Abstract
Neurotransmission depends on the exo-endocytosis of synaptic vesicles at active zones. Synaptobrevin 2 [also known as vesicle-associated membrane protein 2 (VAMP2)], the most abundant synaptic vesicle protein and a major soluble NSF attachment protein receptor (SNARE) component, is required for fast calcium-triggered synaptic vesicle fusion. In contrast to the extensive knowledge about the mechanism of SNARE-mediated exocytosis, little is known about the endocytic sorting of synaptobrevin 2. Here we show that synaptobrevin 2 sorting involves determinants within its SNARE motif that are recognized by the ANTH domains of the endocytic adaptors AP180 and clathrin assembly lymphoid myeloid leukemia (CALM). Depletion of CALM or AP180 causes selective surface accumulation of synaptobrevin 2 but not vGLUT1 at the neuronal surface. Endocytic sorting of synaptobrevin 2 is mediated by direct interaction of the ANTH domain of the related endocytic adaptors CALM and AP180 with the N-terminal half of the SNARE motif centered around M46, as evidenced by NMR spectroscopy analysis and site-directed mutagenesis. Our data unravel a unique mechanism of SNARE motif-dependent endocytic sorting and identify the ANTH domain proteins AP180 and CALM as cargo-specific adaptors for synaptobrevin endocytosis. Defective SNARE endocytosis may also underlie the association of CALM and AP180 with neurodevelopmental and cognitive defects or neurodegenerative disorders.
Keywords: clathrin-mediated endocytosis, structure
Neurotransmission in the brain depends on the calcium-triggered fusion and recycling of neurotransmitter-filled synaptic vesicles (SVs) with the presynaptic membrane at active zones (1). Following their exocytic insertion into the presynaptic membrane, SV proteins need to be retrieved at a precisely defined stoichiometry by endocytosis, a process involving clathrin, adaptors, and other endocytic proteins (2). Fast calcium-triggered SV fusion critically depends on the SV arginine (R)-soluble NSF attachment protein receptor (SNARE) synaptobrevin [or vesicle-associated membrane protein (VAMP)], which by forming a complex with the plasma membrane glutamine (Q)-SNAREs syntaxin and synaptosomal-associated protein (SNAP)-25 (3) drives neuroexocytosis (4, 5). Synapses lacking synaptobrevin 2 display <1% of wild-type release when stimulated by action potential (AP)-mediated calcium influx (6). Proteomic studies have shown that synaptobrevin 2 is a highly abundant SV protein (7) that is exo-endocytically sorted with very high precision (8). Similar observations have been made for other SV proteins, including synaptotagmin and vesicular glutamate transporters (VGLUTs). How such precise sorting of synaptobrevin 2 is achieved has remained enigmatic. Synaptobrevin lacks recognizable linear sorting motifs (9), and unlike other SNARE proteins does not contain a folded N-terminal domain that serves as a targeting determinant in other VAMP family members (10–12).
Genetic data have linked synaptobrevin sorting to the function of the AP180 N-terminal homology (ANTH) domain-containing endocytic protein AP180. Unc-11/AP180 mutants in Caenorhabditis elegans mislocalize synaptobrevin (13, 14), whereas more general endocytic defects are seen in Lap/AP180-deficient Drosophila melanogaster strains (15, 16). Whether these phenotypes reflect a direct association between AP180 family members and synaptobrevin is unknown. In mammals, the ANTH domain family consists of two members, clathrin assembly lymphoid myeloid leukemia (CALM) and AP180. AP180 is exclusively expressed in neurons, where it accumulates at nerve terminals (17). By contrast, CALM is a ubiquitous protein found in both neurons and glia and in nonneuronal tissues (18). Though both CALM and AP180 have been implicated in regulating neurite outgrowth (19), their relationship to SV cycling has not been explored.
Here we demonstrate that endocytic sorting of synaptobrevin 2 is achieved by direct interaction of the ANTH domain of the endocytic adaptors CALM and AP180 with the N-terminal half of the SNARE motif. These data suggest a unique mechanism of SNARE motif-dependent endocytic sorting and identify the ANTH domain proteins AP180 and CALM as cargo-specific adaptors for synaptobrevin endocytosis in the central nervous system. Our work further contributes to the notion that cargo-selective mechanisms operate at synapses to maintain the high fidelity of SV protein sorting and recycling.
Results
Depletion of the ANTH Domain Proteins AP180 or CALM Causes Selective Surface Accumulation of Synaptobrevin 2.
To explore the possible role of the related ANTH domain proteins AP180 and CALM in SV recycling, we knocked down either or both proteins by RNA interference. CALM- or AP180-specific siRNA efficiently down-regulated expression of their corresponding target protein in transfected HEK293 cells (Fig. 1A) and in primary hippocampal neurons in culture (Fig. S1A) (19). Under the conditions used, depletion of CALM or AP180 did not adversely affect electrical excitability of neurons or stimulation-dependent exocytosis as probed by exo-endocytic cycling of the styryl dye FM4-64 (Fig. 1 B and C).
Fig. 1.
Depletion of AP180 and CALM causes surface accumulation of synaptobrevin 2. (A) Efficient siRNA knockdown of AP180 or CALM expression. HEK293 cells transiently expressing rat AP180 were transfected with siRNA specific for rat AP180 (Left) or CALM (Right) and analyzed by immunoblotting. Expression levels of AP180 or CALM are efficiently down-regulated. (B and C) SV exocytosis probed by FM4-64 in AP180-, CALM-, or AP180- and CALM-depleted neurons. (B) Exocytosis kinetics measured by FM4-64 unloading. (Upper) Scheme of the protocol used to load and unload FM4-64. (Lower) Normalized kinetic traces of FM4-64 dye release from synaptic boutons transfected with control siRNA, siRNA directed against AP180 (AP180 KD), CALM (CALM KD), or siRNAs against both proteins. No significant changes in exocytic release kinetics were observed in neurons depleted of AP180, CALM, or of both proteins. (C) Relative amount of FM4-64 internalized and released upon stimulation (ΔF). Data were normalized to the ΔF of nontransfected terminals and represent mean ± SEM. (D) Vesicular vs. surface pools of synaptobrevin 2-pHluorin assessed by acid-base quenching in hippocampal neurons. Synaptobrevin 2-pHluorin accumulates on the neuronal surface following depletion of AP180 (P = 0.0172, n = 15 neurons), CALM (P = 0.0022, n = 27 neurons), or both proteins (P < 0.0001, n = 28 neurons). (E) Same as in D but using vGLUT1-pHluorin as a reporter. (F) Dispersion of synaptobrevin 2-pHluorin along the axon of AP180/CALM-depleted neurons. Selective mislocalization of pHluorin-tagged synaptobrevin 2, but not vGLUT1, along the axon was observed in neurons depleted of AP180 and CALM. (Left) Profiles from linescan analyses of neurons. (Scale bar, 5 μm.) (G and H) Morphometric ultrastructural analysis of cumulative SV size frequency (G) and SV density (H) by electron microscopy. The average size of SVs is significantly increased in terminals from neurons depleted of AP180 or of both AP180 and CALM.
To assess the role of CALM and AP180 in synaptobrevin 2 endocytosis, we made use of superecliptic pHluorin-tagged synaptobrevin 2 (synaptopHluorin). Fluorescence of synaptopHluorin critically depends on pH, and can thus be used to quantitatively analyze the partitioning of synaptobrevin 2 between the plasma membrane and internal SV-localized pools. Fluorescence changes were monitored from active synaptic boutons, i.e., displaying appropriate responses to electrical stimulation expressing synaptopHluorin together with siRNA. Fluorescence quantification after acid quenching and ammonium dequenching revealed the relative ratio of pHluorin molecules present on SVs or stranded on the neuronal cell surface. Depletion of either AP180 or CALM led to a significant increase in the fraction of surface-stranded synaptopHluorin, indicative of its impaired endocytic retrieval. This effect was augmented in neurons depleted of both ANTH domain proteins (Fig. 1D), suggesting that AP180 and CALM serve overlaping functions in synaptobrevin 2 internalization. To test whether loss of CALM or AP180 selectively impairs synaptobrevin 2 endocytosis, or whether other SV proteins are also affected, we assessed the partitioning of pHluorin-tagged vGLUT1, another abundant SV component at excitatory synapses. To our surprise, knockdown of either CALM or AP180 or of both proteins together had no effect on the vesicular-to-surface pool ratio of VGLUT1-pHluorin (Fig. 1E). These effects were corroborated by analyzing the axonal distribution of synaptopHluorin and vGLUT1-pHluorin. A partial dispersion of synaptopHluorin but not of vGLUT1-pHluorin was observed in double-knockdown neurons depleted of both CALM and AP180 (Fig. 1F). Hence, CALM and AP180 exert an overlapping cargo-specific role in the endocytic retrieval of synaptobrevin 2 from the neuronal surface.
Synaptic Vesicle Size Is Altered in AP180-Depleted Neurons.
AP180 (termed Lap in flies) mutants in D. melanogaster display enlarged and heterogenously sized SVs (16). To explore possible alterations in SV size we turned to ultrastructural analysis by electron microscopy. Synapses from transfected neurons were identified by immunogold labeling using antibodies against eGFP cotransfected together with specific siRNA targeting AP180 or CALM. Nerve terminals from AP180- or CALM-depleted neurons were morphologically normal (Fig. S2A) and showed no significant change in SV density compared with those from control neurons (Fig. 1H). However, SVs from AP180 knockdown neurons appeared slightly larger and more heterogenous, as evidenced by the increased variance of SV size (Fig. S2 A–C). Similar alterations were observed in neurons depleted of both AP180 and CALM, whereas SV size was unchanged in CALM-depleted neurons (Fig. 1G and Fig. S2 B and C). As alterations in SV size have also been observed in Lap/AP180 mutants in D. melanogaster (16) and in neurons from synaptobrevin 2 knockout mice (20), these data further support a tight functional link between synaptobrevin 2 and AP180.
Direct Association of the ANTH Domains of AP180 and CALM with the SNARE Motif of Synaptobrevin 2.
The observed functional connection between the ANTH domain proteins CALM and AP180 and synaptobrevin 2 suggested that these proteins might also physically interact. We probed this possibility using immunoprecipitation experiments. Indeed, synaptobrevin 2 coprecipitated with full-length AP180 or its ANTH domain but not with an AP180 deletion mutant lacking the ANTH domain (Fig. 2A). Thus, the ANTH domain is both required and sufficient for synaptobrevin 2 association. To delineate the molecular determinants within synaptobrevin 2 for AP180-ANTH binding, we generated a series of truncation mutants, in which parts of the cytoplasmic domain were deleted. Though deletion of the N-terminal 31 amino acids preceding the SNARE motif had no effect on synaptobrevin association with AP180, deletion mutants lacking the N-terminal part of the SNARE motif had lost this ability (Fig. 2B). Thus, complex formation between AP180 and synaptobrevin 2 is mediated by ANTH-mediated recognition of determinants within the SNARE motif.
Fig. 2.
Recognition of the SNARE motif of synaptobrevin 2 by the ANTH domains of AP180 and CALM. (A) AP180 associates with synaptobrevin 2 via its ANTH domain. HEK293 cells coexpressing synaptobrevin 2-FLAG together with empty vector, full-length AP180 (FL), the ANTH domain alone (ANTH), or a mutant lacking the ANTH domain (ΔANTH) were subjected to immunoprecipitation using anti-FLAG antibodies. Samples were analyzed by immunoblotting. Input: 1% of the starting material. Note that the immunoblot representing input material was exposed longer to allow proper visualization of all bands. (B) Synaptobrevin 2 interacts with AP180 via the N-terminal half of its SNARE motif. HEK293 cells coexpressing AP180 and FLAG-tagged truncation mutants of synaptobrevin 2 were subjected to immunoprecipitation as in A. Complex formation was disrupted by deletion of residues from the N-terminal half of the SNARE motif of synaptobrevin 2 (Δ1–50). (C) Delineation of the minimal AP180-ANTH binding site within synaptobrevin 2 using peptide SPOT arrays. Peptides displaying nonspecific binding are boxed in gray and were excluded from the analysis. Binding peptides are boxed in red and the sequence of these peptides is listed below. (D) Direct binding of synaptobrevin 2 to the ANTH domains of CALM and AP180. (Left) Immobilized GST-ANTH domains derived from CALM or AP180 (10 μg) were incubated with His6-synaptobrevin 2 (1–96) for 1 h at 4 °C. Following extensive washes, samples were analyzed by SDS/PAGE and immunoblotting. (Right) Interaction between GST-synaptobrevin 2 (33–54) comprising the N-terminal half of its SNARE motif immobilized on beads and incubated with His6-ANTH domains. Samples were analyzed by SDS/PAGE and immunoblotting. (E) Dose-dependent binding of purified CALM-ANTH to immobilized synaptobrevin 2 (1–96) analyzed by surface plasmon resonance. Shown are binding curves from a representative experiment after background substraction. RU, resonance units.
To corroborate these data and to identify further binding determinants we turned to cellulose-bound peptide SPOT (pepscan) arrays. To this aim, the amino acid sequence of the synaptobrevin 2 cytoplasmic domain was covered by an array of cellulose-bound 15-mer peptides overlapping in sequence by 14 amino acids each. This matrix was incubated with His6-tagged AP180-ANTH domain and washed extensively, and bound protein was detected by anti-His6 antibodies bound to HRP-conjugated secondary antibodies. These pepscan overlay assays identified a series of interacting peptides that cover the N-terminal half of the SNARE motif of synaptobrevin 2 (amino acids 33–61; Fig. 2C).
Given that AP180 and CALM share a structurally conserved ANTH domain, we probed binding of synaptobrevin 2 to CALM. As expected, the ANTH domain of CALM (CALM-ANTH) fused to GST avidly pulled down recombinant synaptobrevin 2 with an efficiency that even exceeded that of AP180-ANTH (Fig. 2D, Left). Moreover, both His6-CALM-ANTH and His6-AP180-ANTH directly associated with the N-terminal half of the SNARE motif of synaptobrevin 2 (residues 33–54) in direct binding assays using recombinant proteins (Fig. 2D, Right). Avid concentration-dependent binding of CALM-ANTH to synaptobrevin 2 was also seen in surface plasmon resonance experiments (Fig. 2F). However, due to the intrinsic tendency of CALM-ANTH to oligomerize at elevated concentrations, a reliable KD value could not be determined. The observed preference of synaptobrevin 2 for CALM over AP180 (see also below) correlates with the slight decline of CALM expression during postnatal CNS development and with a concomitant increase of AP180 and synaptobrevin 2 levels (Fig. S1B). One might thus speculate that endocytic recycling of synaptobrevin 2 undergoes a developmental switch from a CALM-based high-affinity/low-capacity to an AP180-based low-affinity/high-capacity retrieval system.
Structural Changes Associated with Binding of Synaptobrevin 2 to ANTH Domains.
The structural basis of ANTH domain-mediated recognition of synaptobrevin 2 by CALM and AP180 was monitored site-specifically via 1H-15N heteronuclear single quantum coherence (HSQC) in the absence or presence of the membrane-mimicking detergent dodecylphosphocholine (DPC) (21). For these experiments, 15N-labeled synaptobrevin 2 was used, which showed instructive spectra without ligand. In detergent- and lipid-free buffer, most resonances appear in the random coil region of the 1HN chemical shift range (between 8.0 and 8.5 ppm; Fig. 3 A and B). Signals from residues 81–96 (21) appear at chemical shifts <7.9 ppm, indicating their potential involvement in loops and helical structure. However, their intensity is weak with very small signals for residues 89–91, possibly indicating self-association. Addition of DPC causes a general increase in signal intensity and the occurrence of additional, as well as more pronounced, helical segments; these include part of the N-terminal SNARE motif (e.g., amino acids 41–54; Fig. 3 A and C) (21).
Fig. 3.
NMR spectroscopic analyses of structural changes associated with binding synaptobrevin 2 to CALM-ANTH. (A–C) Sequences of synaptobrevin 2 indicating residues influenced by CALM-ANTH binding as detected in 1H-15N-HSQC spectra of solutions in aqueous buffer (Upper sequence and spectrum in B) and in the presence of DPC (Lower sequence and spectrum in C). Secondary structures are indicated above and below the sequences. In the Upper sequence, all residues are shown in orange whose signals disappear upon addition of CALM-ANTH (cf. also data for AP180-ANTH in Fig. S3). The chemical shift changes observed upon addition of CALM-ANTH in buffer containing DPC were classified into three categories (strong: purple; medium: red; weak: yellow) and displayed in the Lower sequence in A. (D) Structure of synaptobrevin 2 (PDB ID code 2KOG) showing residues 30–92 and the residues affected by CALM-ANTH binding in DPC buffer according to the color code in A.
If 15N-labeled synaptobrevin 2 is titrated with a double-molar excess of CALM-ANTH (Fig. 3 A and B) or AP180-ANTH (Fig. S3) in aqueous solution, the cross-peaks of large segments of synaptobrevin 2 diminish (Fig. 3B) due to an intermediate exchange regime, or due to formation of a large complex. Disappearing signals (Fig. 3A, orange) cluster in the N-terminal half of the SNARE motif (residues 30–68) and in the membrane-proximal part of the cytoplasmic domain (residues 74–96). In line with the results of the pulldown experiments, effects are stronger for CALM-ANTH than for AP180-ANTH (Fig. S3), which demonstrates that both the ANTH domains of CALM and AP180 associate with synaptobrevin 2 via the N-terminal half of the SNARE motif comprising residues 30–68, in agreement with the biochemical studies (Fig. 2).
To study the formation of protein complexes between synaptobrevin 2 and the ANTH domains of CALM or AP180 in a membrane-mimetic environment, titration experiments were carried out in the presence of DPC micelles. Under these conditions, most cross-peaks remained visible in the presence of CALM-ANTH. Furthermore, complex formation between 15N-labeled synaptobrevin 2 and CALM-ANTH resulted in chemical shift changes of those nuclei that participate in binding. Dramatic effects were observed for R47 and N49 (pink); strong effects for L32, A37, Q38, V42, D44, M46, V50, and R56 (red); and weakly changing chemical shifts for T35, K52, and members of the segment 80–91 (yellow). Nearly no chemical shift changes were observed for residues 61–77, except for A74 (Fig. 3 A and C). No clear decision could be taken for V48. A very similar pattern of chemical shift changes was elicited by AP180-ANTH (Fig. S3), indicating that both ligands interact with the same set of residues in synaptobrevin 2. We conclude that the ANTH domains of CALM and AP180 associate with synaptobrevin 2 via the N-terminal half of its amphipathic SNARE helix.
ANTH domain association also elicited alterations in the organization of the C-terminal helix and the juxtamembrane linker of synaptobrevin 2 (Fig. 3). Previous work has identified a cluster of aromatic residues Y88–W90 within this region that might regulate synaptobrevin 2 function (22–24). Our data suggest that this aromatic cluster may form a stable loop structure. However, because the chemical shift-changes induced by CALM-ANTH (Fig. 3 A and C) or AP180-ANTH (Fig. S3) in the presence of DPC are weak, we consider it unlikely that the aromatic cluster directly associates with CALM or AP180.
SNARE Motif-Dependent Endocytic Sorting of Synaptobrevin 2.
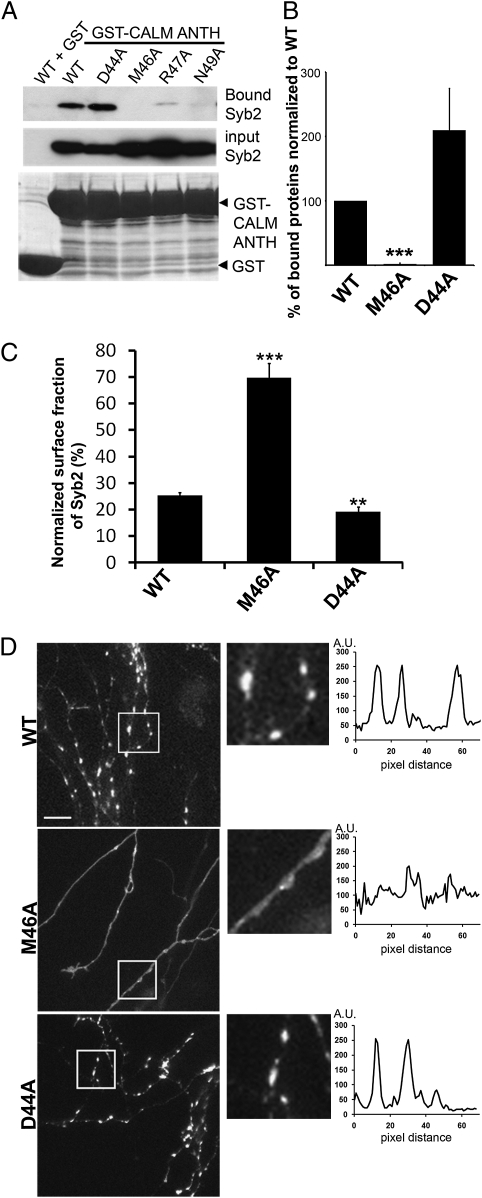
Finally, we functionally probed the physiological relevance of ANTH domain recognition of SNARE motif-based sorting determinants within synaptobrevin 2 for its exo-endocytic cycling. To this aim, we generated site-directed mutants of synaptobrevin 2 harboring mutations within the N-terminal half of the SNARE helix. Based on the NMR spectroscopy analysis, we targeted D44, M46, R47, and N49 for mutagenesis. M46 constitutes a central part of the hydrophic face of the N-terminal half of the amphipathic SNARE helix (3). Substitution of M46A completely abrogated the ability of synaptobrevin 2 to associate with GST-CALM-ANTH (Fig. 4 A and B), whereas mutation of R47 or N49 to alanines greatly diminished binding (Fig. 4A). By contrast, increased binding to CALM-ANTH was observed for D44A (Fig. 4 A and B and Fig. S4B). Because M46A and D44A differentially affected the recognition of synaptobrevin 2 by ANTH domain-containing adaptors, remained competent for SNARE complex formation (Fig. S4A), and have previously been shown to regulate synaptobrevin 2 targeting to synaptic-like microvesicles in PC12 cells (25), these mutants were selected for further analysis.
Fig. 4.
SNARE motif-dependent endocytic sorting of synaptobrevin 2. (A) Mutational analysis of the ANTH domain-binding interface within the SNARE motif of synaptobrevin 2. GST-CALM-ANTH was immobilized on beads and incubated with lysates from HEK293 cells expressing synaptobrevin 2-FLAG carrying the indicated mutations. Samples were analyzed by SDS/PAGE and immunoblotting. (B) Quantification of CALM-ANTH binding to synaptobrevin 2 M46A or D44A as depicted in A. Binding to CALM-ANTH is abolished significantly by M46A and increased by D44A mutations (P < 0.0001 and P = 0.1415, respectively; n = 3 independent experiments). (C) Exo-endocytic cycling of mutant synaptobrevin 2-pHluorin. M46A is seen to accumulate on the neuronal surface (P < 0.0001, n = 9 neurons), whereas D44A shows decreased surface pools (P = 0.0073, n = 19 neurons). (D) Axonal dispersion of synaptobrevin 2 (M46A). Wild-type and D44A synaptobrevin 2-pHluorin display a pronounced concentration at presynaptic boutons, whereas M46A is dispersed along the axon. The experiment was carried out as described in Fig. 1F. (Scale bar, 5μm.)
To probe the effect of these mutations on the partitioning of synaptobrevin 2 between internal and surface pools, we expressed mutant variants of synaptopHluorin in primary hippocampal neurons. Boutons expressing either of these mutant synaptopHluorins (M46A or D44A) exhibited proper responses to electrical stimulation (Fig. S5). As expected from the biochemical analysis, synaptopHluorin carrying the M46A mutation exhibited a dramatic increase in the surface-to-vesicular pool ratio, indicative of its failure to become internalized (Fig. 4C). Conversely, a statistically significant reduction in the surface-to-vesicular pool was observed for D44A (Fig. 4C), likely as a consequence of its increased binding to AP180 and CALM (Fig. 4B and Fig. S4B). These changes were accompanied by a failure of synaptopHluorin-M46A to be enriched at presynaptic vesicle clusters (Fig. 4D). Thus, ANTH domain-based endocytic sorting of synaptobrevin is mediated by the N-terminal half of the SNARE helix centered around M46.
Discussion
We identify here the molecular mechanism by which the SV R-SNARE protein synaptobrevin 2 is endocytosed at the neuronal surface. Combined cell biological and structural biochemical analyses indicate that the N-terminal half of the SNARE motif of synaptobrevin 2 serves as a sorting determinant for recognition by the related ANTH domain-containing endocytic adaptors AP180 and CALM. First, knockdown of AP180 or CALM, or of both proteins together, causes a substantial increase in the fraction of surface-stranded synaptobrevin 2 along the axon. Second, synaptobrevin via determinants within the N-terminal half of its SNARE motif around M46 is directly bound to and recognized by the ANTH domain of CALM or AP180, as shown by biochemical assays and NMR spectroscopy analysis. Third, mutations within synaptobrevin 2 that affect the ability of the protein to interact with CALM or AP180 lead to corresponding changes in the efficiency with which it is endocytosed from the neuronal surface. Thus, endocytic recycling of synaptobrevin 2 is achieved by ANTH domain-mediated recognition of the N-terminal half of the SNARE motif.
This is a unique mechanism for SNARE sorting that is substantially different from the post-Golgi trafficking recently described for the VAMP-family members Vti1b and VAMP7/Ti-VAMP (10–12). In these cases, specific sorting is achieved by the association of the N-terminal Habc domain of Vti1b and the longin domain of VAMP7/Ti-VAMP with their cognate adaptors EpsinR and Hrb, respectively. Synaptobrevin 2 internalization by AP180/CALM by contrast involves determinants within the SNARE motif that overlap with the binding site for syntaxin/SNAP-25 (3, 4). Hence, we predict that NSF-mediated disassembly of SNARE complexes (2, 4, 5) is a prerequisite for endocytic recycling of synaptobrevin 2 via AP180/CALM, consistent with the low abundance of syntaxin and SNAP-25 in purified SVs (7). The comparably weak interaction between synaptobrevin 2 and CALM or AP180 likely is facilitated by avidity effects (26), which could involve local clustering of exocytosed synaptobrevin 2 molecules (27, 28) and/or lateral cross-linking of ANTH adaptor-SNARE complexes via the assembling clathrin scaffold (29, 30).
The results reported here further support and extend previous genetic studies that have suggested a role for AP180 family members in synaptobrevin sorting and SV endocytosis in invertebrates (14–16, 31, 32). Similar to what has been observed in D. melanogaster (16), AP180-depleted neurons display alterations in SV size, a phenotype reminiscent of that seen in neurons derived from synaptobrevin 2 knockout mice (20). Irregularly shaped and sized vesicles and pits have also been observed in CALM-depleted fibroblasts (33). Paired with the observation that synaptobrevin knockout mice suffer from delayed replenishment of SV membranes following hypertonic sucrose stimulation (20), these data indicate a close physical and functional partnership between synaptobrevin and the AP180 family members CALM and AP180. Why two distinct endocytic proteins, CALM and AP180, are used to retrieve synaptobrevin from the neuronal surface in mammals remains to be determined but could be related to the developmental regulation of their expression profiles and concomitant changes in exo-endocytic cycling of SV proteins.
A critical hallmark of the SV cycle is the tight coupling between exo- and endocytosis (1). Such coupling ensures a close balance between exo- and endocytosed membrane and is required to maintain SV protein composition over multiple cycles. How such coupling is achieved remains unknown, but may involve adaptor-dependent sorting mechanisms (34, 35) and/or SV protein clustering (27). Our data show that at least in the case of synaptobrevin, the ANTH domain-containing adaptors AP180 and CALM operate in a cargo-specific manner to selectively retrieve surface-stranded brevin molecules, whereas sorting of other SV cargo such as vGLUT1 procedes unperturbed. These observations favor a scenario in which coordinated activity of cargo-specific adaptors maintains SV composition. Such cargo-specific SV sorting adaptors include AP180 and CALM (this study), stonin 2 (36), and perhaps endophilin (37), among other to-be-identified proteins.
Finally, it is tempting to speculate that the function of AP180 and CALM in retrieving surface-stranded synaptobrevin from the neuronal surface may be related to their role in neurodegenerative disorders, including Alzheimer's disease (38, 39). Further work is needed to investigate this possibility in detail.
Materials and Methods
SI Materials and Methods available online include plasmids, siRNAs, antibodies, cell culture and transfection, protein purification, binding assays, immunoprecipitation, SPOT synthesis and binding studies, surface plasmon resonance (SPR) measurements, pHluorin imaging, FM4-64 imaging, immunostaining of primary neurons, and electron microscopy.
Preparation of Detergent Extracts from Mouse Brain.
Hippocampi of mice at various ages were isolated and minced by a douncer for 15–20 strokes at 3.9 × g in lysis buffer [20 mM Hepes (pH 7.4), 100 mM KCl, 2 mM MgCl2, 5 mM EDTA, 1% Triton X-100, and 1 mM PMSF] supplemented with protease inhibitor mixture (Sigma) and 10 μM ALLN (Calbiochem). After 30 min on ice, the lysate was centrifuged at 20,800 × g for 10 min at 4 °C. The resulting supernatant was collected and its concentration was determined by the Bradford assay.
Protein Purification, Binding Assays, and Immunoprecipitation.
Recombinant proteins were purified using standard protocols. As a final purification step, recombinant proteins were subjected to size exclusion chromatography in buffer A [150 mM NaCl, 5 mM DTT, 1 mM EDTA, and Mes (pH 6.0)] for NMR spectroscopy and in buffer B [20 mM Tris (pH 7.5) and 150 mM NaCl] for SPR measurements. Binding assays and immunoprecipitation experiments were essentially done as described in Diril et al. (36). See SI Materials and Methods for further details.
pHluorin Imaging.
Primary neuronal cultures were prepared from hippocampi of P1–P3 Wistar rats. On 6–8 days in vitro (DIV), neurons were transfected by calcium phosphate transfection (Promega Inc.). On DIV 12–15, neurons expressing synaptopHluorin or vGLUT1-pHluorin were live-imaged using a charge-coupled device camera (AxioCam; Carl Zeiss, Inc.) on an inverted microscope (Axiovert 200M; Carl Zeiss, Inc.) essentially as described previously (36, 40). See SI Materials and Methods for full details. The fraction of surface pool was calculated by the following equation; psurface = [SpH]surface/([SpH]surface + [SpH]vesicle) = (Fphysio. – Facidic)/(Fphysio. – Facidic) + (Fbasic – Fphysio.). A total of 200 APs (20 Hz, 100 mA) were used to stimulate the neurons.
NMR Spectroscopy.
1H-15N HSQC spectra of 15N-labeled synaptobrevin 2 were recorded on a Bruker DRX-600 NMR spectrometer in aqueous solution at 18 °C, in complex with DPC at 30 °C. Protein concentration was 0.5–1 mM in a buffer consisting of 150 mM NaCl, 5 mM DTT, 1 mM EDTA, 10% D2O, 20 mM Mes at pH 6.0, and optionally 200 mM DPC. CALM-ANTH or AP180-ANTH was added stepwise up to twofold stoichiometric excess. Spectra were processed with Topspin and analyzed in Sparky version 3.114 (41). Assignments were taken from BMRB entry 16514 (21). Shift tolerances of 0.05 ppm were given for spectra of DPC-bound synaptobrevin 2. Structures were plotted using University of California, San Francisco, Chimera (http://www.cgl.ucsf.edu/chimera/) (42).
Supplementary Material
Acknowledgments
We thank T. Südhof, R. Edwards, and R. Jahn for plasmids. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB958/A01, FOR806, and Exc 257-Neurocure and the European Science Foundation. S.J.K. is the recipient of a PhD fellowship from the Max Delbrück Center for Molecular Medicine.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107067108/-/DCSupplemental.
References
- 1.Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat Rev Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- 2.Murthy VN, De Camilli P. Cell biology of the presynaptic terminal. Annu Rev Neurosci. 2003;26:701–728. doi: 10.1146/annurev.neuro.26.041002.131445. [DOI] [PubMed] [Google Scholar]
- 3.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 4.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 5.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoch S, et al. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- 7.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 8.Mutch SA, et al. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci. 2011;31:1461–1470. doi: 10.1523/JNEUROSCI.3805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traub LM. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 10.Chaineau M, Danglot L, Proux-Gillardeaux V, Galli T. Role of HRB in clathrin-dependent endocytosis. J Biol Chem. 2008;283:34365–34373. doi: 10.1074/jbc.M804587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller SE, Collins BM, McCoy AJ, Robinson MS, Owen DJ. A SNARE-adaptor interaction is a new mode of cargo recognition in clathrin-coated vesicles. Nature. 2007;450:570–574. doi: 10.1038/nature06353. [DOI] [PubMed] [Google Scholar]
- 12.Pryor PR, et al. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell. 2008;134:817–827. doi: 10.1016/j.cell.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dittman JS, Kaplan JM. Factors regulating the abundance and localization of synaptobrevin in the plasma membrane. Proc Natl Acad Sci USA. 2006;103:11399–11404. doi: 10.1073/pnas.0600784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nonet ML, et al. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Mol Biol Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bao H, et al. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, et al. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- 17.Morgan JR, et al. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. J Neurosci. 1999;19:10201–10212. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao PJ, Zhang P, Mattson MP, Furukawa K. Heterogeneity of endocytic proteins: Distribution of clathrin adaptor proteins in neurons and glia. Neuroscience. 2003;121:25–37. doi: 10.1016/s0306-4522(03)00431-7. [DOI] [PubMed] [Google Scholar]
- 19.Bushlin I, et al. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. J Neurosci. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deák F, Schoch S, Liu X, Südhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- 21.Ellena JF, et al. Dynamic structure of lipid-bound synaptobrevin suggests a nucleation-propagation mechanism for trans-SNARE complex formation. Proc Natl Acad Sci USA. 2009;106:20306–20311. doi: 10.1073/pnas.0908317106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kweon DH, Kim CS, Shin YK. Regulation of neuronal SNARE assembly by the membrane. Nat Struct Biol. 2003;10:440–447. doi: 10.1038/nsb928. [DOI] [PubMed] [Google Scholar]
- 23.Quetglas S, et al. Calmodulin and lipid binding to synaptobrevin regulates calcium-dependent exocytosis. EMBO J. 2002;21:3970–3979. doi: 10.1093/emboj/cdf404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fdez E, et al. A role for soluble N-ethylmaleimide-sensitive factor attachment protein receptor complex dimerization during neurosecretion. Mol Biol Cell. 2008;19:3379–3389. doi: 10.1091/mbc.E08-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grote E, Hao JC, Bennett MK, Kelly RB. A targeting signal in VAMP regulating transport to synaptic vesicles. Cell. 1995;81:581–589. doi: 10.1016/0092-8674(95)90079-9. [DOI] [PubMed] [Google Scholar]
- 26.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 27.Opazo F, et al. Limited intermixing of synaptic vesicle components upon vesicle recycling. Traffic. 2010;11:800–812. doi: 10.1111/j.1600-0854.2010.01058.x. [DOI] [PubMed] [Google Scholar]
- 28.Roy R, Laage R, Langosch D. Synaptobrevin transmembrane domain dimerization-revisited. Biochemistry. 2004;43:4964–4970. doi: 10.1021/bi0362875. [DOI] [PubMed] [Google Scholar]
- 29.Ford MG, et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 30.Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci. 2004;117:9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- 31.Burston HE, et al. Regulators of yeast endocytosis identified by systematic quantitative analysis. J Cell Biol. 2009;185:1097–1110. doi: 10.1083/jcb.200811116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- 33.Meyerholz A, et al. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- 34.Jung N, Haucke V. Clathrin-mediated endocytosis at synapses. Traffic. 2007;8:1129–1136. doi: 10.1111/j.1600-0854.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 35.Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- 36.Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell. 2006;10:233–244. doi: 10.1016/j.devcel.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Voglmaier SM, et al. Distinct endocytic pathways control the rate and extent of synaptic vesicle protein recycling. Neuron. 2006;51:71–84. doi: 10.1016/j.neuron.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 38.Harold D, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu F, Matsuoka Y, Mattson MP, Yao PJ. The clathrin assembly protein AP180 regulates the generation of amyloid-beta peptide. Biochem Biophys Res Commun. 2009;385:247–250. doi: 10.1016/j.bbrc.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pechstein A, et al. Regulation of synaptic vesicle recycling by complex formation between intersectin 1 and the clathrin adaptor complex AP2. Proc Natl Acad Sci USA. 2010;107:4206–4211. doi: 10.1073/pnas.0911073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goddard TD, Kneller DG. SPARKY 3. 2008. Available at http://www.cgl.ucsf.edu/home/sparky/
- 42.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.