellular aging, in young adults. The predicted decrease in LTL was unchanged after adjusting for several key potential confounding factors and was particularly pronounced in women. The magnitude of the effect equates to a difference of approximately 0.41 SD units (and 0.68 SD units among female subjects), and translates clinically in terms of the lymphocyte aging rate to an approximate difference of 3.5 y (and 5 y among female subjects). Many questions remain, such as those regarding the exact mechanisms underlying prenatal programming of telomere length and the directionality of the associations between prenatal stress, telomere length, and later health outcomes (i.e., whether shortened telomeres precede and play a causal role in the onset of insulin resistance, immune dysfunction, and other markers of physiological dysregulation in prenatally stressed individuals, or whether prenatal stress, through a common mechanism, simultaneously influences telomere length and physiological function). To address these questions, a multilevel approach is required that includes molecular and cellular studies, the use of appropriate animal models, and well designed prospective, longitudinal human studies. Nonetheless, the results of the present study are an important first step, and they add further evidence to the growing awareness that predisease pathways for common, complex age-related disorders may have their foundations very early in life.
Methods
Sample.
The study sample consisted of 94 subjects: 45 whose mothers experienced a high level of psychological stress during the index pregnancy (as detailed later) constituted the PSG and 49 whose mothers had a healthy, uneventful index pregnancy constituted the CG. All subjects were of Western European decent. The characteristics of the study population are provided in Table 1.
Procedures.
Study participants were recruited in Trier, Germany, through an announcement in local newspapers and a solicitation to local university students. Before entering the study, the absence of acute or chronic health conditions was ascertained by self-report and confirmed by a medical examination. All subjects were nonsmokers and reported to be medication-free except for oral contraceptives. A copy of the prenatal medical record (which is provided to every mother during her prenatal care) was obtained from each participant. From this record, information was abstracted about maternal age, parity, obstetric complications (e.g., gestational diabetes, hypertension/preeclampsia, infection), birth outcomes (length of gestation/preterm birth and birth weight/small-for-gestational-age birth, height, and head circumference at birth), and newborn complications. Participants received a modest monetary compensation on completion of the study assessments. All investigations were conducted in accordance with the guidelines described in the Declaration of Helsinki, the study protocol was approved by the ethics committee of the German Psychological Society, and written informed consent was obtained from all participants.
Prenatal Stress Measurement.
We adopted a conservative strategy for the conceptualization of prenatal stress (as described elsewhere; e.g., refs. 31, 34). Briefly, we defined a high level of prenatal psychosocial stress exposure as the presence of major negative life events that occurred to the mother during her index pregnancy (i.e., after conception and before birth). We selected a list of negative life events that are considered highly stressful across individuals [e.g., death or sudden severe illness of an immediate family member, loss of primary residence (34)]. All subjects underwent semistructured interviews about maternal exposure to these major negative life events during gestation and were instructed to review the items with their mothers before the interview. To minimize potential self-selection bias and retrospective after-the-fact recall bias, subjects were not informed about the study hypotheses, all subjects received the same information before entering the study, and the experimenters were blind to subject group status (PSG or CG).
Measurement of Potential Postnatal Confounders.
As it is possible that prenatal stress exposure is associated with adverse postnatal experiences such as the presence of other stressors and adverse conditions during childhood, we assessed and controlled for these potential confounding factors, including the presence of traumatic events in childhood (including death of a close relative, separation from a parent, and sexual or physical abuse), self-reported quality of maternal care, and concurrent levels of chronic stress and depressive symptoms at the time of the study assessments (refs. 31, 34 describe the measures).
Telomere Length Assay.
Blood was drawn from the participants in the early afternoon and stored at −80 °C for the assessment of LTL. An additional peripheral blood sample was collected at the same time point to determine total number of leukocytes, lymphocytes, as well as lymphocyte subpopulations [CD3−/CD19+ (B cells), CD3+/CD4+ (T-helper cells), CD3+/CD8+ (T-cytotoxic cells), CD3−/CD16, 56+ (NK cells); methods described in ref. 32)]. PSG and CG subjects did not differ significantly in any of these measures.
Whole blood samples were shipped for telomere length assays to the Blackburn laboratory at the University of California, San Francisco. The laboratory personnel were blinded to group membership status of the blood samples (PSG or CG). DNA was extracted by using a QIAamp DNA blood mini kit. Measurement of relative telomere lengths (i.e., T/S ratios) by quantitative PCR were adapted from a validated published method (69) and performed as described previously (70). The conversion from T/S ratio to base pairs was calculated based on the mean telomeric restriction fragment length from Southern blot analysis and the slope of the plot of mean telomeric restriction fragment length versus T/S for these samples. This was expressed as the following formula:
Data Analysis.
We first determined whether the effects of prenatal stress exposure and other covariates were statistically significant by using unadjusted and fully adjusted regression models. Next, for statistically significant effects, we calculated a standardized effect size (Cohen d effect size statistic) to place effects expressed in the original raw units of measurement (i.e., T/S ratio) in the more generic and dimensionless context of SD units (71). Finally, we expressed significant effect sizes in terms of their clinical or practical significance [i.e., difference in years of cellular aging (34)].
To examine the associations between prenatal stress and LTL, unadjusted and fully adjusted regression models were conducted, beginning with simple bivariate relationships between prenatal stress and other potential covariates and telomere length (i.e., unadjusted models) and then adding variables that could confound the association or that could be in the causal pathway. These variables included subject characteristics that have been associated with LTL in previous studies (i.e., age, BMI, sex), birth weight percentile (as a marker of other intrauterine conditions over and above prenatal stress exposure), postnatal/early-life adversity (i.e., childhood trauma exposure and perceived maternal care), and current chronic stress and depressive symptom levels. Because sex-specific effects of prenatal stress exposure have been reported for other outcomes in previous studies (72, 73), we were interested in addressing this question in the context of LTL. However, given the relatively smaller number of men and larger variation in the distribution of LTL compared with women in our study population, we were unable to model a statistical interaction between prenatal stress exposure and sex. We instead ran the same sets of regression models separately stratified by sex.
The unstandardized regression (β) coefficients from these models indicate the change in the T/S ratio associated with prenatal stress exposure and a one-unit change in other predictor variables. All statistical analyses were run using SPSS software (version 18), and the significance level was set at an α level of 0.05.
Supplementary Material
Acknowledgments
We thank Claudia Buss, Daniel L. Gillen, Keith M. Godfrey, Christopher W. Kuzawa, and James M. Swanson for useful discussions. This study was supported by US Public Health Service (National Institutes of Health) Grants R01 HD-06028 and P01 HD-047609 (to P.D.W.) and R01 HD-065825 (to S.E.), and by the Barney and Barbro Fund (E.H.B.).
Footnotes
Conflict of interest statement: E.S.E., J.L., and E.H.B. are cofounders of Telome Health, a company focused on telomere measurement.
This article is a PNAS Direct Submission.
See Author Summary on page 13377.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107759108/-/DCSupplemental.
References
- 1.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 2.Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison PT. Fetal programming and fetal psychology. Infant Child Dev. 2010;19:6–20. [Google Scholar]
- 4.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 5.Gluckman PD, et al. Towards a new developmental synthesis: Adaptive developmental plasticity and human disease. Lancet. 2009;373:1654–1657. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 6.Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA. 2007;298:1685–1687. doi: 10.1001/jama.298.14.1685. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 8.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epel E. Telomeres in a life-span perspective: A new “psychobiomarker”? Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- 10.Chan SW, Blackburn EH. Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol Cell. 2003;11:1379–1387. doi: 10.1016/s1097-2765(03)00174-6. [DOI] [PubMed] [Google Scholar]
- 11.Frenck RW, Jr, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Epel ES, Blackburn E. Telomeres, telomerase, stress and aging. In: Bernston GG, Cacioppo JT, editors. Handbook of Neuroscience for the Behavioral Sciences. New York: Wiley; 2009. [Google Scholar]
- 13.Blackburn EH. Telomere states and cell fates. Nature. 2000;408:53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 14.Serrano AL, Andrés V. Telomeres and cardiovascular disease: Does size matter? Circ Res. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- 15.Mather KA, Jorm AF, Parslow RA, Christensen H. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66:202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 16.Jaskelioff M, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parks CG, et al. Telomere length, current perceived stress, and urinary stress hormones in women. Cancer Epidemiol Biomarkers Prev. 2009;18:551–560. doi: 10.1158/1055-9965.EPI-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damjanovic AK, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J Immunol. 2007;179:4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epel ES, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31:277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Kiecolt-Glaser JK, Glaser R. Psychological stress, telomeres, and telomerase. Brain Behav Immun. 2010;24:529–530. doi: 10.1016/j.bbi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22:600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ornish D, et al. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs TL, et al. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36:664–681. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Tyrka AR, et al. Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biol Psychiatry. 2010;67:531–534. doi: 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiecolt-Glaser JK, et al. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Donovan A, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.01.035. 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drury SS, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68:e21–e22. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, et al. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS ONE. 2010;5:e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 31.Entringer S, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:498.e491–498.e497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- 33.Entringer S, et al. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55:292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. FEBS Lett. 1999;448:4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- 36.Tarry-Adkins JL, et al. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J. 2009;23:1521–1528. doi: 10.1096/fj.08-122796. [DOI] [PubMed] [Google Scholar]
- 37.Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J. 2008;22:2037–2044. doi: 10.1096/fj.07-099523. [DOI] [PubMed] [Google Scholar]
- 38.Biron-Shental T, et al. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR) Early Hum Dev. 2010;86:451–456. doi: 10.1016/j.earlhumdev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Raqib R, et al. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr. 2007;85:845–852. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- 40.Eisenberg DT. An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects. Am J Hum Biol. 2011;23:149–167. doi: 10.1002/ajhb.21127. [DOI] [PubMed] [Google Scholar]
- 41.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuzawa CW, Gluckman PD, Hanson MA, Beedle A. Evolution, developmental plasticity, and metabolic disease. In: Stearns SC, Koella JC, editors. Evolution in Health and Disease. 2nd Ed. Oxford: Oxford Univ Press; 2008. pp. 253–264. [Google Scholar]
- 43.Kuzawa CW, Quinn EA. Developmental origins of adult function and health: Evolutionary hypotheses. Annu Rev Anthropol. 2009;38:131–147. [Google Scholar]
- 44.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–610. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 46.Antonow-Schlorke I, et al. Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA. 2011;108:3011–3016. doi: 10.1073/pnas.1009838108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aviv A, Aviv H. Telomeres and essential hypertension. Am J Hypertens. 1999;12:427–432. doi: 10.1016/s0895-7061(98)00202-7. [DOI] [PubMed] [Google Scholar]
- 48.Demerath EW, Cameron N, Gillman MW, Towne B, Siervogel RM. Telomeres and telomerase in the fetal origins of cardiovascular disease: A review. Hum Biol. 2004;76:127–146. doi: 10.1353/hub.2004.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol. 2010;106:323–336. doi: 10.1016/j.pbiomolbio.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 50.Okuda K, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 53.Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: specific effects or induction of general susceptibility? Psychol Bull. 2004;130:115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- 54.Coe CL, Lubach GR, Karaszewski JW. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol Neonate. 1999;76:301–310. doi: 10.1159/000014172. [DOI] [PubMed] [Google Scholar]
- 55.Coe CL, Lubach GR, Karaszewski JW, Ershler WB. Prenatal endocrine activation alters postnatal cellular immunity in infant monkeys. Brain Behav Immun. 1996;10:221–234. doi: 10.1006/brbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 56.Carrero JJ, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263:302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 57.Fitzpatrick AL, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 58.Gardner JP, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- 59.Valdes AM, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 60.Al-Attas OS, et al. Adiposity and insulin resistance correlate with telomere length in middle-aged Arabs: The influence of circulating adiponectin. Eur J Endocrinol. 2010;163:601–607. doi: 10.1530/EJE-10-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 62.Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 63.Bowman ME, et al. Corticotropin-releasing hormone-binding protein in primates. Am J Primatol. 2001;53:123–130. doi: 10.1002/1098-2345(200103)53:3<123::AID-AJP3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 64.Jaffe RB. Role of human fetal adrenal gland in the initiation of parturition. In: Smith R, editor. The Endocrinology of Parturition. Newcastle, Australia: Karger; 2001. [Google Scholar]
- 65.Hill AB. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hoefler M. The Bradford Hill considerations on causality: A counterfactual perspective. Emerg Themes Epidemiol. 2005;2:11. doi: 10.1186/1742-7622-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Phillips CV, Goodman KJ. The missed lessons of Sir Austin Bradford Hill. Epidemiol Perspect Innov. 2004;1:3. doi: 10.1186/1742-5573-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward AC. The role of causal criteria in causal inferences: Bradford Hill's “aspects of association”. Epidemiol Perspect Innov. 2009;6:2. doi: 10.1186/1742-5573-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kraemer HC, et al. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 72.Bhatnagar S, Lee TM, Vining C. Prenatal stress differentially affects habituation of corticosterone responses to repeated stress in adult male and female rats. Horm Behav. 2005;47:430–438. doi: 10.1016/j.yhbeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Bowman RE, et al. Sexually dimorphic effects of prenatal stress on cognition, hormonal responses, and central neurotransmitters. Endocrinology. 2004;145:3778–3787. doi: 10.1210/en.2003-1759. [DOI] [PubMed] [Google Scholar]