Abstract
Cyclic AMP (cAMP) and calcium are ubiquitous, interdependent second messengers that regulate a wide range of cellular processes. During development of neuronal networks they are critical for the first step of circuit formation, transducing signals required for axon pathfinding. Surprisingly, the spatial and temporal cAMP and calcium codes used by axon guidance molecules are unknown. Here, we identify characteristics of cAMP and calcium transients generated in growth cones during Netrin-1–dependent axon guidance. In filopodia, Netrin-1–dependent Deleted in Colorectal Cancer (DCC) receptor activation induces a transient increase in cAMP that causes a brief increase in calcium transient frequency. In contrast, activation of DCC in growth cone centers leads to a transient calcium-dependent cAMP increase and a sustained increase in frequency of calcium transients. We show that filopodial cAMP transients regulate spinal axon guidance in vitro and commissural axon pathfinding in vivo. These growth cone codes provide a basis for selective activation of specific downstream effectors.
Keywords: cyclic AMP dynamics, calcium dynamics, compartmentalization, photoactivated adenylyl cyclase alpha, cyclic AMP oscillations
Cyclic AMP (cAMP) is a major cellular second messenger that activates and integrates multiple intracellular signaling pathways. Microdomains of cAMP are generated in cardiac myocytes (1), regional domains of cAMP are present in neurons (2, 3), and cAMP diffusion is restricted by rapid degradation by phosphodiesterases (4) that limit the duration of cAMP signaling (5, 6). However, little is known about spatial compartmentalization and temporal dynamics of cAMP in neuronal growth cones, despite the importance of cAMP for axon pathfinding in response to a wide range of molecular guidance cues, including Netrin-1 (7–11), semaphorins (12), Slits, and ephrins (13).
Calcium is another ubiquitous second messenger involved in axon pathfinding, mediating responses to axon guidance molecules. In addition to axon steering, calcium modulates axon outgrowth and retraction (14, 15). A sustained gradient of calcium across the growth cone is thought to be generated by asymmetric activation of axon guidance receptors, and to be the relevant calcium signal for axon pathfinding (16). Spontaneous fast and spatially restricted filopodial calcium transients are also sufficient to steer axons (17, 18). The frequency of slower transients in the entire growth cone controls the rate of axon extension in vivo and in vitro (19). The regulation of these transients by axon guidance cues has not been investigated.
Filopodia are critical for axon pathfinding (20), and clues to their operation are provided by subcellular localization of signaling components. Filopodial enrichment of regulatory subunit II of protein kinase A, a major effector of cAMP, is required for growth cone attraction mediated by intracellular gradients of cAMP or PACAP (21). Modulation of spontaneous calcium transients in filopodia regulates axon turning (17). Temporal regulation of cAMP synthesis is also important for axon responses to ephrin-As (14), and the frequency of calcium transients in the growth cone center that includes the lamellipodium regulates the rate of axon extension (19). However, little is known about regulation of filopodial second messenger signals and their temporal dynamics by diffusible axon guidance cues.
We have investigated the subcellular localization, temporal dynamics, and local interactions of cAMP and calcium signals generated in growth cones in response to Netrin-1, the principal axon guidance molecule required for attraction of commissural axons by the spinal floor plate. We report a major difference in cAMP/calcium interplay between filopodia and the growth cone center. Optogenetic elevation of cAMP in filopodia but not in growth cone centers drives growth cone attraction. Both filopodial and growth cone center signaling pathways are mediated by activation of the same Netrin-1 receptor, Deleted in Colorectal Cancer (DCC). We show that cAMP dynamics are also essential for midline crossing of commissural axons in vivo.
Results
We identified spatial and temporal second-messenger codes that regulate axon guidance using dissociated cell cultures of Xenopus spinal neurons. Application of a local Netrin-1 gradient to growth cones of these cultured neurons attracts axons in a cAMP- and calcium-dependent manner that requires the DCC Netrin-1 receptor (8, 16) mediating both attraction and repulsion in Xenopus spinal neurons (22). In contrast to previous studies (16, 23), we did not include serum in the culture medium because it blocked some of the growth cone calcium signals observed in vivo (Fig. S1 A and B). The response to Netrin-1 and its dependence on cAMP and calcium were robust: Netrin-1 is an attractant in the control condition, converted into a repellent when adenylyl cyclase is inhibited, and does not affect the direction of axon growth in the absence of extracellular calcium (Fig. S1C).
We used a plasma membrane-targeted Epac2-camps (pmEpac2-camps) FRET probe (24) to monitor fluctuations in cAMP concentration. Growth cones expressing pmEpac2-camps responded to bath application of forskolin, an activator of transmembrane adenylyl cyclases, with an increase in the CFP:YFP ratio (Fig. S2 and Movie S1). Although we did not calibrate the sensor, we demonstrated that saturation was not reached with the resting concentration of cAMP in growth cones (Fig S2). Targeting pmEpac2-camps to the plasma membrane reduces differences in fluorescence intensity between the center of the growth cone and filopodia, and allows monitoring of cAMP in both compartments (Fig. S3). However, measurement of cAMP levels with this Epac-based sensor could miss detection of cAMP levels that would be revealed with a PKA-based sensor.
Dynamics of cAMP and Calcium Signals in Filopodia.
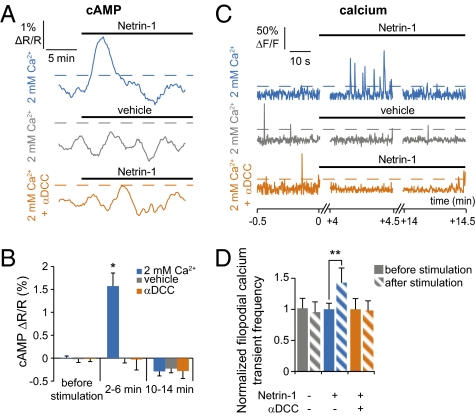
When growth cones were stimulated by Netrin-1, cAMP increased transiently in filopodia ∼1 min after stimulation, reaching a maximum by ∼4 min (Fig. 1A). Although stimulation was maintained, the cAMP level returned to baseline ∼10 min after the onset of Netrin-1 application. This effect was not due to desensitization of the probe, because pmEpac2-camps reported a sustained increase in cAMP concentration (Fig. S2). The cAMP response to Netrin-1 was blocked when it was replaced with a vehicle or when cultures were preincubated in a solution containing a function-blocking antibody against the DCC Netrin-1 receptor (Fig. 1 A and B).
Fig. 1.
In filopodia, Netrin-1 induces a brief cAMP transient and a brief increase in frequency of calcium transients. (A and B) The cAMP concentration increases transiently in filopodia after growth cone stimulation with Netrin-1 (blue trace), whereas no signal is detected when Netrin-1 stimulation is replaced by application of vehicle (gray trace) or when neurons are preincubated with a DCC function-blocking antibody (1 μg/mL, orange trace). Average of 10 or more growth cones. Signal is considered positive when its amplitude exceeds twice the SD of the baseline for 5 min before stimulation (dashed lines). (C and D) Netrin-1 induces a transient increase in frequency of filopodial calcium transients (blue trace) that is absent when Netrin-1 is replaced by a vehicle (gray trace) or in the presence of the anti-DCC function-blocking antibody (1 μg/mL, orange trace). Signals are considered positive when they exceed 20% ΔF/F0 (dashed lines; greater than twice the SD of the baseline). n ≥ 60 filopodia. Error bars, SEM; *P < 0.05, **P < 0.01. (B and D) Paired Student t test.
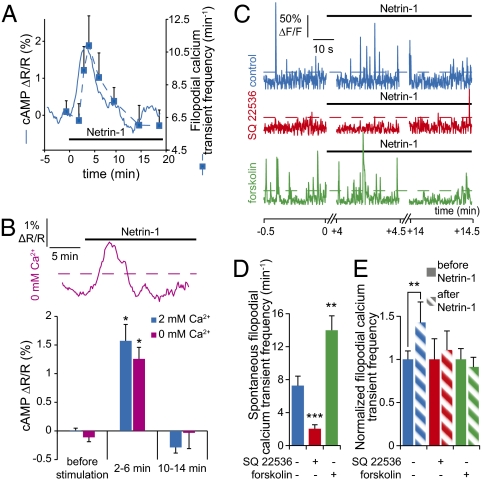
The frequency of fast filopodial calcium transients (17) followed the same dynamics as the cAMP transient (Figs. 1 C and D and 2A, Fig. S4, and Movie S2). Starting from a baseline (6.8 ± 0.7 min−1) before Netrin-1 stimulation, the frequency began rising ∼2 min after the initiation of stimulation and achieved its maximum (10.9 ± 1.8 min−1) ∼4 min after the onset of Netrin-1 application; it returned to baseline after ∼14 min (Fig. 2A and Movie S2). Stimulation with vehicle or exposure to anti-DCC prevented this increase in filopodial calcium transient frequency (Fig. 1 C and D). Ratiometric calcium imaging that avoids artifacts due to volume changes confirmed the Netrin-1–induced increase in frequency of filopodial calcium transients. The increase in frequency was greater in proximal than in distal filopodia (Fig. S4).
Fig. 2.
The Netrin-1–induced elevation of cAMP drives generation of filopodial calcium transients. (A) The increase in calcium transient frequency (dashed trace) follows the increase in cAMP (solid trace). (B) Calcium-free medium does not affect the transient Netrin-1–induced elevation of cAMP. (A and B) Average of 10 or more growth cones. Dashed line as in Fig. 1A. (C and D) Spontaneous filopodial calcium transients occur less frequently in SQ22536-treated growth cones (red trace) and more often in forskolin-treated neurons (green trace). (C) Dashed lines as in Fig. 1C. (E) Application of the transmembrane adenylyl cyclase inhibitor SQ22536 or forskolin prevents the increase in frequency of calcium transients induced by Netrin-1 (blue trace). (D and E) n ≥ 60 filopodia. Error bars, SEM; *P < 0.05, **P < 0.01, ***P < 0.001. (B and D) Paired Student t test. (E) ANOVA.
cAMP Drives Generation of Filopodial Calcium Transients.
Because cAMP and calcium regulate each other and modulate axon turning (25), we investigated the interplay between cAMP and calcium during Netrin-1–induced signaling in filopodia. To determine whether cAMP regulates production of filopodial calcium transients, we suppressed its synthesis with SQ22536, a blocker of transmembrane adenylyl cyclases, and stimulated its synthesis with forskolin. SQ22536 reduced and forskolin increased the frequency of spontaneous filopodial calcium transients (Fig. 2 C and D). In contrast, both treatments precluded the Netrin-1–induced increase in frequency (Fig. 2 C and E). Blocking transmembrane adenylyl cyclases prevents the Netrin-1–dependent increase in calcium transient frequency, whereas constantly stimulating them likely elevates cAMP to a level that induces the maximal calcium transient frequency so that further stimulation by Netrin-1 is not possible. These experiments indicate that Netrin-1–dependent stimulation of filopodial calcium transients is driven by transient elevation of cAMP. We monitored cAMP in Netrin-1–stimulated growth cones in calcium-free medium to determine whether calcium influx regulates cAMP synthesis. The absence of calcium did not affect the cAMP response, suggesting that the Netrin-1–dependent increase in cAMP concentration is independent of extracellular calcium (Fig. 2B). A role for calcium release from intracellular stores is not excluded.
cAMP and Calcium Dynamics in Growth Cone Centers.
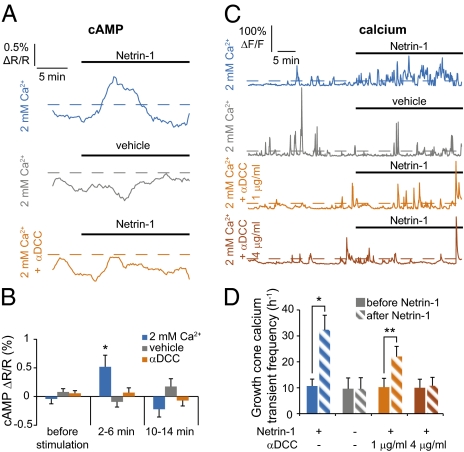
We next focused on the dynamics of signaling events occurring in the center of the growth cone, excluding filopodia. Application of Netrin-1 to growth cones elicited an increase in cAMP concentration in growth cone centers with a time course delayed and prolonged compared with that in filopodia, starting to rise ∼2 min after stimulation and reaching its maximum by ∼5 min before decaying (Fig. 3 A and B). This increase was blocked by replacement of Netrin-1 with vehicle or with the DCC function-blocking antibody. The frequency of calcium transients in the growth cone center increased in a sustained manner upon stimulation with Netrin-1 (Movie S3), but not in response to vehicle, and was fully suppressed by a higher concentration of anti-DCC (Fig. 3 C and D).
Fig. 3.
In the growth cone center, Netrin-1 induces a longer cAMP transient and a sustained increase in frequency of calcium transients that are larger and longer in duration. (A and B) The cAMP concentration increases transiently in the growth cone center after Netrin-1 stimulation (blue trace), whereas no signal is detected when Netrin-1 stimulation is replaced by application of culture medium (gray trace) or after incubation with a function-blocking antibody to DCC (1 μg/mL, orange trace). Average of 10 or more growth cones. Dashed line as in Fig. 1A. (C and D) Netrin-1 stimulation induces a sustained increase in frequency of calcium transients in growth cone centers (blue trace). The spontaneous calcium transient frequency is not affected by application of vehicle to growth cones (gray trace). The Netrin-1–induced increase in frequency is blocked after preincubation with 4 μg/mL of anti-DCC antibody (brown trace). n ≥ 5 growth cones. (A and C) Dashed lines as in Fig. 1 A and C. Error bars, SEM; *P < 0.05, **P < 0.01. (B) Paired Student t test. (D) Wilcoxon test.
Calcium Drives Elevation of cAMP in Growth Cone Centers.
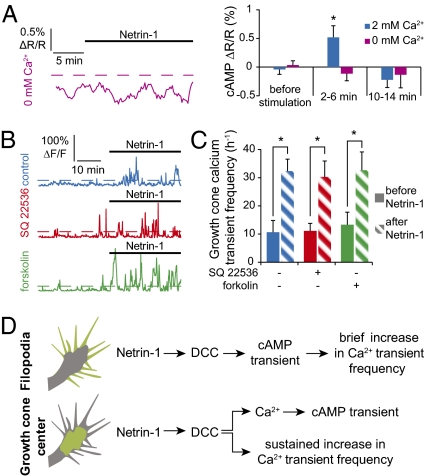
Growing neurons in calcium-free culture medium blocked both the Netrin-1–induced increase in cAMP concentration (Fig. 4A) and the calcium transients (26). In contrast, neither blockade of cAMP synthesis by SQ22536 nor forskolin-induced increase of cAMP concentration affected the frequency of calcium transients in the growth cone center in response to Netrin-1 (Fig. 4 B and C). Thus, the elevation of cAMP is downstream of calcium, and the order of cAMP and calcium signaling is inverted between the filopodia and growth cone center (Fig. 4D).
Fig. 4.
The Netrin-1–induced calcium drives elevation of cAMP in the center of the growth cone. (A) Calcium-free culture medium blocks the transient elevation of cAMP in growth cone centers. Average of 10 or more growth cones. Dashed line as in Fig. 1A. (B and C) Application of the transmembrane adenylyl cyclase inhibitor SQ22536 (red trace) or forskolin (green trace) does not affect the Netrin-1–dependent elevation of calcium transient frequency. (B) Dashed lines as in Fig. 1C. Error bars, SEM; *P < 0.05. (A) Paired Student t test. (C) Wilcoxon test. (D) Summary of signaling pathways. In filopodia, Netrin-1 drives a transient increase of cAMP concentration through its DCC receptor; cAMP elicits a brief rise in frequency of filopodial calcium transients. In growth cone centers, Netrin-1, through the DCC receptor, drives a transient calcium-dependent increase in cAMP and a sustained cAMP-independent increase in the frequency of calcium transients.
cAMP Transients in Filopodia Steer Axons.
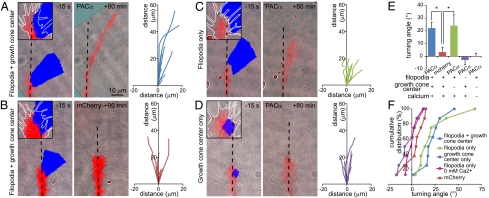
To investigate further the spatial code by which cAMP regulates axon guidance, we used mCherry-tagged photoactivated adenylyl cyclase (27) (mCherry-PACα; Fig. S5A and Movie S4) to generate localized elevations of cAMP mimicking the duration of those stimulated by Netrin-1 (27). Exposure of quadrants of growth cones expressing mCherry-PACα to blue light for 3 min every 20 min induced turning of axons toward the stimulated side (Fig. 5 A, E, and F), mimicking the effect of Netrin-1 in similar culture conditions (8, 16, 22, 23) (Fig. S1) and demonstrating that asymmetrical and pulsatile elevations of cAMP are sufficient for axon turning. The same stimulation protocol applied to mCherry-expressing growth cones had no effect on the direction of axon outgrowth (Fig. 5 B, E, and F). We next determined the contributions of cAMP elevations in filopodia and growth cone centers. Mimicking the Netrin-1–driven cAMP increase in filopodia on one side of the growth cone induced axon turning toward the stimulated side (Fig. 5 C, E, and F). In contrast, increasing cAMP in filopodia on one side of growth cones in culture medium lacking calcium did not induce axon turning (Fig. 5 E and F), in agreement with the dependence of filopodial calcium transients on cAMP signals (Fig. 4D). Stimulation of one side of the growth cone center in the presence of extracellular calcium also failed to induce turning (Fig. 5 D–F). Local stimulation of PACα produces an asymmetric and transient elevation of cAMP (Fig. S5 B and C), likely because of hydrolysis by phosphodiesterases (4). Thus, filopodial cAMP signals that mimic the duration of those elicited by Netrin-1 are sufficient to steer growth cone turning. In contrast, asymmetric elevations of cAMP in the growth cone center are not sufficient to induce growth cone turning. Overlap of excitation wavelengths of pmEpac2-camps and PACα prevented comparison of the amplitude of PACα-induced cAMP transient with that elicited by Netrin-1.
Fig. 5.
Optogenetically generated cAMP transients in filopodia but not growth cone centers encode growth cone turning in vitro. (A) A brief cAMP elevation induced by blue light illumination of one side of a PACα-expressing growth cone is sufficient to induce a change in direction of axon outgrowth (Left). Trajectories of PACα-expressing axons stimulated for 3 min three times per hour in filopodia and growth cone center (Right). The initial orientation of the axons (before stimulation) is aligned with the y axis and superimposed on the same origin. (B) Illumination of filopodia and the growth cone center expressing mCherry does not induce growth cone turning (Left). Trajectories from mCherry-expressing growth cones are evenly distributed around the initial orientation of the axon (Right). (C) Stimulation of PACα in filopodia alone is sufficient to induce axon turning toward the stimulated side of the growth cone. (D) Elevation of cAMP in the growth cone center does not stimulate axon turning. (E) Average turning angle and (F) cumulative distribution of angles of axons expressing PACα or mCherry and illuminated on filopodia, growth cone center, or in both. (E and F) n ≥ 8 growth cones. Error bars, SEM; *P < 0.05, Kruskal–Wallis test.
cAMP Transient Frequency Codes Growth Cone Responsiveness.
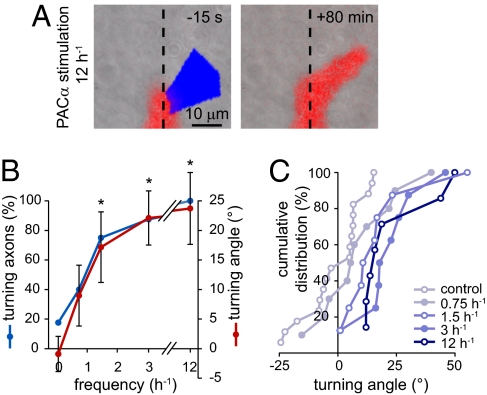
We examined temporal coding of axon turning by cAMP by exposing mCherry-PACα–expressing growth cones to pulses of blue light at frequencies of 0–12 h−1. These growth cones did not turn spontaneously in the absence of stimulation. Increasing the frequency of cAMP transients led to an increase in the percentage of turning axons, and the maximum response was achieved with a frequency of three transients per hour, similar to that of spontaneous transients observed in cell bodies (5) (Fig. 6). Neither the turning success rate (percentage of axons turning more than 7.5°) nor the turning angle was affected by a further increase in frequency. Moreover, the frequency of cAMP transients did not affect the turning angle of individual, responsive axons (Fig. S6), suggesting that the frequency of cAMP transients codes for an all-or-none switch, inducing growth cones to turn. The distinct thresholds for individual growth cones may reflect cell type-specific sensitivity for axon guidance cues.
Fig. 6.
Temporal coding of turning by optogenetically generated cAMP transients. (A) High-frequency cAMP elevations (12 h−1) induced by blue light illumination of one side of a PACα-expressing growth cone are sufficient to induce a change in direction of axon outgrowth. (B) Turning success rate (percentage of axons turning more than 7.5°) and turning angle after cAMP pulses at frequencies of 0–12 h−1. n ≥ 7 growth cones. Error bars, SEM; *P < 0.05, Kruskal–Wallis test. (C) Cumulative distribution of angles of PACα-expressing axons illuminated at frequencies of 0–12 h−1.
Sustained Changes in cAMP Levels Disrupt Commissural Axon Pathfinding in Vivo.
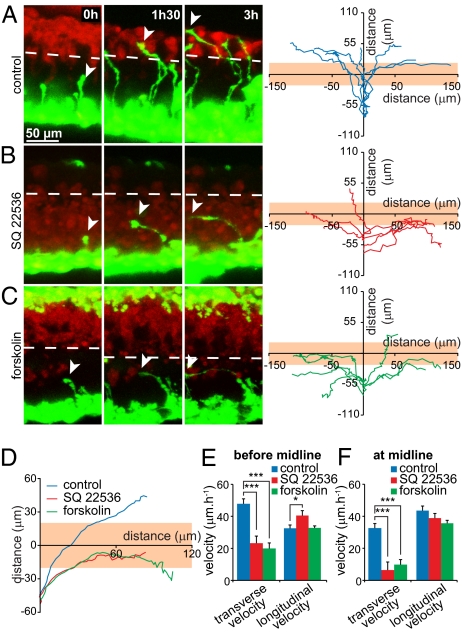
Netrin-1 is the major attractive axon guidance molecule required for commissure formation in a wide range of animal species, including Caenorhabditis elegans, Drosophila melanogaster, and mammals (28–30). Calcium signaling is required for proper ventral midline crossing by commissural axons in the Xenopus spinal cord (31), but the role of cAMP in their guidance has been contentious. In vitro, cAMP induces a switch in the response to Netrin-1 (8), but genetic deletion of soluble adenylyl cyclase fails to produce a defect in guidance of spinal commissural neurons in mice (32). We imaged growth of Xenopus laevis spinal commissural axons to determine whether cAMP signaling regulates axon guidance in vivo (Fig. S7A). In control conditions, commissural axons grow to the midline and cross it before turning anteriorly or posteriorly (31) (Fig. 7 A and D and Fig. S7B). Decreasing cAMP levels with SQ22536 prevents axons from crossing the midline. The majority of axons join the floor plate before turning and growing longitudinally (Fig. 7 B and D and Fig. S7B), indicating that cAMP signaling is required for normal commissural axon guidance. A sustained increase in cAMP concentration generated by application of forskolin leads to a similar defect (Fig. 7 C and D and Fig. S7B). Analysis of growth cone velocity indicates that cAMP signaling is required for commissural growth cone pathfinding, with only a modest effect on axon outgrowth (Fig. 7 E and F). Elimination of cAMP modulation both with SQ22536 and forskolin suggests that appropriate guidance of spinal commissural axons requires critical cAMP dynamics.
Fig. 7.
Disruption of cAMP signaling prevents midline crossing of commissural axons in vivo. (A) Alexa 488 dextran-filled commissural axons imaged in the intact spinal cord of control embryos grow to the ventral midline (dashed line at the Alexa 568 border), cross it, and turn rostrally or caudally along the contralateral ventral fascicle (Left). Trajectories of control growth cones (Right). Orange horizontal bar represents the floor plate. (B) Commissural axons in a SQ 22536-treated spinal cord grow to the ventral midline but fail to cross it. (C) Treating the spinal cord with forskolin also prevents axons from crossing the midline. (A–C) n > 25 growth cones. All trajectories are shown in Fig. S7B. (D) Trajectories of individual axons were aligned to obtain an average trajectory for each experimental condition, and rostrally and caudally turning trajectories were superimposed. Averaged trajectories demonstrate a failure of midline crossing when spinal cords are treated with SQ22536 or forskolin. More than 25 trajectories were scored for each condition. (E) To distinguish effects on axon directionality and growth, we quantified the transverse velocity (toward the midline) and the longitudinal velocity (parallel to the midline) of axons from spinal cords exposed to control, SQ22536-, or forskolin-containing medium. The transverse velocity was reduced by high or low cAMP concentration before the midline and (F) further suppressed within a 40-μm–wide area around the ventral midline. In contrast, the longitudinal velocity was only slightly affected by modifying the cAMP concentration. (E and F) Error bars, SEM; ***P < 0.001, *P < 0.05, ANOVA.
Local Pulses of cAMP Regulate Axon Pathfinding in Vivo.
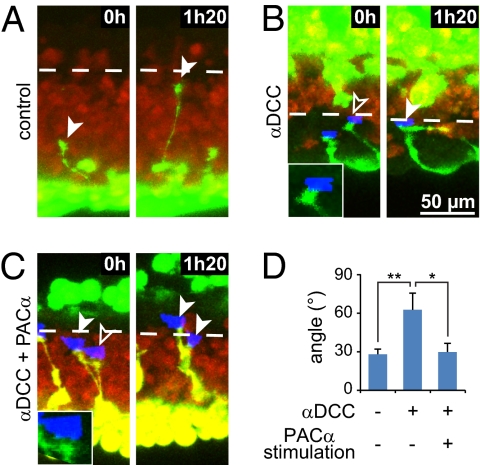
To determine whether the spatial localization and temporal dynamics of cAMP identified in vitro regulate axon pathfinding in vivo, we expressed PACα in half of an X. laevis embryo and imaged spinal commissural axons approaching the ventral midline (Fig. S7A). Control axons grow perpendicular to the midline (Fig. 8 A and D). Blockade of Netrin-1 signaling using the DCC function-blocking antibody causes axons to turn away from the perpendicular to the midline, likely because of the response to other axon guidance cues not present in culture (Fig. 8 B and D). Local and pulsatile blue light illumination of the distal part of PACα-expressing growth cones, in an area equivalent to filopodia location in vitro, is sufficient to maintain the orientation of axon outgrowth toward the midline despite blockade of DCC (Fig. 8 C and D). Thus, restricted and transient elevations of cAMP can replace Netrin-1 signaling to maintain commissural axon growth directionality in vivo, using the cAMP codes described in vitro.
Fig. 8.
Local and pulsatile optogenetic elevation of cAMP concentration rescues defects of commissural axon pathfinding in vivo. (A) Before crossing the ventral midline, Alexa 488 dextran-filled commissural axons grow perpendicular to the midline (dashed line at the Alexa 568 border). Arrowheads identify growth cones. (B) Application of a blocking antibody against DCC prevents axons from growing perpendicular to the midline. Blue light illumination of growth cones that do not express PACα (blue areas; uninjected side of the animal, not labeled with Alexa 568) does not rescue this defect. (Inset) Outlined arrowhead indicates enlarged growth cone. (C) Local and pulsatile illumination (blue areas; 3 min three times per hour) of PACα-expressing growth cones (PACα and Alexa 568-coinjected side of the animal) maintain the orientation of axon growth toward the midline. (D) Mean angle of axon trajectory deviation from the perpendicular to the midline. Twelve or more trajectories were scored for each condition. Error bars, SEM; **P < 0.01, *P < 0.05, ANOVA.
Discussion
Our experiments identify spatial and temporal second-messenger codes regulating signaling in growth cones in response to a guidance molecule. Use of an Epac-based FRET probe (24) enables detection of small signal-averaged cAMP transients in growth cones. Netrin-1 elicits brief, extracellular calcium-independent elevation of cAMP in filopodia that stimulates increases in filopodial calcium transients. Netrin-1 also stimulates brief calcium-dependent elevation of cAMP and prominent calcium transients in growth cone centers. Thus, the relation between cAMP and calcium signaling depends on the domain of the growth cone. cAMP transients imposed in filopodia control the turning response by regulating the frequency of filopodial calcium transients. Previous studies of axon turning initiated by calcium signals in lamellipodia may have stimulated calcium signals in filopodia as well (16, 25). In contrast, cAMP transients generated in the growth cone center do not participate in axon steering. Calcium transients stimulated by Netrin-1 in growth cone centers bear close resemblance in amplitude, duration, and frequency to those that regulate axon extension (19, 33).
Our findings extend compartmentalization of cAMP from cardiac myocytes, neurons, and a variety of cell lines (1–3, 34) to growth cones and demonstrate how partitioning of signaling enables regulation of dual functions by a single ligand. Differences in calcium and cAMP signal generators (calcium channels and adenylyl cyclases) are common and might be the basis of our observations. Different subtypes of adenylyl cyclases are targeted to distinct microdomains and can account for compartmentalization of cAMP signals (34). Different adenylyl cyclases can account for distinct signals: the genetic deletion of soluble adenylyl cyclase does not affect commissural axon trajectories (32) although it is required for Netrin-1–dependent axon outgrowth (11), but alteration of transmembrane adenylyl cyclase activity induces the defects in spinal commissural axon guidance reported here.
Uneven distribution of cAMP- or calcium-responsive elements is also likely to contribute to compartmentalization of downstream second-messenger signaling. PKA and Epac, two of the main cAMP effectors, regulate distinct signaling pathways during axon guidance (7) and are confined to specific subcellular compartments (35). The difference in cAMP affinity of PKA and members of the Epac family further distinguishes these cAMP-dependent signaling pathways (7, 36). It is still unclear whether the FRET probe we used reflects cAMP signaling independently of downstream effectors or is specific to one of the cAMP pathways, likely an Epac-dependent pathway, because the sensor is based on Epac2. A PKA-based sensor might reveal a different distribution of signals in growth cones in response to Netrin-1.
We show that the cAMP and calcium codes used to induce growth cone steering are based on transient signals of these second messengers. Netrin-1–induced calcium transients have not been reported. This discrepancy may have resulted from the use of serum-containing culture medium that blocks calcium transients in growth cone centers observed in vivo (19) (Fig. S1A) and from the high image acquisition rate required to detect fast filopodial transients. Use of serum-free medium leads to observation of growth cone center calcium transients in Xenopus, Helisoma, and mammalian CNS neurons in vitro (26, 37–39). Signaling by transients allows different downstream effectors to be sensitive to different frequencies. Conversion of transients into persistent effects has been described for synaptic plasticity, in which the cAMP pathway is required for long-term changes (40, 41). Transient signals have been proposed to lead to a long-lasting effect via a network of interacting signaling pathways in which cAMP and calcium are nodes (42). Integrators needed to persistently activate downstream effectors could include calcineurin for regulation of axon outgrowth by growth cone center calcium transients (43) and calpain for signals in filopodia (18). Our results suggest that the integrator for cAMP is activated above a threshold frequency of transients, but not by an optimal frequency.
Extinction of filopodial cAMP and calcium signals during sustained stimulation can account for desensitization of growth cones to guidance cues and zig-zag navigation (23). Desensitization would allow long-range pathfinding and adaptation of axons to different concentrations of guidance cues, and the same molecules would enable sustained control of axon extension. This corroborates the dependence on IP3 receptor activation of axon outgrowth (similar to growth cone center calcium transients) but not axon steering (dependent on filopodial calcium transients) (37).
Imposing a constant high or low concentration of cAMP in vivo leads to similar defects in trajectories of spinal commissural axons, indicating the importance of accurate regulation of cAMP levels. cAMP regulates several signaling pathways important for commissural axon guidance, such as initiation of repulsion by semaphorins after midline crossing by decreasing PKA activity (44), and responses of axons to a midline attractant such as Netrin-1 (this study). Sensitivity to repulsive cues is driven by a sustained change in overall concentration of cAMP (44) that may regulate targeting of receptors to the cell surface (45). We find that local axon pathfinding driven by Netrin-1 in vivo can be regulated by spatially and temporally restricted cAMP signals identified in vitro. This finding suggests that it is possible to steer growth cones in vivo through focal manipulation of intracellular signals.
The signaling events we describe may mediate responses to other guidance cues. We propose that cAMP and calcium transients encode distinct growth-cone behaviors based on their localization in subcellular domains and on their frequency. Guidance cues may activate one or the other of these pathways based on distinct receptors or clusters of receptors (22, 37) in restricted regions that confine the activation of downstream pathways (46).
Materials and Methods
Photostimulation of PACα was achieved by high-frequency, repetitive illumination of selected compartments of the growth cone using a 488-nm laser line. Imaging of spinal commissural axons in vivo was performed using embryos injected with Alexa 568 in a single blastomere at the two-cell stage (to label the midline) and with Alexa 488 in both V1 blastomeres at the eight-cell stage (to label the dorsal spinal cord and commissural axons). The ventral surface of the spinal cord was then imaged after abdomen removal (Fig. S7A). A detailed description of experimental procedures is provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. M. Roe for the gift of pmEpac2 cAMPs generated from the Epac2-camps sensor of Dr. M. J. Lohse, and Dr. G. Nagel for the gift of PACα. We are grateful to members of our laboratory for thoughtful discussion and to Darwin Berg, Michaël Demarque, Davide Dulcis, Patricia Gaspar, David Gomez-Varela, Kurt Marek, and Yimin Zou for helpful critical reading of the manuscript. This work was supported by Fondation pour la Recherche Médicale and Marie Curie fellowships (to X.N.) and by National Institutes of Health Grant NS15918 (to N.C.S.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100247108/-/DCSupplemental.
References
- 1.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 2.Hockberger P, Yamane T. Compartmentalization of cyclic AMP elevation in neurons of Aplysia californica. Cell Mol Neurobiol. 1987;7:19–33. doi: 10.1007/BF00734987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shelly M, et al. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science. 2010;327:547–552. doi: 10.1126/science.1179735. [DOI] [PubMed] [Google Scholar]
- 4.Zaccolo M, et al. Restricted diffusion of a freely diffusible second messenger: Mechanisms underlying compartmentalized cAMP signalling. Biochem Soc Trans. 2006;34:495–497. doi: 10.1042/BST0340495. [DOI] [PubMed] [Google Scholar]
- 5.Gorbunova YV, Spitzer NC. Dynamic interactions of cyclic AMP transients and spontaneous Ca2+ spikes. Nature. 2002;418:93–96. doi: 10.1038/nature00835. [DOI] [PubMed] [Google Scholar]
- 6.Dunn TA, et al. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–12815. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–15444. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ming GL, et al. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 9.Höpker VH, Shewan D, Tessier-Lavigne M, Poo M, Holt C. Growth-cone attraction to netrin-1 is converted to repulsion by laminin-1. Nature. 1999;401:69–73. doi: 10.1038/43441. [DOI] [PubMed] [Google Scholar]
- 10.Corset V, et al. Netrin-1-mediated axon outgrowth and cAMP production requires interaction with adenosine A2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 11.Wu KY, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–1264. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terman JR, Kolodkin AL. Nervy links protein kinase A to plexin-mediated semaphorin repulsion. Science. 2004;303:1204–1207. doi: 10.1126/science.1092121. [DOI] [PubMed] [Google Scholar]
- 13.Nicol X, Muzerelle A, Rio JP, Métin C, Gaspar P. Requirement of adenylate cyclase 1 for the ephrin-A5-dependent retraction of exuberant retinal axons. J Neurosci. 2006;26:862–872. doi: 10.1523/JNEUROSCI.3385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicol X, et al. cAMP oscillations and retinal activity are permissive for ephrin signaling during the establishment of the retinotopic map. Nat Neurosci. 2007;10:340–347. doi: 10.1038/nn1842. [DOI] [PubMed] [Google Scholar]
- 15.Yamada RX, et al. Long-range axonal calcium sweep induces axon retraction. J Neurosci. 2008;28:4613–4618. doi: 10.1523/JNEUROSCI.0019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98. doi: 10.1038/47507. [DOI] [PubMed] [Google Scholar]
- 17.Gomez TM, Robles E, Poo M, Spitzer NC. Filopodial calcium transients promote substrate-dependent growth cone turning. Science. 2001;291:1983–1987. doi: 10.1126/science.1056490. [DOI] [PubMed] [Google Scholar]
- 18.Robles E, Huttenlocher A, Gomez TM. Filopodial calcium transients regulate growth cone motility and guidance through local activation of calpain. Neuron. 2003;38:597–609. doi: 10.1016/s0896-6273(03)00260-5. [DOI] [PubMed] [Google Scholar]
- 19.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 20.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Han L, Tiwari P, Wen Z, Zheng JQ. Spatial targeting of type II protein kinase A to filopodia mediates the regulation of growth cone guidance by cAMP. J Cell Biol. 2007;176:101–111. doi: 10.1083/jcb.200607128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 23.Ming GL, et al. Adaptation in the chemotactic guidance of nerve growth cones. Nature. 2002;417:411–418. doi: 10.1038/nature745. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaev VO, Bünemann M, Hein L, Hannawacker A, Lohse MJ. Novel single chain cAMP sensors for receptor-induced signal propagation. J Biol Chem. 2004;279:37215–37218. doi: 10.1074/jbc.C400302200. [DOI] [PubMed] [Google Scholar]
- 25.Ooashi N, Futatsugi A, Yoshihara F, Mikoshiba K, Kamiguchi H. Cell adhesion molecules regulate Ca2+-mediated steering of growth cones via cyclic AMP and ryanodine receptor type 3. J Cell Biol. 2005;170:1159–1167. doi: 10.1083/jcb.200503157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schröder-Lang S, et al. Fast manipulation of cellular cAMP level by light in vivo. Nat Methods. 2007;4:39–42. doi: 10.1038/nmeth975. [DOI] [PubMed] [Google Scholar]
- 28.Ishii N, Wadsworth WG, Stern BD, Culotti JG, Hedgecock EM. UNC-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron. 1992;9:873–881. doi: 10.1016/0896-6273(92)90240-e. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell KJ, et al. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 30.Serafini T, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 31.Shim S, et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci. 2005;8:730–735. doi: 10.1038/nn1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore SW, et al. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J Neurosci. 2008;28:3920–3924. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 34.Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 35.Gloerich M, Bos JL. Epac: Defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 36.Peace AG, Shewan DA. New perspectives in cyclic AMP-mediated axon growth and guidance: The emerging epoch of Epac. Brain Res Bull. 2011;84:280–288. doi: 10.1016/j.brainresbull.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Hutchins BI, Kalil K. Wnt5a induces simultaneous cortical axon outgrowth and repulsive axon guidance through distinct signaling mechanisms. J Neurosci. 2009;29:5873–5883. doi: 10.1523/JNEUROSCI.0183-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez TM, Snow DM, Letourneau PC. Characterization of spontaneous calcium transients in nerve growth cones and their effect on growth cone migration. Neuron. 1995;14:1233–1246. doi: 10.1016/0896-6273(95)90270-8. [DOI] [PubMed] [Google Scholar]
- 39.Williams DK, Cohan CS. Calcium transients in growth cones and axons of cultured Helisoma neurons in response to conditioning factors. J Neurobiol. 1995;27:60–75. doi: 10.1002/neu.480270107. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 41.Tzounopoulos T, Janz R, Südhof TC, Nicoll RA, Malenka RC. A role for cAMP in long-term depression at hippocampal mossy fiber synapses. Neuron. 1998;21:837–845. doi: 10.1016/s0896-6273(00)80599-1. [DOI] [PubMed] [Google Scholar]
- 42.Bhalla US, Iyengar R. Emergent properties of networks of biological signaling pathways. Science. 1999;283:381–387. doi: 10.1126/science.283.5400.381. [DOI] [PubMed] [Google Scholar]
- 43.Graef IA, et al. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–670. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 44.Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard JF, et al. Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: A novel mechanism regulating commissural axon extension. J Neurosci. 2004;24:3040–3050. doi: 10.1523/JNEUROSCI.4934-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsuki T, Ailani D, Hiramoto M, Hiromi Y. Intra-axonal patterning: Intrinsic compartmentalization of the axonal membrane in Drosophila neurons. Neuron. 2009;64:188–199. doi: 10.1016/j.neuron.2009.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.