Abstract
Corticotropin-releasing hormone (CRH) and growth hormone-releasing hormone (GHRH), primarily characterized as neuroregulators of the hypothalamic-pituitary-adrenal axis, directly influence tissue-specific receptor-systems for CRH and GHRH in the endocrine pancreas. Here, we demonstrate the expression of mRNA for CRH and CRH-receptor type 1 (CRHR1) and of protein for CRHR1 in rat and human pancreatic islets and rat insulinoma cells. Activation of CRHR1 and GHRH-receptor significantly increased cell proliferation and reduced cell apoptosis. CRH stimulated both cellular content and release of insulin in rat islet and insulinoma cells. At the ultrastructural level, CRHR1 stimulation revealed a more active metabolic state with enlarged mitochondria. Moreover, glucocorticoids that promote glucose production are balanced by both 11b-hydroxysteroid dehydrogenase (11β-HSD) isoforms; 11β-HSD–type-1 and 11β-HSD–type-2. We demonstrated expression of mRNA for 11β-HSD-1 and 11β-HSD-2 and protein for 11β-HSD-1 in rat and human pancreatic islets and insulinoma cells. Quantitative real-time PCR revealed that stimulation of CRHR1 and GHRH-receptor affects the metabolism of insulinoma cells by down-regulating 11β-HSD-1 and up-regulating 11β-HSD-2. The 11β-HSD enzyme activity was analyzed by measuring the production of cortisol from cortisone. Similarly, activation of CRHR1 resulted in reduced cortisol levels, indicating either decreased 11β-HSD-1 enzyme activity or increased 11β-HSD-2 enzyme activity; thus, activation of CRHR1 alters the glucocorticoid balance toward the inactive form. These data indicate that functional receptor systems for hypothalamic-releasing hormone agonists exist within the endocrine pancreas and influence synthesis of insulin and the pancreatic glucocorticoid shuttle. Agonists of CRHR1 and GHRH-receptor, therefore, may play an important role as novel therapeutic tools in the treatment of diabetes mellitus.
Keywords: islet proliferation, regenerative therapy, metabolic syndrome
Receptor systems for the corticotropin-releasing hormone (CRH) and the growth hormone-releasing hormone (GHRH) are central regulators of the hypothalamic-pituitary-adrenal axis (HPA-axis). There is, however, increasing evidence for the presence of tissue-specific receptor systems for CRH and GHRH in several tissues involved in the regulation of immune and cardiovascular systems and energy homeostasis (1–5). Recently, a functional CRH receptor system expressing both CRH-receptors type 1 and type 2 (CRHR1 and CRHR2) was described in the endocrine pancreas (6, 7). The CRH receptors belong, together with the glucagon-like peptide-1 receptor (GLP-1R) and the glucose-dependent insulinotropic polypeptide GIP receptor (GIPR), to the family of class B G protein-coupled receptors (6). Activation of CRHR1 by CRH has been demonstrated to stimulate Ca2+ influx and to increase intracellular Ca2+ concentrations in rat pancreatic islets (8, 9) and to stimulate exocytosis of insulin containing granules in mouse pancreatic β-cells (6). In addition, CRH-induced insulin release could be enhanced by coincubation with vasopressin, suggesting complex mechanisms of insulin release in pancreatic islets (10). Therefore, a functional CRH-receptor system within the endocrine pancreas, which mediates insulin secretion, may be important in hypoglycemic episodes occurring in stress-related situations or disorders. Circulating levels of CRH correlate well with stressful situations or situations of disturbed adrenal steroidogenesis, such as pregnancy, psoriasis, Addison disease, inflammation, and bleeding (11–13), and therefore might influence insulin secretion in these situations. Moreover, CRH-mediated stimulation of insulin secretion through the preferentially activated CRHR1 seems to occur predominantly during intermediate to high ambient glucose (6). This finding may suggest a nutrition- or food-related regulation of insulin release similar to the incretins stimulating the GLP-1R and GIPR in pancreatic islets (7). Furthermore, recent evidence suggests that activation of CRHR1 initiates MAPK-signaling (14) and leads to proliferation of rat neonatal β-cells, suggesting a possible beneficial role for CRH in augmentation of β-cell mass (6).
CRHR1 of the anterior pituitary provides a major target in the regulation of circulating glucocorticoids (GC) levels, which induce various features of the metabolic syndrome, including the impairment of β-cell function and of insulin resistance (15, 16). GCs mediate their effects through the specific intracellular GC receptor (GR) present in almost all cell types, including pancreatic β-cells (17). Recently, GCs have been shown to inhibit the expression of CRHR1 and incretin receptors on pancreatic islets (7). The available levels of the biologically active GCs are regulated by 11β-hydroxysteroid dehydrogenase (11β-HSD). Two different isoenzymes of 11β-HSD have been characterized; 11β-HSD type 1 and type 2 (11β-HSD-1 and 11β-HSD-2) (18). Isoenzyme 11β-HSD-1 interconverts active GC (corticosterone in rodents, cortisol in humans) to their metabolites (11-dehydrocorticosterone in rodents, cortisone in humans). There is evidence for an increased expression and activity of the type-1 isoenzyme in diabetic islets, which leads to increased local tissue concentrations of active GCs in diabetic rodents (19). Moreover, it has been shown that selective inhibition of 11β-HSD-1 decreases blood glucose concentrations in hyperglycemic mice (20). The type-2 isoenzyme converts active GCs to their inactive form, thus protecting the glucocorticoid and mineralcorticoid receptor against occupancy by active GCs, thereby leading to insulin resistance and diabetes (21). The inhibition of 11β-HSD activity by carbenoxolone and resultant reduction in intracellular cortisol concentrations produced an increased insulin sensitivity and decreased glucose production (22). Therefore, inhibition of 11β-HSD-1 expression and activity may provide a previously unexplored therapeutic tool of an antidiabetic drug therapy.
Based on these findings, we addressed several questions in this study regarding a tissue-specific receptor system for hypothalamic-releasing hormones in the endocrine pancreas. Is there a functional CRH/CRHR system in human pancreatic islets influencing metabolic activity of pancreatic β-cells? Does CRH contribute to enhanced islet mass through an antiapoptotic or proliferative effect? How does the effect of CRH compare with that of the GHRH-agonist JI-36, recently identified as a strong effector for survival and proliferation of pancreatic islets (5)? Which role for regulating the intracellular cortisone/cortisol conversion and maintaining an adequate exposure of GC is played by the 11β-HSD enzyme system in pancreatic islets? Finally, most importantly, is there a protective mechanism allowing CRH to influence insulin synthesis and augmentation of β-cell mass in the presence of elevated GC levels?
Results
CRH and CRHR1 Are Expressed in Insulinoma Cells and in Rat and Human Pancreatic Islets.
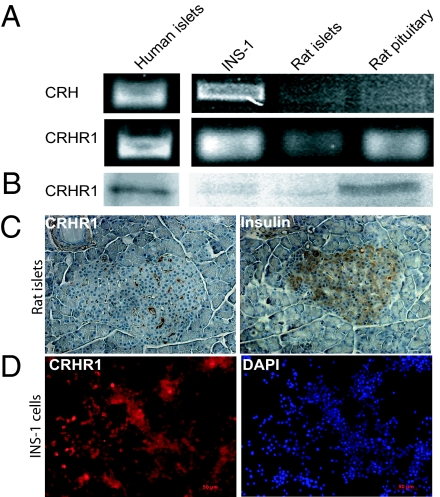
RT-PCR analysis revealed the expression of CRH and CRHR1 mRNA in rat insulinoma (INS)-1 cells, primary rat islets, and primary human islets (Fig. 1A). Western blot analysis verified the expression of CRHR1 protein (55 kDa) in INS-1 cells, rat islets and human islets (Fig. 1B). Immunohistochemical analysis demonstrated CRHR1 expression in rat islets (Fig. 1C) and INS-1 cells (Fig. 1D). Pancreatic β-cells in rat islets were identified by immunostaining for insulin and nuclei of INS-1 cells were stained with DAPI.
Fig. 1.
CRH and CRHR1 expression on insulinoma cells and pancreatic islets. (A) RT-PCR analysis of CRH and CRHR1 and (B) Western blot analysis of the expression of CRHR1 in INS-1 cells, rat islets, and human islets. Rat pituitary was used as positive control and β-actin was used as loading control. (C) Immunohistochemical staining of CRHR1 in serial sections of rat pancreas that were counterstained with insulin to detect pancreatic islets. (D) Immunohistochemical staining of CRHR1 in INS-1 cells (red dye), which were costained with DAPI for the visualization of cell nuclei (blue dye). A representative analysis is shown.
Ultrastructural Analysis of Insulinoma Cells Cultured with Hypothalamic Peptides.
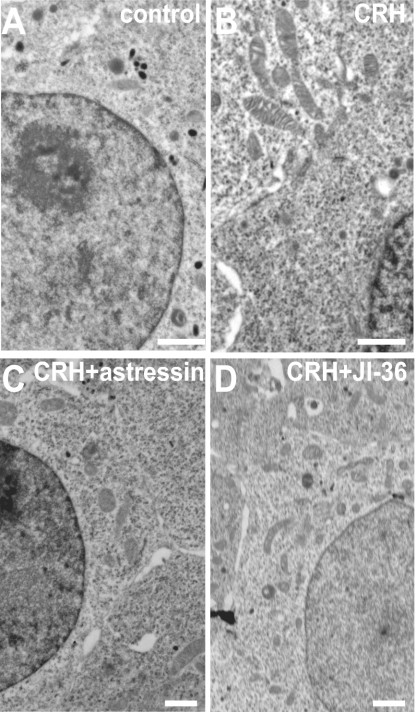
INS-1 cells under normal culture conditions were characterized by insulin-containing secretory granules in the cytoplasm (Fig. 2A). Treatment of INS-1 cells with 10−10 M CRH resulted in a reduction of secretory granules and in enlargement of mitochondria (Fig. 2B). These morphological changes indicate an enhanced metabolic state after CRH stimulation. Culture of INS-1 cells in the presence of 10−10 M CRH and the CRH-antagonist astressin (10−6 M) resulted in a decreased size of mitochondria (Fig. 2C), indicating a reduced metabolic state of INS-1 cells. Furthermore, INS-1 cells were cultured with CRH, in combination with the GHRH-agonist JI-36 (10−6 M) as a positive control. Stimulation of the GHRH receptor is known to increase the active metabolic state of INS-1 cells (5). However, activation of both receptors, the CRHR1 and the GHRH-R, leads to increased number of mitochondria (Fig. 2D). Cells cultured with DMSO as solvent control showed no difference to control cells.
Fig. 2.
Ultrastructural analysis of insulinoma cells. (A) Untreated INS-1 cells show insulin-containing secretory granules in the cytoplasm. (B) In contrast, INS-1 cells cultured with CRH (10−10 M) revealed lower numbers of secretory granules and enlarged mitochondria. (C) Additional cultivation with CRH-antagonist astressin resulted in lower number and size of mitochondria. (D) Cultivation of INS-1 cells with CRH in combination with the GHRH-agonist JI-36 leads to increased amount of mitochondria. These signs indicate a more active metabolic state of the INS-1 cells cultured with CRH. A representative analysis is shown. (Scale bars, 1 μm.)
Stimulation of Insulinoma Cells with Hypothalamic Peptides CRH and Agonist JI-36 Promotes Proliferation and Reduces Apoptosis.
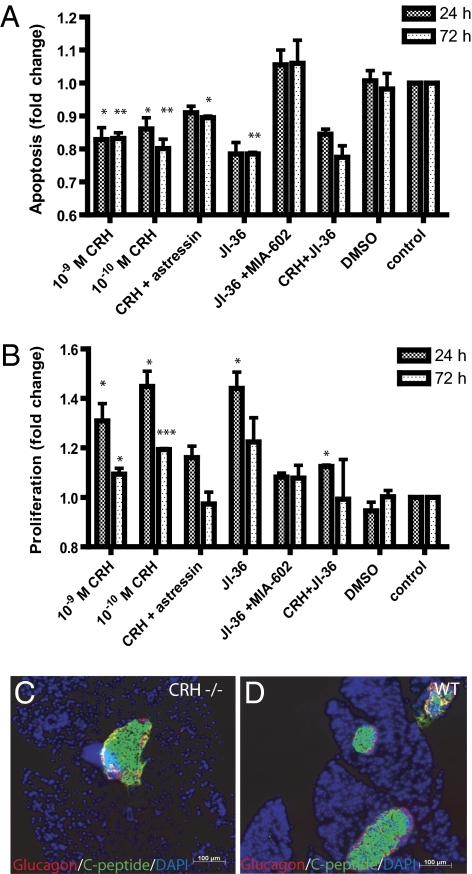
INS-1 cells were cultured with CRH (10−6 M – 10−12 M) or with CRH in combination with the CRH-antagonist, astressin (10−6 M), for 24 or 72 h to analyze the effects on apoptosis and proliferation. Activation of CRHR1 with CRH resulted in a significant reduction of caspases 3/7 activity with a maximal antiapoptotic effect of 19.8 ± 6.9% in contrast to untreated INS-1 cells. This effect was observed after 72 h in the presence of 10−10 M CRH (Fig. 3A). Moreover, CRH had a significant and dose-dependent effect on proliferation of INS-1 cells, as revealed by BrdU incorporation. In contrast to untreated cells, treatment with CRH resulted in enhancement of proliferation by 44.8 ± 10.7% after 24-h cultivation (Fig. 3B). Coincubation with the CRH-antagonist astressin (10−6 M), partly abrogated the anti-apoptotic and proliferative effects. The GHRH-agonist JI-36 was applied as a positive control because of its antiapoptotic and proliferative effect on INS-1 cells (5). Stimulation of GHRH receptor (GHRH-R) reduced apoptosis by 21.5 ± 4.9% and increased proliferation by 44.0 ± 11.5%. Effects of JI-36 could be completely blocked by the GHRH-antagonist MIA-602. However, cultivation of INS-1 cells with CRH in combination with JI-36 resulted in an antiapoptotic and a slight proliferative effect. Because CRH dose-dependently increased cell proliferation and conversely decreased the rate of apoptosis in INS-1 cells with a maximum effect at 10−10 M, CRH was used at 10−10 M in all following experiments. Thus, the effects of CRH on proliferation and apoptosis were evaluated at concentrations of 10−6 M to 10−12 M and incubation times of 24, 48, 72, and 96 h; however, only the concentrations of 10−9 M and 10−10 M caused significant effects compared with control cells. DMSO as solvent control had no effect on proliferation or apoptosis. In further support of the effects of CRH on the endocrine pancreas, we identified by immunohistochemical staining decreased number of pancreatic islets in CRH-negative (CRH−/−) mice (Fig. 3C) compared with wild-type mice (Fig. 3D). Moreover, serum insulin levels were also lower in the CRH−/− mice.
Fig. 3.
Effects of hypothalamic peptides on apoptosis and proliferation of insulinoma cells. (A) Apoptosis as indicated by activity of caspases 3/7 was significantly reduced by 19.8 ± 6.9% after 72-h cultivation with 10−10 M CRH in contrast to untreated cells. Cultivation of INS-1 cells with CRH and the CRH-antagonist, astressin, partly blocked the effect of CRH alone. The GHRH-agonist JI-36 was used as positive control, decreasing the apoptosis by 21.5 ± 4.9%. This effect could be blocked completely by the GHRH-antagonist MIA-602. The combination of CRH with JI-36 revealed 22.5 ± 4.9% reduced apoptosis (n = 3). (B) Evaluation of cell proliferation was performed by BrdU incorporation, whereby CRH (10−10 M) revealed significant increase in proliferation by 44.8 ± 10.7% after 24-h cultivation in contrast to control. Astressin revealed a blockage of the proliferative effect of CRH. The positive control, JI-36, revealed an increased proliferation of 44.0 ± 11.5%. However, this proliferative effect was blocked by the GHRH-antagonist MIA-602. Cultivation of INS-1 cells with CRH plus JI-36 showed no effect on proliferation (n = 3). CRH treatment increased dose-dependently cell proliferation and conversely decreased the rate of apoptosis in INS-1 cells, with the maximum effect at 10−10 M. DMSO was used as solvent control in same dilution as the substance. (*P < 0.05; **P < 0.01; ***P < 0.001) Immunohistochemical staining of pancreatic islets for glucagon and C-peptide indicates lower islet number in (C) CRH −/− mice than in (D) control animals (WT).
Stimulation of CRHR1 Leads to Glucose-Stimulated Insulin Secretion in Insulinoma Cells and Rat Islets.
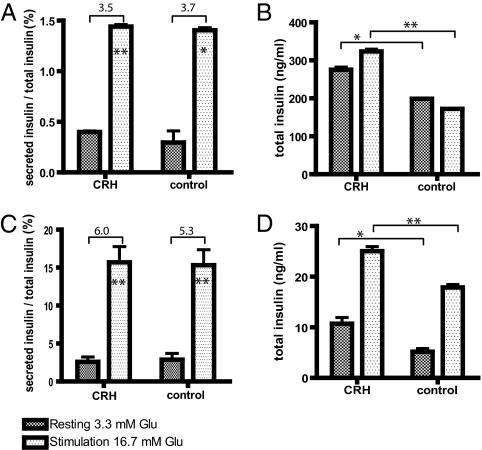
Functional testing of INS-1 cells was performed by measuring glucose-stimulated insulin secretion and insulin synthesis (Fig. 4). INS-1 cells and rat islets were exposed to 10−10 M CRH for 24 and 48 h before glucose stimulation. At basal glucose concentration (3.3 mM), INS-1 cells and rat islets treated with CRH released insulin into the culture media at similar rates as untreated controls. Upon stimulation with high glucose (16.7 mM), insulin release from INS-1 cells, treated and untreated with CRH, was significantly increased by 3.5- and 3.7-fold, respectively, relative to basal glucose concentration (Fig. 4A). Treatment with CRH, however, resulted in 63% increase in total insulin content compared with untreated INS-1 cells (Fig. 4B). This effect of CRH on the insulinoma cell line, INS-1, was reproduced in primary rat islet cells. Rat islets, CRH-treated and untreated, responded to high glucose with a stimulation indices of 6.0 and 5.3, respectively (Fig. 4C). However, culturing rat pancreatic islets with CRH resulted in an increase of total insulin by ∼40% compared with untreated islets (Fig. 4D). At basal glucose concentrations, total insulin of rat islets treated with CRH was 10.72 ± 2.16 ng/mL. In contrast, control rat islets contained 5.17 ± 1.43 ng/mL of insulin. Upon stimulation with high glucose, treatment with CRH resulted in 25.05 ± 1.56 ng/mL total insulin compared with control islets, which contained 17.87 ± 0.96 ng/mL insulin. Overall, pretreatment with CRH resulted in enhanced total insulin production, thus contributing to the restoration of normoglycemia.
Fig. 4.
Effect of CRH on glucose-stimulated insulin secretion and synthesis of insulinoma cells and rat islets after 24-h cultivation. After equilibration at 3.3 mM glucose, INS-1 cells and rat islets were stimulated with high-glucose concentration of 16.7 mM for 1 h. Glucose challenge resulted in significantly increased insulin release. (A) The stimulation index of INS-1 cells (insulin-release basal glucose vs. insulin-release high glucose) was 3.5 and 3.7 of CRH-treated and untreated cells, respectively (n = 3). (B) CRH treatment of INS-1 cells resulted in an increased amount of total insulin (cellular insulin content + released insulin) by 63% compared with untreated INS-1 cells (n = 3). (C) Rat pancreatic islets revealed a stimulation index of 6.0 and 5.3 of CRH treated and untreated islets, respectively (n = 3). (D) However, rat islets cultured with CRH resulted in an increased amount of total insulin by 40% compared with untreated rat pancreatic islets (n = 3). (*P < 0.05; **P < 0.01; ***P < 0.001).
11β-HSD-1 and 11β-HSD-2 Are Expressed in Insulinoma Cells and in Rat and Human Pancreatic Islets.
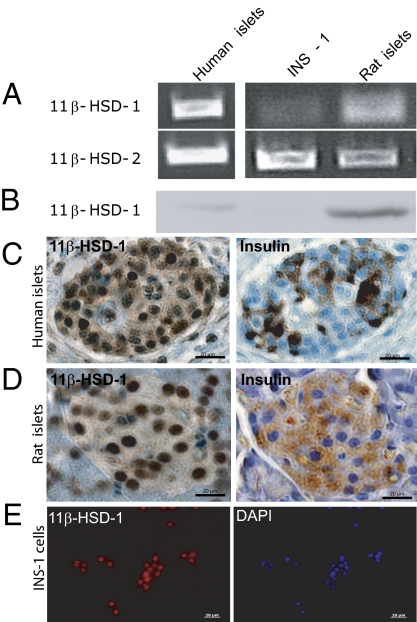
RT-PCR analysis revealed mRNA expression of 11β-HSD-1 and 11β-HSD-2 in rat INS-1 cells, primary rat islets and primary human islets (Fig. 5A). Western blot analysis documented the protein expression of 11β-HSD-1 (35 kDa) in INS-1 cells, rat islets, and human islets (Fig. 5B). In addition, the 11β-HSD-1 protein was detected by immunohistochemical staining in human pancreatic islets (Fig. 5C), rat pancreatic islets (Fig. 5D), and INS-1 cells (Fig. 5E). To confirm the localization of 11β-HSD-1 to pancreatic β-cells, costaining for insulin was performed. Microscopic analysis revealed that all insulin-positive cells stained for 11β-HSD-1.
Fig. 5.
11β-HSD-1/2 expression on insulinoma cells and pancreatic islets. (A) RT-PCR analysis of 11β-HSD-1 and 11β-HSD-2 and (B) Western blot analysis of the expression of 11β-HSD-1 on INS-1 cells, rat islets, and human islets. Immunohistochemical staining of 11β-HSD-1 in human islets (C), rat islets (D), and INS-1 cells (E) (red dye). Serial sections of human and rat pancreas were counterstained with insulin to detect pancreatic islets. INS-1 cells were costained with DAPI for the visualization of cell nuclei (blue dye). A representative analysis is shown.
Influence of Hypothalamic Peptides CRH and Agonist JI-36 on Expression of mRNA for 11β-HSD-1 and 11β-HSD-2.
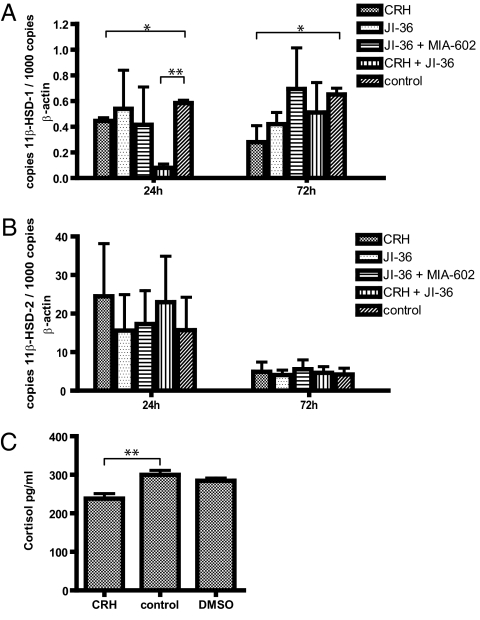
INS-1 cells were cultured with 10−10 M CRH, 10−6 M JI-36, 10−6 M JI-36 plus 10−6 M MIA-602, or with 10−10 CRH and 10−6 M JI-36 for 24 and 72 h and changes in mRNA expression for 11β-HSD-1 and 11β-HSD-2 were determined by quantitative RT-PCR. mRNA levels for 11β-HSD-1 in INS-1 cells cultured with CRH were reduced after 24 and 72 h by 23.3% and 56.9%, respectively, in comparison with untreated INS-1 cells. Cultivation with the GHRH-agonist JI-36 for 72 h revealed a reduced mRNA expression for 11β-HSD-1 by 35.4% in contrast to control. Combination of both hypothalamic-releasing hormone agonists decreased the expression level by 86.2% (Fig. 6A). Furthermore, treatment with CRH resulted in increased mRNA expression for 11β-HSD-2 by 55.9% and 16.1% of cells cultured for 24 and 72 h, respectively (Fig. 6B). Cultivation of INS-1 cells with CRH and JI-36 for 24 h increased mRNA expression for 11β-HSD-2 by 46.1%.
Fig. 6.
Effects of CRH and agonist JI-36 on the 11β-HSD-1/2 enzyme system in insulinoma cells. (A) INS-1 cells were cultured for 24 and 72 h with CRH, the GHRH-agonist JI-36, with and without the antagonist MIA-602 and CRH in combination with JI-36. Treatment of INS-1 cells with CRH or JI-36 decreased mRNA levels for 11β-HSD-1 by 56.9% and 35.4% after 72-h cultivation, respectively. Combination of both hypothalamic agonists for 24 h revealed 86.2% reduced mRNA expression for 11β-HSD-1. (B) CRH treatment with or without JI-36 increased the mRNA expression for 11β-HSD-2 in INS-1 cells by 55.9% and 46.1% after 24-h cultivation, respectively (n = 4). Values of 11β-HSD-1/2 mRNA are shown relative to expression of β-actin. (C) Activity of 11β-HSD enzyme was analyzed by measuring the production of active GCs. Levels of the active GC cortisol were measured in culture media of rat INS-1 cells, pretreated with CRH (10−10 M) for 24 h and 500 nM cortisone for 3 h. Following CRH treatment, active GC levels were significantly reduced (n = 3). DMSO treatment served as solvent control. (*P < 0.05; **P < 0.01).
Stimulation of CRHR1 in Insulinoma Cells Results in Reduced GC Levels.
Specific activity of 11β-HSD enzyme was analyzed by measuring the production of active GCs. Levels of the active GC cortisol were measured in culture media of rat INS-1 cells, pretreated with CRH (10−10 M) for 24 h and cortisone (500 nM) for 3 h. Cortisol is produced locally from cortisone because of the activity of the enzyme 11β-HSD-1. Following treatment with CRH, levels of active GC were significantly reduced by 27.7%, in contrast to untreated INS-1 cells, indicating either a reduced activity of 11β-HSD-1 or an increased activity of 11β-HSD-2 (Fig. 6C). DMSO as solvent control had no effect on cortisol levels.
Discussion
Hypothalamic-pituitary regulation of the endocrine pancreas was proposed by the pioneering work of Bernardo Houssay more than half century ago (23). However, only now is a better understanding is emerging of the complex interaction of hypothalamic-releasing hormones and their input from neuronal networks. Thus, we are beginning to understand the intricate communication between the neuroendocrine stress system and the metabolic regulation of energy expenditure, insulin secretion, and glucose homeostasis, which involves multiple hormones, including at least two hypothalamic hormones. For example, we have previously shown that an agonistic analog of GHRH promoted the proliferation and survival of pancreatic β-cell islets (5). In collaborative studies in 1984, one of the authors (A.V.S.) participated in the demonstration of immunoreactive CRH in the endocrine pancreas (24), although its functions were then unknown. Recently, Huising et al. (6) showed that CRH receptors, expressed on pancreatic β-cells, promote β-cell proliferation and potentiate insulin secretion.
The findings of our present study support the existence of a functionally relevant receptor system for CRH and GHRH in both rodent and human pancreatic islet cells. In addition to stimulating β-cell proliferation and insulin synthesis in a glucose-dependent manner, CRH increases viability of β-cells and reduces apoptosis in a dose-dependent manner. This effect was comparable in magnitude to the effect of GHRH agonists previously demonstrated in vitro and in vivo (5). The proliferative and antiapoptotic effect was partially reversed by the CRH-antagonist astressin. Furthermore, in vivo studies have shown that mice with genetic CRH deficiency had lower islet cell numbers, supporting our in vitro findings. The CRH-deficient mice had reduced serum insulin levels, as indicated in a previous report (25). These results raise the possibility for a significant role of CRH in pancreas development. This finding may provide a protective mechanism preventing GC-induced cell death and impairment of β-cell function. In addition to its primary action on the pituitary, CRH has been implicated in the regulation of many extrapituitary cell systems. As examples, the skin and also adipose tissue seem to possess a complete and functional local CRH/CRHR system (4, 26).
The capacity of CRH to induce insulin secretion and cell proliferation, even as enhancing the viability of β-cells and decreasing programmed cell death, may suggest a potential therapeutic use for agonists of CRHR1 in patients with diabetes. At the level of the pituitary, however, activation of CRHR1 by CRH leads to stimulation of adrenocorticotropic hormone-secretion and GC release from the adrenal cortex. GCs mediate all features of the metabolic syndrome by inducing fat cell maturation and obesity, as well as insulin resistance in peripheral tissues. More recently, it became evident that GCs also have direct impact on β-cell function (7). Thus, overexpression of the GR and GR-signaling leads to an increased apoptotic rate, and exposure of patients to GCs results in the inhibition of several parameters of β-cell function. These parameters include both clinical and model-based parameters of β-cell function based on calculations of glucose, insulin, and c-peptide concentrations in serum obtained during standardized meal tests, before and during steroid treatment (27). These findings are in accordance with the observation that patients who receive GCs following islet transplantation will have the benefits of immunoprotection and immune suppression, but will also suffer from the diabetogenic effects of GCs, which include β-cell impairment. Consistent with this finding, GCs have recently been shown to significantly inhibit the expression of incretin receptors and CRHR1 (7). Our data raise the possibility that the direct actions of CRH on the pancreatic islet cells, possibly as part of a local stress-response system, may have beneficial effects, particularly in states of increased systemic GC levels. In recent years it has, however, become clear, that human metabolic make up includes an enzyme system providing protection from locally high active GC levels based on the differential regulation of the 11β-HSD enzyme system. Currently, selective 11β-HSD-1 inhibitors, which allow the reduction of local GC concentrations by blocking the conversion of cortisone to cortisol, are being applied in clinical trials as a new therapeutic strategy for the treatment of the metabolic syndrome (28, 29). It has been shown that 11β-HSD-1 mediates GC activation and insulin release in pancreatic islets (30). A classic blocker of the 11β-HSD system, carbenoxolone, reverses the inhibition of insulin release (22). Furthermore, increased expression and activity of 11β-HSD occurs in diabetic islets (19). A down-regulation of 11β-HSD activity by CRH has been previously shown in adipose tissue (31).
In the present article, we show that rodent and human pancreatic islets include a tissue-specific 11β-HSD enzyme system that is affected by the pancreatic CRH receptor and the GHRH-R system. The endocrine pancreas expresses both type 1 and type 2 enzymes of the 11β-HSD system. Moreover, CRH, through CRHR1, and agonist JI-36, through the GHRH-R, increased the mRNA expression of 11β-HSD-2 while decreasing the mRNA expression of 11β-HSD-1. Consistent with these findings, stimulation of CRHR1 reduced the active GC levels, demonstrating a reduced enzyme activity for 11β-HSD-1 or an increased enzyme activity for 11β-HSD-2. Ultrastructural analysis of insulinoma cells treated with CRH revealed a reduced number of insulin-containing secretory granules and enlarged mitochondria, demonstrating an increased insulin turnover and metabolic activity in these cells. Therefore, regulation of the pancreatic 11β-HSD enzyme system by hypothalamic-releasing hormone agonists may protect the pancreatic islets against the increased GC levels present in diabetic patients. Moreover, it may provide a mechanistic link between the neuroendocrine stress system and islet integrity. This regulation may also produce a mechanism to maintain the beneficial effects of pancreatic CRHR1 or GHRH-R activation on insulin secretion and islet mass in the presence of an activated HPA-axis and the corresponding rise in GC levels.
Materials and Methods
Animals.
Housing and care of CRH−/− and wild-type mice were according to National Institutes of Health and European Union guidelines. Mice were kept at a 12-h light-dark cycle and had ad libitum access to standard chow diet (Harlan; Rodent Diet 2018) and water. The CRH−/− mouse line was generated as previously described (32). CRH−/− mice were raised in C57/Bl6 background and were obtained by crossing of heterozygous strains; their wild-type littermates, CRH+/+, were used as controls. The genotype of every animal was established by PCR, as previously described. All procedures were approved by the Animal Care and Use Committee of the Biomedical Research Foundation of the Academy of Athens. Serum insulin was measured using an RIA kit (Millipore) according to the manufacturer's instructions. Adult mice (2–4 mo old) were anesthetized and pancreata were dissected in cold PBS, fixed at 4 °C for 4 h, dehydrated, and paraffinized.
Rat INS-1 Insulinoma Cells.
Rat INS-1 cells were cultured in RPMI medium 1640 (PAA) supplemented with 2 mM l-glutamine, 10% FBS, 1 mM Na-Pyruvate, 50 μM 2-mercaptoethanol, and 100 U/mL penicillin-streptomycin (Gibco) in a humidified 5% CO2/95% O2 atmosphere at 37 °C. Fresh medium was added every second day to the culture flasks, cells were passaged once per week.
Isolation of Rat and Human Pancreatic Islets.
Pancreatic islets were isolated as previously described (5). Purified rat islets were maintained in culture media (CMRL 1066; Mediatech) supplemented with 10% FBS at 37 °C in a 5% CO2 incubator. Human islets were cultured at 37 °C in a 5% CO2 incubator in CMRL 1066 (Mediatech) containing 2.5% human serum albumin. Volume and purity were determined by microscopic sizing after staining with dithizone (Sigma-Aldrich).
Chemicals.
CRH was purchased from Ferring, dissolved in DMSO, and used at a concentration of 10−6 M – 10−12 M. The CRH-antagonist astressin was purchased from Bachem, dissolved in DMSO, and used at a final concentration of 10−6 M. The GHRH-agonist Jl-36, and GHRH-antagonist MIA-602 were synthesized in the A.V.S. laboratory and used at a concentration of 10−6 M.
Exposure of Insulinoma Cells and Rat Pancreatic Islets to Agonists and Antagonists for Hypothalamic-Releasing Hormones.
INS-1 cells were grown for 72 h before stimulation with agonists or antagonists. Islets were collected immediately after the isolation procedure and divided into three treatment groups: (i) culture media without supplementation, (ii) culture media with vehicle (DMSO) as a solvent control, and (iii) culture media containing CRH. Media change of INS-1 cells was performed every second day and of islets every 24 h.
Glucose-Stimulated Insulin Secretion of Insulinoma Cells and Rat Islets.
Rat islets or expanded INS-1 cells were preincubated in oxygenated Krebs Ringer bicarbonate buffer (137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4-7H2O, 2.5 mM CaCl2-2H2O, 25 mM NaHCO3, 0.25% BSA), and included 3.3 mM glucose at 37 °C for 1 h. Afterward, buffer was replaced with fresh oxygenated Krebs Ringer bicarbonate buffer containing either 3.3 mM glucose or 16.7 mM glucose, and cells were incubated for 1 h at 37°. Supernatant was collected and cells were lysed by 0.15% HCl. Secreted insulin and cellular insulin content was measured by RIA using the rat insulin RIA kit (Millipore). Secreted insulin values were normalized to total insulin. Stimulation index reflects the fold-change of insulin release under basal glucose condition compared with high-glucose conditions.
For details of quantitative real-time PCR, Western blot analysis, immunohistochemistry and immunofluorescence, electron microscopy, cell apoptosis assay, cell proliferation assay, 11β-HSD activity assay, statistical analysis, and RNA isolation and RT-PCR, see SI Text. For primers used in expression analysis, see Table S1.
Supplementary Material
Acknowledgments
We thank Linda Gebauer for her technical help, Silke Zeugner for her assistance with immunohistochemistry, Doreen Streichert for help with electron microscopy, and Martina Haberland for help in preparation of the manuscript. This work was supported by Grants KFO 252/1 and BR1179/4-1 of the Deutsche Forschungsgemeinschaft (to M.D.B., S.R.B., and B.L.); by a grant of the German Federal Ministry of Education and Research to the German Center for Diabetes Research (to S.R.B. and B.L.); by the Deutsche Forschungsgemeinschaft Center for Regenerative Therapies Dresden Cluster of Excellence (S.R.B., B.L., and M.D.B.); and by a grant of the German Federal Ministry of Education and Research AUS 10/802 (to S.R.B.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110965108/-/DCSupplemental.
References
- 1.Audhya T, Jain R, Hollander CS. Receptor-mediated immunomodulation by corticotropin-releasing factor. Cell Immunol. 1991;134(1):77–84. doi: 10.1016/0008-8749(91)90332-6. [DOI] [PubMed] [Google Scholar]
- 2.Clifton VL, Owens PC, Robinson PJ, Smith R. Identification and characterization of a corticotrophin-releasing hormone receptor in human placenta. Eur J Endocrinol. 1995;133:591–597. doi: 10.1530/eje.0.1330591. [DOI] [PubMed] [Google Scholar]
- 3.Perrin M, et al. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seres J, et al. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- 5.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huising MO, et al. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci USA. 2010;107:912–917. doi: 10.1073/pnas.0913610107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huising MO, et al. Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets. Endocrinology. 2011;152:138–150. doi: 10.1210/en.2010-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kageyama K, et al. Modulation of Ca2+ influx by corticotropin-releasing factor (CRF) family of peptides via CRF receptors in rat pancreatic beta-cells. Peptides. 2006;27:1814–1819. doi: 10.1016/j.peptides.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Kanno T, Suga S, Nakano K, Kamimura N, Wakui M. Corticotropin-releasing factor modulation of Ca2+ influx in rat pancreatic beta-cells. Diabetes. 1999;48:1741–1746. doi: 10.2337/diabetes.48.9.1741. [DOI] [PubMed] [Google Scholar]
- 10.O'Carroll AM, Howell GM, Roberts EM, Lolait SJ. Vasopressin potentiates corticotropin-releasing hormone-induced insulin release from mouse pancreatic beta-cells. J Endocrinol. 2008;197:231–239. doi: 10.1677/JOE-07-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Keane V, et al. Increased pituitary-adrenal activation and shortened gestation in a sample of depressed pregnant women: A pilot study. J Affect Disord. 2011;130:300–305. doi: 10.1016/j.jad.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Yi SS, et al. Regulatory mechanism of hypothalamo-pituitary-adrenal (HPA) axis and neuronal changes after adrenalectomy in type 2 diabetes. J Chem Neuroanat. 2010;40(2):130–139. doi: 10.1016/j.jchemneu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Heller MM, Lee ES, Koo JY. Stress as an influencing factor in psoriasis. Skin Therapy Lett. 2011;16(5):1–4. [PubMed] [Google Scholar]
- 14.Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–1729. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- 15.Lenzen S, Bailey CJ. Thyroid hormones, gonadal and adrenocortical steroids and the function of the islets of Langerhans. Endocr Rev. 1984;5:411–434. doi: 10.1210/edrv-5-3-411. [DOI] [PubMed] [Google Scholar]
- 16.Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. 1992;15:1413–1422. doi: 10.2337/diacare.15.10.1413. [DOI] [PubMed] [Google Scholar]
- 17.Fischer B, et al. Immunohistochemical localization of the glucocorticoid receptor in pancreatic beta-cells of the rat. Endocrinology. 1990;126:2635–2641. doi: 10.1210/endo-126-5-2635. [DOI] [PubMed] [Google Scholar]
- 18.Stewart PM, Krozowski ZS. 11 beta-hydroxysteroid dehydrogenase. Vitam Horm. 1999;57:249–324. [PubMed] [Google Scholar]
- 19.Duplomb L, et al. Increased expression and activity of 11beta-HSD-1 in diabetic islets and prevention with troglitazone. Biochem Biophys Res Commun. 2004;313:594–599. doi: 10.1016/j.bbrc.2003.11.160. [DOI] [PubMed] [Google Scholar]
- 20.Alberts P, et al. Selective inhibition of 11beta-hydroxysteroid dehydrogenase type 1 decreases blood glucose concentrations in hyperglycaemic mice. Diabetologia. 2002;45:1528–1532. doi: 10.1007/s00125-002-0959-6. [DOI] [PubMed] [Google Scholar]
- 21.Hollis G, Huber R. 11β-Hydroxysteroid dehydrogenase type 1 inhibition in type 2 diabetes mellitus. Diabetes Obes Metab. 2011;13(1):1–6. doi: 10.1111/j.1463-1326.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: Studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- 23.Houssay BA. Nobel Lecture. 1949. Available at http://nobelprize.org/nobel_prizes/medicine/laureates/1947/houssay-lecture.html. Accessed July 27, 2011.
- 24.Petrusz P, Merchenthaler I, Maderdrut JL, Vigh S, Schally AV. Corticotropin-releasing factor (CRF)-like immunoreactivity in the vertebrate endocrine pancreas. Proc Natl Acad Sci USA. 1983;80:1721–1725. doi: 10.1073/pnas.80.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeong KH, Sakihara S, Widmaier EP, Majzoub JA. Impaired leptin expression and abnormal response to fasting in corticotropin-releasing hormone-deficient mice. Endocrinology. 2004;145:3174–3181. doi: 10.1210/en.2003-1558. [DOI] [PubMed] [Google Scholar]
- 26.Krause K, Schnitger A, Fimmel S, Glass E, Zouboulis CC. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm Metab Res. 2007;39(2):166–170. doi: 10.1055/s-2007-961811. [DOI] [PubMed] [Google Scholar]
- 27.van Raalte DH, et al. Acute and 2-week exposure to prednisolone impair different aspects of beta-cell function in healthy men. Eur J Endocrinol. 2010;162:729–735. doi: 10.1530/EJE-09-1034. [DOI] [PubMed] [Google Scholar]
- 28.Morgan SA, Tomlinson JW. 11Beta-hydroxysteroid dehydrogenase type 1 inhibitors for the treatment of type 2 diabetes. Expert Opin Investig Drugs. 2010;19:1067–1076. doi: 10.1517/13543784.2010.504713. [DOI] [PubMed] [Google Scholar]
- 29.Ge R, Huang Y, Liang G, Li X. 11Beta-hydroxysteroid dehydrogenase type 1 inhibitors as promising therapeutic drugs for diabetes: Status and development. Curr Med Chem. 2010;17:412–422. doi: 10.2174/092986710790226147. [DOI] [PubMed] [Google Scholar]
- 30.Davani B, et al. Type 1 11beta-hydroxysteroid dehydrogenase mediates glucocorticoid activation and insulin release in pancreatic islets. J Biol Chem. 2000;275:34841–34844. doi: 10.1074/jbc.C000600200. [DOI] [PubMed] [Google Scholar]
- 31.Hochberg Z, Friedberg M, Yaniv L, Bader T, Tiosano D. Hypothalamic regulation of adiposity: The role of 11beta-hydroxysteroid dehydrogenase type 1. Horm Metab Res. 2004;36:365–369. doi: 10.1055/s-2004-814570. [DOI] [PubMed] [Google Scholar]
- 32.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.