Abstract
Anopheles gambiae sensu stricto is the major vector of malaria, a disease with devastating consequences for human health. Given the constant spread of the disease, alternative approaches to the use of insecticides are urgently needed to control vector populations. Females of this species undergo large behavioral changes after mating, which include a life-long refractoriness to further insemination and the induction of egg laying in blood-fed individuals. Genetic control strategies aimed at impacting Anopheles fertility through the release of sterile males are being advocated to reduce the size of mosquito field populations. Such strategies depend on the ability of the released sterile males to mate successfully with wild females and to switch off the female receptivity to further copulation. Here we evaluate the role of sperm in regulating female behavioral responses after mating in An. gambiae. We developed spermless males by RNAi silencing of a germ cell differentiation gene. These males mated successfully and preserved standard accessory gland functions. Females mated to spermless males exhibited normal postcopulatory responses, which included laying large numbers of eggs upon blood feeding and becoming refractory to subsequent insemination. Moreover, spermless males induced transcriptional changes in female reproductive genes comparable to those elicited by fertile males. Our data demonstrate that, in contrast to Drosophila, targeting sperm in An. gambiae preserves normal male and female reproductive behavior for the traits and time frame analyzed and validate the use of approaches based on incapacitation or elimination of sperm for genetic control of vector populations to block malaria transmission.
Keywords: oviposition, reproduction, sexual refractoriness, postmating behavior, insect
In the fight against arthropod-borne infectious diseases that affect humans, genetic control of the vector provides an attractive addition to the armory of tools already available. The release of sterile males to reduce the size of field populations (sterile insect technique, SIT) (1) is being suggested for many disease vectors, due to increased insecticide resistance in field populations that is reducing the effectiveness of chemical control. Anopheles gambiae sensu stricto females, the major vectors of human malaria, are good targets for SIT, as they mate a single time in their lifespan (2). After copulation, females of this species experience significant behavioral changes, which include the induction of egg laying in blood-fed individuals and a drastic reduction in their receptivity to further insemination (3). A sterile mating would therefore completely eliminate reproduction for the duration of their lives.
Important prerequisites for successful SIT in An. gambiae are that the released males must be fully competitive for mating and that they induce refractoriness to further copulations for the female lifespan, to ensure life-long sterility (4). Recent molecular and genetic advances are providing unique tools to generate male-only populations and to bring about sterility in males through genetic means that avoid the fitness costs caused by irradiation (5, 6). However, progress in our understanding of the mechanisms shaping male reproductive success and female postmating responses has been slow. A deeper knowledge of the processes that ensure fertility and regulate mating behavior in male and female mosquitoes would facilitate the deployment of genetic control of these vectors, which to date have been applied to Anopheles species with limited success (7).
The two major postmating responses in Anopheles females, the stimulation of oviposition in blood-fed individuals and the induction of refractoriness to further insemination, are likely to be triggered by factors transferred by the male during sex: sperm, generated in the testes and stored by the female in a dedicated receptacle named spermatheca, and seminal secretions, produced by the male accessory glands (MAGs). Seminal secretions are transferred as a gelatinous mating plug (8), which is digested in the atrium (uterus) of females within 24 h of its transfer. Mating plug formation is achieved by the cross-linking activity of a MAG-specific transglutaminase (TGase) on other MAG-expressed proteins (9). The processes of mating plug transfer and sperm storage are intimately coupled with one another: Females mated to males deficient for the plug-forming TGase (and therefore incapacitated in the coagulation and transfer of the mating plug) are not capable of correctly storing sperm in their spermathecae (9).
Female postmating responses are well characterized in Drosophila melanogaster, where mating induces a 1-wk-long refractoriness to further copulation and enhances the levels of egg laying. A wealth of studies has demonstrated that postmating behavior in the fruit fly is transiently induced by MAG proteins (in particular Acp70A, also known as Sex Peptide) transferred to the female reproductive tract (reviewed in ref. 10), whereas sperm transfer is required to extend these responses beyond the first day (11–13). Males lacking sperm induce normal refractoriness to mating and oviposition for 1 d postcopulation, after which virgin-like mating and egg-laying behaviors are resumed. This “sperm effect” is indirect and is mediated by MAG proteins bound to sperm tails that are slowly released after copulation (11, 12, 14).
In An. gambiae, some evidence about the relative role of sperm and seminal fluids in modulating female postmating behavior has been gathered in the past 40 y on the basis of indirect methods such as the use of hybrid males, forced matings, injections of tissue extracts, and surgical implantation or removal of tissues, although its interpretation is difficult and somewhat controversial (3, 15, 16). Although these studies suggest, if not irrefutably, a role for MAG secretions, the role of sperm in triggering and/or maintaining postmating responses remains uncertain. Hybrid An. gambiae/An. melas males with degenerate testes were still capable of inducing oviposition and refractoriness to mating in crosses with An. gambiae females (17), although the possibility that some dysfunctional sperm may have leaked into the spermathecae cannot be excluded. On the other hand, in a more recent study, it was reported that oviposition in An. gambiae requires an intact spermatheca filled with sperm. When the spermatheca was surgically removed or physically damaged to release the sperm, mated females did not oviposit (18).
Regardless of its triggers, the female response to mating in An. gambiae is characterized by conspicuous transcriptional changes, mostly in the atrium and spermatheca, where a large number of genes are strongly induced or repressed during the first day following copulation (19). The spermatheca experiences a fast induction of genes encoding nutrient transporters (vitellogenins and apolipoproteins) and factors preventing oxidative damage (peroxidases), suggesting a role of these genes in ensuring sperm viability and function (19). The atrium in comparison appears primed for insemination and expresses in its virgin status a number of genes, among which are many proteases, which are strongly down-regulated following copulation and may be involved in processing of the mating plug (9, 19).
Here we report molecular and genetic studies aimed at assessing the role of sperm in modulating female behavior after mating. Using males in which sperm development has been aborted by targeting a gene required for early germ cell differentiation (zero population growth, zpg) (20), we demonstrate that spermless males induce bona fide postmating responses in An. gambiae females.
Results
Injections of Double-Stranded RNA Targeting zpg (Dszpg) Do Not Affect the Functionality of the Male Accessory Glands.
Spermless males (Spm−) were developed by RNA interference (RNAi), performing injections of dszpg in embryos from an An. gambiae transgenic line. As described in Materials and Methods, these males exhibited very small testes, and microscopic analysis confirmed that their gonads did not contain any sperm cells (Fig. S1). Before using Spm− males to assess the role of sperm in inducing postmating responses in females, we ensured that dszpg injections had not affected the normal secretion and transfer of seminal fluids produced by the other principal male reproductive tissues, the MAGs. In all cases analyzed, females mated to Spm− males showed the presence of a normal mating plug in their atrium, indicating standard function of the male glands. Furthermore, we analyzed by quantitative reverse transcription PCR the expression levels of three MAG-specific genes coding for major mating plug proteins, including the plug-forming TGase (9, 21), in Spm− males compared with uninjected control males (Spm+). In a number of independent samples, we found no difference in the expression levels of MAG genes between Spm+ and Spm− males (two-tailed Mann–Whitney test, P > 0.05) (Fig. S2). As expected, no sperm was detected in the spermatheca of females mated to Spm− males (Fig. S3). Altogether, these data show that dszpg injections, although generating males with no sperm, had no negative effects on the production, secretion, and function of MAG proteins. This result allowed us to validate the use of Spm− males to assess the role of sperm in inducing postmating responses in An. gambiae females.
Sperm Are Not Required to Trigger Oviposition.
To characterize the role of sperm in stimulating oviposition, we compared the egg-laying behavior of females mated to Spm+ and Spm− males. A total of 55 Spm− males were generated in two sets of dszpg injections and used in mating experiments with virgin females. A similar number of Spm+ males were used as controls. Mating couples were captured during copulation in experimental and control groups, and mated females were blood fed and placed into single cups for oviposition. As an additional control, age-matched virgin females were also blood fed and allowed to lay eggs. No difference was observed in the ability of Spm− and Spm+ males to mate, and similar numbers of mated couples were obtained in each group.
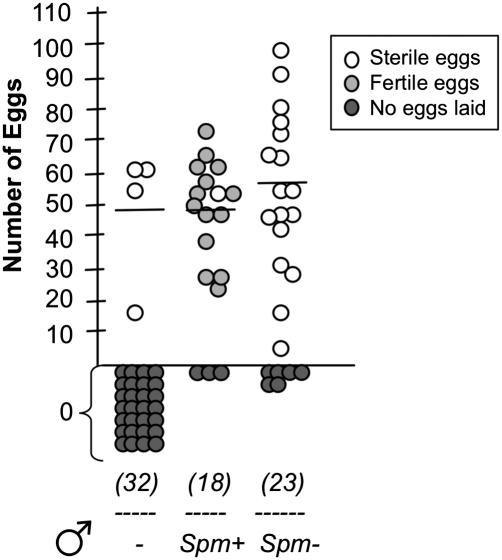
In the group containing females mated to spermless males, 74% of females laid eggs, with an average of 58 eggs/female (Fig. 1). None of the egg batches hatched, as expected given that the females had not received sperm from their mates. The spermathecae of females mated to Spm− males were dissected after oviposition, and in all cases no spermatozoa could be detected, confirming that sperm transfer had not occurred (Fig. S3). In the control group, 83% of females mated to Spm+ males oviposited, laying on average 49 eggs/female. These eggs were fertile, as females had received sperm from the male. No significant difference in egg laying could be found between the two groups (two-tailed Mann–Whitney test, P > 0.05). Virgin females did not lay eggs, with the exception of a small percentage (13%) that laid (sterile) egg batches, as sometimes observed with older virgins (Fig. 1). Compared with the low rate of oviposition observed in virgin females, the vast proportion of females that laid eggs after mating with Spm− males demonstrates that sperm transfer is not required to trigger egg laying in An. gambiae females (chi-square test, P < 0.0001).
Fig. 1.
Sperm is not required to induce oviposition. Virgin females were mated to males with sperm (Spm+) or males with no sperm (Spm−), and after blood feeding the number of eggs laid by each female was counted. A control group of virgin blood-fed females was also included in the analysis (−). The numbers in parentheses indicate the total number of mated females used in the analysis (in two independent experiments). The average number of eggs laid is indicated, excluding females that did not oviposit.
Females Mated to Spermless Males Become Refractory to Further Insemination.
We then assessed whether sperm play a role in reducing female receptivity to further mating. Virgin females were mated to Spm− or control Spm+ males. Two days following this initial copulation (to provide enough time for mating plug digestion), mated females from both groups were placed in cages containing an excess of wild-type males to allow remating to occur. After 2 d with wild-type individuals, females were blood fed and allowed to lay eggs individually. If sperm were essential for switching off the female receptivity to further mating, we would expect that females receiving no sperm when mating to spermless individuals would remate and become inseminated by wild-type males, thereby producing fertile egg batches. In the control experiments, we analyzed the progeny of females initially mated to Spm+ males (derived, as well as the Spm− males, from a DsRed-marked transgenic line, as described in Materials and Methods) and then exposed to wild-type males. These controls would allow us to assess the frequency of multiple inseminations in this setting, measured as the number of females producing mixed transgenic and wild-type progeny. The occurrence of multiple mating, which is a rare event in the field (3), has instead been reported in laboratory cages (22).
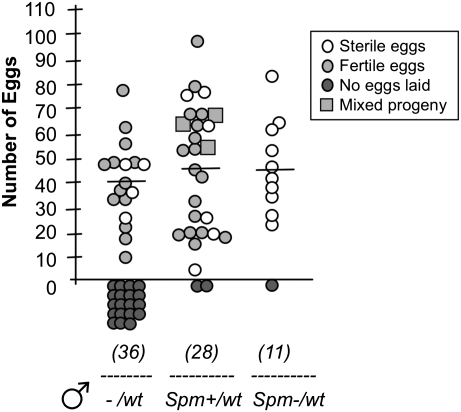
From two independent experiments, we obtained a total of 11 females that were mated to Spm− males and reexposed to wild-type males. None of the 10 egg batches (91%) obtained from these females hatched, indicating that insemination by wild-type males had not occurred (Fig. 2). In the control group, 26 of 28 females (93%) mated to Spm+ males laid eggs. Among the 20 egg batches that hatched, 17 showed the presence of the transgenic DsRed allele alone, as expected if only the initial mating had occurred. Three egg batches produced both transgenic and wild-type larvae, indicating reinsemination at a frequency of 15%, consistent with previous laboratory studies (22) (Fig. 2).
Fig. 2.
Females mated to spermless males are refractory to further insemination. Virgin females were captured while mating to males with sperm (Spm+) or males with no sperm (Spm−), and after 2 d mated females were placed with wild-type (wt) males for a further 2 d. A control group of virgin females was also mated to wild-type males for 2 d (−/wt). After blood feeding, females were allowed to oviposit, the number of eggs laid was counted, and hatched larvae were screened for transgenic (DsRed) and wild-type phenotypes to assess occurrence of reinsemination. Mixed progeny refers to the presence of both transgenic and wild-type alleles, indicative of reinsemination. The numbers in parentheses indicate the total number of females used in the analysis (in two independent experiments). The average number of eggs laid is indicated, excluding females that did not oviposit.
Females mated to spermless males were 6–7 d old when they were exposed to wild-type males to assess the occurrence of remating. We verified that aged-matched 6- to 7-d-old virgin females could become inseminated when placed with wild-type males for 2 d. Even at a low male:female ratio (using a 1.8-fold excess of females, compared with a 2.5- to 10-fold excess of males in the remating experiments), virgin females mated and laid fertile eggs, showing that insemination occurs in females of that age (Fig. 2). A significant difference was found between the number of fertile females in this control group and in the group mated to spermless males (Fisher's exact test, P < 0.02). Altogether, these data show that females do not require the presence of sperm as a signal for switching off their mating receptivity for at least 4 d following mating.
Spermless Males Induce Transcriptional Responses in Females.
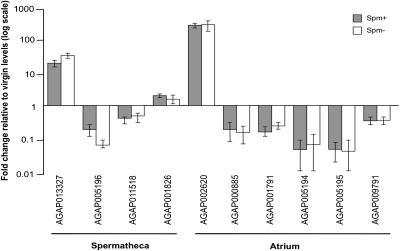
We then assessed whether the extensive transcriptional changes normally triggered by mating in the female lower reproductive tract are dependent upon sperm transfer. We established mating experiments between virgin females and Spm− or Spm+ males and dissected the atrium and spermatheca at 24 h postcopula and in age-matched virgins. Quantitative reverse transcription PCR was used to analyze the expression levels of all genes previously shown to be modulated in the female reproductive tract at that time point after mating (19). Among the genes analyzed, four were regulated exclusively or predominantly in the spermatheca (the heme peroxidase AGAP013327, the serine protease AGAP005196, the ABC transporter AGAP011518, and the apolipophorin AGAP001826), and six were predominantly regulated in the atrium (a gene of unknown function switched on by mating, AGAP002620, and five down-regulated proteases).
Regardless of their tissue of expression, no significant differences in the transcription levels of the 10 genes were detected in Spm+ and Spm− matings (Fig. 3, two-tailed Mann–Whitney test, P > 0.05). These results suggest that spermless males are capable of modulating the female transcriptional response to mating in a comparable manner to control males.
Fig. 3.
Transcriptional regulation of female reproductive genes by Spm+ and Spm− males. Shown is the expression profile of female reproductive genes 24 h after copulation with Spm+ or Spm− males, relative to virgin levels (indicated by the horizontal line set at 1). The data represent three biological replicates run in duplicate by qRT-PCR. Error bars indicate SEM. Genes are represented by their vectorbase identifiers (www.vectorbase.org). The predominant tissue of expression (spermatheca or atrium) is indicated.
Sperm Do Not Play a Role in Sustaining Long-Term Oviposition Behavior.
To ascertain whether the absence of sperm could reduce the time span of the female response to mating in Anopheles, we compared the number of eggs laid by females that were blood fed 24 h and 4 d after mating with spermless males (Figs. 1 and 2). If the oviposition response were short-lived in the absence of sperm, we would expect the egg-laying rate to decline rapidly within a few days of copulation to reach the levels detected in virgins, as observed in Drosophila. No difference was instead found in the number of eggs laid by the two groups (58 and 47 eggs/female laid at 24 h and 4 d postmating, respectively; two-tailed Mann–Whitney test, P > 0.05), indicating that the presence of sperm is not needed to sustain long-term egg-laying behavior.
Discussion
The data reported here show that An. gambiae mosquitoes do not rely on sperm for triggering postmating responses in females, namely the stimulation of oviposition and the induction of refractoriness to further insemination. By generating males without sperm through the RNAi-mediated silencing of zpg, a gene needed for germ cell development, we examined the role of sperm in female postcopulatory behavior without affecting the function of the male accessory glands, where seminal fluids are produced. These spermless males appeared competent for mating, were fully capable of inducing oviposition, and switched off female receptivity to further insemination. Matings with sperm-deficient males achieved complete sterility, as the 34 females mated to them produced no progeny.
Previous experiments had suggested that a spermatheca filled with sperm was necessary to activate at least one female postmating response: Mated An. gambiae females did not oviposit when their spermatheca was surgically removed or damaged to release sperm (18). As our data show that egg laying is independent of sperm transfer, a possible explanation for this discrepancy is that signaling from the spermatheca (rather than the presence of sperm in the storage receptacle) is needed for the occurrence of this female postmating response.
Spermless males induced transcriptional changes in the female lower reproductive tract similar to those caused by fertile individuals. Although we were expecting to see normal transcriptional modulation of genes in the atrium, the observation of transcriptional changes in genes expressed in the spermatheca was perhaps more surprising. In Drosophila, more female genes were differentially regulated after mating as a result of sperm transfer (549 genes) compared with MAG proteins alone (160 genes) (23). In our system, none of the 10 genes analyzed showed sperm-dependent regulation after mating. Although firm conclusions cannot be drawn given the limited number of genes available for our analysis (19), these findings suggest that the mating-induced modulation of expression of reproductive genes is strongly coupled to female postmating behavior, as both are maintained in copulations that fail to achieve sperm transfer. Further studies will be needed to verify the extent of the lack of sperm-mediated regulation at the whole-genome scale and to establish its biological significance.
Our results reveal that Anopheles and Drosophila males use at least partially different strategies to modulate female physiology and behavior. In Drosophila, the slow release of MAG proteins bound to sperm tails is needed to extend the female responses to mating from 1 d up to a week (11, 12, 14). In An. gambiae instead, sperm transfer does not appear to be necessary for prolonging the normal female behavior beyond the first day after copulation. No difference was found in the egg-laying ability of females mated to spermless males at 1 d compared with 4 d after mating, and female mating receptivity was abolished for at least 4 d after copulation. Although we cannot absolutely conclude that the mating refractoriness induced in females by spermless males is permanent, our data highlight major differences in the postmating physiology of Anopheles and Drosophila.
The different roles that sperm play in Drosophila and Anopheles female behavior may reflect the distinct challenges faced by males from polyandrous compared with monandrous species in terms of sperm competition and cryptic female choice. The evolution of monogamy is complex and involves selection acting on both the female and the male, for example on sperm volume in males and in females to avoid costly male manipulation. Males in Drosophila transfer only limited sperm volume (24) (perhaps because they withhold sperm for further matings); therefore there is a real risk the female will exhaust sperm supplies and will need to remate. The sperm in the fruit fly may therefore have been selected to signal their presence to the female to reduce the probability she remates. Although the genetic bases of monandry in An. gambiae females are unknown, the fact that they normally mate once suggests that sperm will not be selected continuously to signal their presence. Moreover, as successful sperm storage is dependent upon mating plug formation (9), it seems reasonable that Anopheles females might use the presence of a complete mating plug in their uterus rather than a full spermatheca to assess successful insemination by a competent male.
The results presented here point to a crucial role for MAG secretions as regulators of female postmating responses in Anopheles. Previous work, based on the injection of accessory gland extracts or on the use of hybrid males with degenerate reproductive organs, has provided contradictory evidence about MAG function (3, 15, 16). The experimental prevention of plug transfer or the generation of MAG-depleted males will be needed to determine conclusively whether MAG secretions are the major modulators of female behavior.
Our data validate targeting sperm as an excellent means for reducing the fertility of field populations. Spermless males maintain normal mating behavior and induce sterility in females, two critical prerequisites for the design of control strategies involving SIT (where sterility is induced either through irradiation or genetically). In SI Text we describe a population dynamic model to assess the consequences of remating for the size of releases required for population eradication. Even low levels of remating have a major negative impact on the number of individuals required for successful eradication (see example in Fig. S4), and where remating is frequent then this control method is not feasible. The model supports the use of males with genetically ablated testes as an SIT control strategy and shows the critical importance of maintaining normal female nonreceptivity to further mating. It also shows that the number of insects that will be required for successful control depends on the relative competitiveness of manipulated and wild-type males, something that must be assessed under field conditions. There is evidence from other systems that sperm production is metabolically costly (25), and there is thus a possibility that manipulated males might even be at an advantage compared with wild-type individuals.
A detailed understanding of the molecular basis of fertility and reproductive behavior in An. gambiae will be essential to the successful implementation of genetically based vector control strategies to reduce the scourge of malaria in Africa.
Materials and Methods
Generation of Spermless Males.
A total of 9,605 embryos from an An. gambiae transgenic line in which sperm had been marked with an eGFP fluorescent marker (26) were injected in a number of RNAi injection experiments essentially performed as previously described (27). Embryos were injected with 1.5 μg/μL of double-stranded RNA (dsRNA, approximate injected volume 0.1–0.3 nL), targeting the putative An. gambiae ortholog of zpg (AGAP006241) (28), a gene determining germ cell development (20). The dszpg was prepared according to the manufacturer's guidelines (Ambion), using a PCR template that was amplified from mosquito cDNA using primers with T7 overhangs (forward primer 5′-TAATACGACTCACTATAGGGCTCGTGAACGTGATCTTTTCC-3′ and reverse primer 5′-TAATACGACTCACTATAGGGCGGCCCGACGAAGTGG-3′) (28). The expression of the eGFP fluorescent marker in the sperm cells (6) allowed us to screen at the pupal stage for male individuals that did not develop sperm. Pupae were screened using a Nikon inverted microscope (Eclipse TE200) at a wavelength of 563 nm for DsRed (the selectable marker, to make sure the individual was transgenic) and 488 nm for eGFP expression, to visualize the presence/absence of sperm in male pupae. Of the 172 adult males obtained in the dszpg injection experiments, 96 were spermless (Spm−) (corresponding to 1% of total injected embryos and to 56% of the number of adult males surviving injections). These males exhibited very small testes and microscopic analysis confirmed that their gonads did not contain any sperm cells (Fig. S1). As a control for the direct effects of dszpg on sperm development, we injected 1,214 embryos with dsRNA targeting the bacterial gene LacZ. All surviving adult males (∼40 individuals) had fully developed sperm in the testes, showing that the impairment of sperm development in dszpg injections was due specifically to silencing of the zpg gene. Spermless males were used in mating experiments with virgin females, to perform oviposition and remating assays.
Isolation of Mating Couples.
An. gambiae mosquitoes from the G3 strain were used as the wild-type colony providing virgin females for all experiments. Mating couples were isolated essentially as described (19). Briefly, male (Spm+ or Spm−) and female (wild-type) pupae were separated under a dissection microscope (Nikon SMZ1000) and allowed to eclose in separate cages. After eclosion, mating was induced by introducing 2- to 3-d-old virgin females into cages containing control uninjected virgin males having normal sperm (Spm+) or virgin spermless (Spm−) males (4–6 d old). Mating pairs were observed and isolated when they dropped to the ground, gently placing modified Falcon tubes over the couple without disturbing the copulation process. After completion of copulation, mating pairs were removed from cages and the females (and in some cases the males) analyzed.
Oviposition Assay.
Females mated to control (Spm+) or spermless (Spm−) males were blood fed and placed into single plastic cups aligned with a 5-cm strip of filter paper inside and filled with ∼50 mL water at the bottom. The strip ensures that laid eggs remain hydrated and enable larvae to hatch into the water. Only females that had fully engorged in blood were used for the analyses. The numbers of eggs laid and of hatched larvae were recorded in two independent experiments.
Reinsemination Assay.
Virgin females were captured in two separate experiments while mating to Spm+ or Spm− males (homozygotes for the DsRed selectable marker) as described above and allowed to recover for 48 h. After these 48 h, females mated to Spm+ and Spm− were then placed in separate cages containing a 2.5- to 10-fold excess of wild-type males for 48 h to allow remating to occur. After 48 h exposure with wild-type males, females were blood fed and allowed to lay individual egg batches (as described in the oviposition assay). In females initially mated to Spm+ males, all progeny should be DsRed+ unless reinsemination (indicated by the presence of wild-type larvae) had occurred. In females initially mated to Spm− males, eggs should be sterile except in the event of remating with wild-type males, which would produce fertile (wild-type) progeny. We verified that age-matched virgin females could become inseminated when mated to wild-type males for 2 d at the time of the second mating for their sisters. Even at low male:female ratios (1:1.8), virgin females readily mated and laid fertile eggs.
RNA Extraction and cDNA Synthesis.
For tissue-specific analyses, female atria/spermathecae or male accessory glands (dissected at 24 h postmating from five mated females or males, respectively) were isolated in RNAlater (Ambion), and RNA was extracted from three biological replicates using TRI reagent (Ambion) according to the manufacturer's guidelines. The samples were subsequently treated with DNase I (Invitrogen) to avoid DNA contamination. The cDNA synthesis was performed in a 100-μL reaction volume containing 1× First Strand buffer, 5 mM DDT, 0.5 mM dNTPs, 2.5 μM random hexamers, 40 units RNaseOut recombinant ribonuclease inhibitor, and 125 units of M-MLV reverse transcriptase (all reagents from Invitrogen).
Quantitative Reverse Transcription PCR.
Quantification of tissue-specific gene expression was obtained using Fast SYBR Green Master Mix (Applied Biosystems) by the ΔΔCt method of relative quantification. Three biological repeats were run in duplicates on a StepOnePlus Real-Time thermocycler (Applied Biosystems) in 15-μL reactions as previously described (19). Gene expression was normalized against the ribosomal protein L19 (AGAP004422), which is stably expressed and is not regulated by mating.
Supplementary Material
Acknowledgments
We thank Elena Levashina, Francesco Baldini, Paolo Gabrieli, and Robert W. Shaw for helpful suggestions with the manuscript. We thank David Rogers, Francesco Baldini, Hazel Williams, Ann Cronin, Robert W. Shaw, and Wilfredo Mathias for help with laboratory and mosquito procedures. This work was funded by a Medical Research Council Career Development Award (Agreement ID 78415, File G0600062) and by the European Community FP7 Collaborative Project 223601 “Malvecblok” (to F.C.). J.T. was supported by a Grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health initiative. H.C.J.G. is part of the Research and Policy for Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104738108/-/DCSupplemental.
References
- 1.Knipling EF. Sterile-male method of population control. Science. 1959;130:902–904. doi: 10.1126/science.130.3380.902. [DOI] [PubMed] [Google Scholar]
- 2.Tripet F, Touré YT, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68:1–5. [PubMed] [Google Scholar]
- 3.Klowden MJ. Sexual receptivity in Anopheles gambiae mosquitoes: Absence of control by male accessory gland substances. J Insect Physiol. 2001;47:661–666. doi: 10.1016/s0022-1910(00)00127-x. [DOI] [PubMed] [Google Scholar]
- 4.Whitten M, Mahon R. Misconceptions and constraints. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht, The Netherlands: Springer; 2005. pp. 601–626. [Google Scholar]
- 5.Windbichler N, Papathanos PA, Crisanti A. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality in Anopheles gambiae. PLoS Genet. 2008;4:e1000291. doi: 10.1371/journal.pgen.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23:1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 7.Benedict MQ, Robinson AS. The first releases of transgenic mosquitoes: An argument for the sterile insect technique. Trends Parasitol. 2003;19:349–355. doi: 10.1016/s1471-4922(03)00144-2. [DOI] [PubMed] [Google Scholar]
- 8.Giglioli MEC, Mason GF. The mating plug of anopheline mosquitoes. Proc R Entomol Soc Lond. 1966;41:123–129. [Google Scholar]
- 9.Rogers DW, et al. Transglutaminase-mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol. 2009;7:e1000272. doi: 10.1371/journal.pbio.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravi Ram K, Wolfner MF. Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr Comp Biol. 2007;47:427–445. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- 11.Chapman T, et al. The sex peptide of Drosophila melanogaster: Female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA. 2003;100:9923–9928. doi: 10.1073/pnas.1631635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Kubli E. Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA. 2003;100:9929–9933. doi: 10.1073/pnas.1631700100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manning A. The control of sexual receptivity in female Drosophila. Anim Behav. 1967;15:239–250. doi: 10.1016/0003-3472(67)90006-1. [DOI] [PubMed] [Google Scholar]
- 14.Peng J, et al. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol. 2005;15:207–213. doi: 10.1016/j.cub.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 15.Bryan JH. Further studies on consecutive matings in the Anopheles gambiae complex. Nature. 1972;239:519–520. doi: 10.1038/239519a0. [DOI] [PubMed] [Google Scholar]
- 16.Shutt B, Stables L, Aboagye-Antwi F, Moran J, Tripet F. Male accessory gland proteins induce female monogamy in anopheline mosquitoes. Med Vet Entomol. 2010;24:91–94. doi: 10.1111/j.1365-2915.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- 17.Bryan JH. Results of consecutive matings of female Anopheles gambiae species B with fertile and sterile males. Nature. 1968;218:489. doi: 10.1038/218489a0. [DOI] [PubMed] [Google Scholar]
- 18.Klowden MJ. Switchover to the mated state by spermathecal activation in female Anopheles gambiae mosquitoes. J Insect Physiol. 2006;52:679–684. doi: 10.1016/j.jinsphys.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 19.Rogers DW, et al. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA. 2008;105:19390–19395. doi: 10.1073/pnas.0809723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tazuke SI, et al. A germline-specific gap junction protein required for survival of differentiating early germ cells. Development. 2002;129:2529–2539. doi: 10.1242/dev.129.10.2529. [DOI] [PubMed] [Google Scholar]
- 21.Dottorini T, et al. A genome-wide analysis in Anopheles gambiae mosquitoes reveals 46 male accessory gland genes, possible modulators of female behavior. Proc Natl Acad Sci USA. 2007;104:16215–16220. doi: 10.1073/pnas.0703904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomulski L. Polyandry in nulliparous Anopheles gambiae mosquitoes (Diptera: Culicidae) Bull Entomol Res. 1990;80:393–396. [Google Scholar]
- 23.McGraw LA, Gibson G, Clark AG, Wolfner MF. Genes regulated by mating, sperm, or seminal proteins in mated female Drosophila melanogaster. Curr Biol. 2004;14:1509–1514. doi: 10.1016/j.cub.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 24.Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- 25.Hayward A, Gillooly JF. The cost of sex: Quantifying energetic investment in gamete production by males and females. PLoS ONE. 2011;6:e16557. doi: 10.1371/journal.pone.0016557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papathanos PA, Windbichler N, Menichelli M, Burt A, Crisanti A. The vasa regulatory region mediates germline expression and maternal transmission of proteins in the malaria mosquito Anopheles gambiae: A versatile tool for genetic control strategies. BMC Mol Biol. 2009;10:65. doi: 10.1186/1471-2199-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catteruccia F, et al. Stable germline transformation of the malaria mosquito Anopheles stephensi. Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- 28.Magnusson K, et al. Transcription regulation of sex-biased genes during ontogeny in the malaria vector Anopheles gambiae. PLoS ONE. 2011;6:e21572. doi: 10.1371/journal.pone.0021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.