Abstract
The removal of the neural tube in salamander embryos allows the development of nerve-free aneurogenic limbs. Limb regeneration is normally nerve-dependent, but the aneurogenic limb regenerates without nerves and becomes nerve-dependent after innervation. The molecular basis for these tissue interactions is unclear. Anterior Gradient (AG) protein, previously shown to rescue regeneration of denervated limbs and to act as a growth factor for cultured limb blastemal cells, is expressed throughout the larval limb epidermis and is down-regulated by innervation. In an aneurogenic limb, the level of AG protein remains high in the epidermis throughout development and regeneration, but decreases after innervation following transplantation to a normal host. Aneurogenic epidermis also shows a fivefold difference in secretory gland cells, which express AG protein. The persistently high expression of AG in the epithelial cells of an aneurogenic limb ensures that regeneration is independent of the nerve. These findings provide an explanation for this classical problem, and identify regulation of the epidermal niche by innervation as a distinctive developmental mechanism that initiates the nerve dependence of limb regeneration. The absence of this regulation during anuran limb development might suggest that it evolved in relation to limb regeneration.
Keywords: axolotl, newt, parabiosis
Limb regeneration in salamanders proceeds by formation of the limb blastema, a growth zone of mesenchymal progenitor cells apposed by a specialized wound epithelium. The peripheral nerve branches are transected during amputation and, as in all vertebrates, the axons regenerate in association with ensheathing Schwann cells (1, 2). Regeneration of the salamander limb is absolutely dependent on concomitant nerve regeneration, and is abrogated by transecting the axons at the level of the plexus supplying the limb, an operation referred to as denervation (3). The events of wound healing and generation of blastemal cells are both apparently unaffected by prior denervation, but the subsequent proliferation of the blastemal cells is severely curtailed (4–6). The critical role of the regenerating nerve supply can also be demonstrated in contexts that do not depend on prior amputation of a limb (7). For example, in the axolotl accessory limb model, the deflection of a major peripheral nerve into the flank of an animal carrying an appropriate skin graft at that location leads to outgrowth of a supernumerary limb (8). The regenerating axons and the wound epithelium are key accessory “niche” tissues for the blastemal cells, and the interplay between these two tissues is a critical feature of regeneration (9, 10). Classical studies on the newt limb showed that the requirement for an adequate density of regenerating axons could be met by either sensory or motor nerves (11), and neither nerve impulses nor transmitter release were necessary (12). Nerve dependence is therefore an unconventional property that does not conform to familiar ideas of nerve cell specificity or function. It is observed in other contexts of regeneration such as the fish fin and barbel (13), the starfish arm (14), and the primary body axis in polychaete worms (15). It is possible that nerve dependence of regeneration may have evolved in different animals as a mechanism to ensure that the regenerate will become functionally innervated (16).
The phenomena underlying nerve dependence of salamander limb regeneration offer a striking example of how the development of a structure can determine the mechanism of regeneration. It is possible to remove a large section of the neural tube from a salamander embryo before the limb bud develops (17). The limb develops normally without innervation, although skeletal muscle is missing from later stages (18). Other innervated structures such as the lateral line (19) or mechanosensory Merkel cells (20) also develop normally in an aneurogenic larva. The aneurogenic limb (ANL) regenerates in the absence of the nerve (21), a property that has been repeatedly verified (22–24), thus providing a paradoxical exception to nerve dependence. If an ANL is transplanted in place of the forelimb of a normal larva, the brachial nerves grow into the transplant and establish connections. This leads to an abrupt transition from nerve independence to dependence, such that an ANL that becomes innervated will now not regenerate after denervation (25). When nerves grow into the limb bud or into the ANL at a later stage of development, they apparently abrogate the mechanism for regeneration that is seen in the ANL, thus establishing a state of nerve dependence that persists through the remainder of larval development and adulthood. One interesting insight into the mechanism at a tissue level is that substitution of normal limb epidermis for the ANL epidermis prevents regeneration, but a “collar” of aneurogenic epidermis will rescue such a limb as long as the wound epithelium is derived from it after amputation (24). Although these observations point to the epidermis as an important target for nerve-dependent regulation, it is clear that resolution of these issues depends on the understanding of nerve dependence at a cellular and molecular level.
We have recently identified the newt Anterior Gradient protein (nAG) as a strong candidate to mediate the nerve dependence of limb regeneration (26). nAG was identified as a binding partner for the surface protein Prod 1 (27). AG proteins contain a thioredoxin-fold and an N-terminal signal sequence that can lead to secretion. They are widely expressed in secretory epithelia, and homozygous mutant mice are defective in mucous secretion and lack goblet cells (28, 29). They are also implicated in several examples of mammalian adenocarcinoma (30–32). The activity of human AGR2 protein on lung adenocarcinoma cells in culture depends on stimulation of amphiregulin expression by activation of the Hippo signaling pathway coactivator YAP1 (33). When the axons regenerate after amputation of the newt limb, the nAG protein is up-regulated first in the Schwann cells of the distal nerve sheath and then in gland cells underlying the wound epidermis (26). AG proteins were first identified in the context of the Xenopus cement gland (34), and ectopic expression of the Xenopus molecule was shown to induce the formation of a gland as well as being expressed by it (35). In newts, the glands underlying the wound epithelium apparently discharge into the blastema by a holocrine mechanism, and nAG is expressed in their secretory granules as detected by immunoelectron microscopy (36). In a denervated newt blastema, nAG is not expressed by the Schwann cells and the glands do not form in the wound epithelium. If the protein is expressed by electroporation of a plasmid into the distal stump, this induces the appearance of the nAG-positive glands and, more strikingly, rescues the denervated blastema so that it completes the proximodistal axis and forms digits. This activity of the protein is probably exerted directly on the blastemal cells, because cultured newt blastemal cells respond to the secreted recombinant protein by entering S phase (26). These observations make it important to investigate the expression of the AG protein in normal limb development and in the ANL. This may provide insights into the developmental mechanisms discussed above. Our results provide a coherent explanation for the ANL and attendant phenomena, in addition to strongly supporting the relevance of AG to nerve dependence.
Results
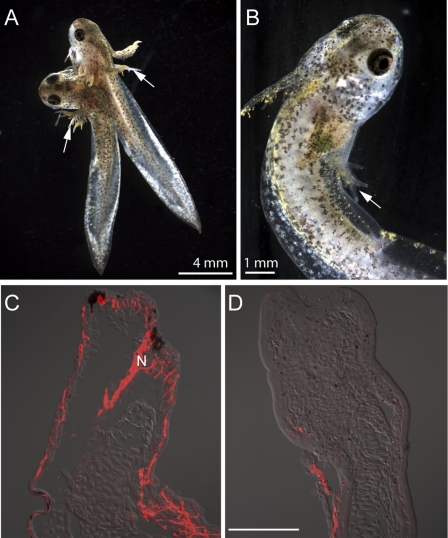
Aneurogenic larvae of the spotted salamander Ambystoma maculatum were generated by parabiosis followed by removal of the neural tube in one member of the pair. After further development, this yielded one aneurogenic and one paired normal limb (Fig. 1A). In this rapidly developing species it is also possible to generate ANL by removal of the neural tube without parabiosis, as noted in earlier work (23, 24) (Fig. 1B). The degree of limb innervation was evaluated by staining sections with antibody to acetylated tubulin. Normal limbs showed dense cutaneous innervation (Fig. 1C), whereas nerve staining was either residual or absent in ANL (Fig. 1D).
Fig. 1.
Generation of aneurogenic larvae. The extent of innervation was analyzed by the reactivity of neuron-specific tubulin antibody. (A) Parabiosis was performed at stage 24 and the neural tube was removed from the larva on the left at stage 29. The forelimb of both aneurogenic (Left) and normal host (Right) larvae is indicated by an arrow. The parabiotic pair has reached developmental stage 46. (B) An aneurogenic larva (stage 46) generated by complete removal of the neural tube at stage 28. The arrow identifies the three-digit forelimb. (C) Longitudinal section of the forelimb of a normal host larva showing the distribution of nerve (red). Note the extensive innervation to the larval skin. (D) A section of the forelimb of an aneurogenic larva that lacks innervation. The images (C and D) are a composite overlay of fluorescence with differential interference contrast (DIC). N, nerve. [Scale bar, 200 μm (C and D).]
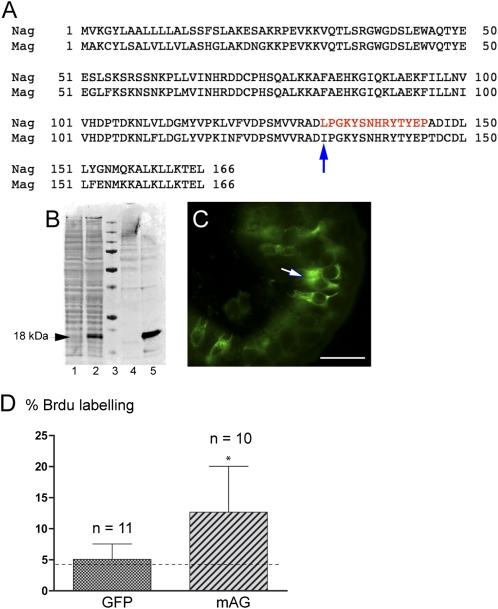
Aneurogenic larvae were significantly easier to derive and maintain in A. maculatum compared with the axolotl or the newt Pleurodeles waltl used earlier (22). We therefore isolated cDNA clones encoding mAG (Fig. 2A), the A. maculatum ortholog of nAG (newt) and aAG (axolotl). The peptide corresponding to residues 132–145 in nAG, which differs by only one residue in mAG (Fig. 2A), was used previously to generate affinity-purified antibodies (26, 36), and this reagent, named 224, reacted satisfactorily with mAG. After transfection of Cos 7 cells with a plasmid expressing mAG, an immunoreactive band of ∼18 kDa was detected in the cell lysate and conditioned medium (Fig. 2B, lanes 2 and 5) but not in untransfected cells (Fig. 2B, lanes 1 and 4). The 224 antibody reacted specifically with sections of A. maculatum tissue, including the intestinal epithelium, where it reacted with goblet cells (Fig. 2C). The recombinant nAG protein was shown previously to stimulate S-phase entry in primary cultures of dissociated newt limb blastemal cells (26). Cos 7 cells were transfected with mAG or GFP plasmids and the conditioned media were concentrated and assayed in parallel on cultured newt blastemal cells. The mAG-transfected medium gave a significant stimulation of S-phase labeling compared with GFP-transfected or the background of control medium (Fig. 2D).
Fig. 2.
Characterization of A. maculatum Anterior Gradient protein and its biological activity. (A) Protein sequence alignment of newt (nAG) and A. maculatum (mAG) Anterior Gradient proteins. The red letters indicate the peptide (14 amino acids) in nAG that has been used to generate a rabbit antibody referred to as 224. The peptide differs at one amino acid (arrow) from the respective sequence in mAG. (B) An immunoblot showing that mAG is a secreted protein. Cos 7 cells were cotransfected with plasmids expressing mAG or GFP. The culture medium and the cell lysate were collected at 48 h after transfection and analyzed by Western blotting. The mAG protein is present in the medium (lane 5) and is found at a lower level in the cell lysate (lane 2). Lanes 1 and 4 are cell lysate and medium, respectively, from parallel cultures where the Cos 7 cells were transfected with GFP alone. Lane 3, molecular weight marker. (C) A cross-section of the small intestine of A. maculatum showing the specific reactivity of the peptide antibody 224 with the secretory goblet cells (arrow). (D) Activity of secreted mAG on cultured newt blastemal cells. Blastemal cells growing in microwells were incubated in the presence of 0.05% serum-containing medium, concentrated medium from control protein (GFP) transfections, or concentrated medium from mAG transfection of Cos 7 cells. The cells were labeled with BrdU for 72 h, fixed, and analyzed for S-phase reentry by staining with anti-BrdU antibody. The baseline S-phase reentry level with 0.05% serum medium is indicated by a dotted line. The blastemal cells incubated with mAG show a significant increase in S-phase reentry. (Scale bar, 100 μm.) Error bars, SD. *P < 0.05.
Down-Regulation of mAG Expression After Innervation.
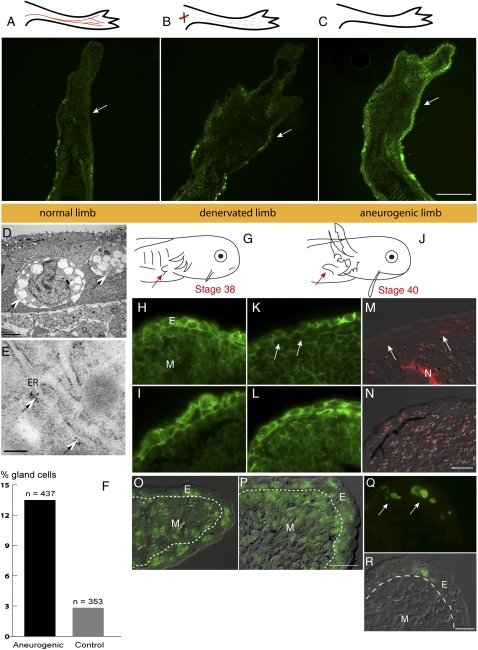
The level of expression of mAG protein in sections of larval limbs was analyzed by indirect immunofluorescence. At stage 46, the forelimb has formed digits and the level of expression in the epidermis is low (Fig. 3A); this was essentially unchanged after denervation of the limb (Fig. 3B). In contrast, the ANL at stage 46 showed strong epidermal expression (Fig. 3C). This difference in AG expression between the normal and aneurogenic epidermis was accompanied by a difference in the incidence of secretory gland cells. These cells were readily visualized in the aneurogenic epidermis by transmission electron microscopy (Fig. 3D), and they expressed mAG as evidenced by specific staining of the endoplasmic reticulum with gold-labeled antibodies (Fig. 3E). They appear to be equivalent to the secretory Leydig cells described in the axolotl and other salamanders (36–38). These gland cells make up over 10% of the cells in the aneurogenic epidermis, and this was fivefold less in the normal epidermis (Fig. 3F).
Fig. 3.
Developmental regulation of mAG protein. Expression of mAG protein in tissue sections was detected by reactivity with 224 antibody. (A) Normal innervated limb. (B) Section of a limb 10 d after denervation. (C) Aneurogenic limb. Note that expression is high throughout the larval epidermis. The epidermis is arrowed in A–C. (D) Electron micrograph showing gland cells (arrows) in an aneurogenic larva at stage 43–44. The unicellular gland cells have large inclusions and are distributed throughout the larval skin. (E) Immunogold labeling of mAG protein in gland cells of an aneurogenic larva. The gold particles are localized to the rough endoplasmic reticulum (arrows). (F) Quantitative distribution of gland cells in aneurogenic and control epidermis. The number of cells with glandular morphology was counted from an entire larval epidermis. The total number of cells was tabulated from two independent samples (stage 43–44). (G) Morphology of the larva at stage 38. The limb bud is indicated by an arrow. (H) mAG expression in larval skin of the limb bud. (I) Expression in an aneurogenic limb bud. (J) Morphology of the stage 40 larva. The limb bud is indicated by an arrow. (K) Section of the limb at stage 40. Arrows point to an area of the epidermis that shows down-regulation by innervation. (L) Aneurogenic epidermis at stage 40 retains a higher level of mAG. (M and N) Neuron-specific antibody staining of K and L with DIC overlay. (O) Section through the distal tip of a normal limb bud at stage 40 showing BrdU incorporation. Note the BrdU-positive cells (green) in the epidermis. The image is an overlay of DIC with fluorescence. (P) BrdU-labeled cells in an aneurogenic limb bud at stage 40. (Q) Apoptotic cells were detected by TUNEL assay in the aneurogenic larval epidermis at stage 40 (arrows). (R) DIC overlay showing the localization within gland cells. E, epidermis; ER, endoplasmic reticulum; M, mesenchyme; N, nerve. [Scale bars, 100 μm (A–C); 5 μm (D); 0.2 μm (E); and 50 μm (H, I, and K–R).]
We propose that the arrival of the nerve in normal limb development leads to down-regulation of the expression of mAG and decreases the number of gland cells that express this protein. Expression was readily detected in sections of the larval epidermis from stage 34 onward, and cells of a secretory nature with large dense vacuoles were also present. The normal and aneurogenic epidermis showed comparable intensity of staining at stage 38, when the innervation has just arrived at the limb (Fig. 3 G–I). At stage 40 (Fig. 3J), the normal limb epidermis showed decreased staining compared with the aneurogenic case (Fig. 3 K and L), and nerve staining of the same sections showed the presence of adjacent nerve branches in the mesenchyme of the normal but not the aneurogenic limb (Fig. 3 M and N).
The expression of mAG in the epidermis is apparently down-regulated during the interval of stages 38–41 in normal development. This could be due to an effect of innervation on the differentiated state of epithelial cells, as well as an effect exerted on dividing progenitor cells. To investigate the cell cycling in the limb, both normal and aneurogenic larvae at stage 40 were labeled for 8 h by injection of BrdU. The sections of normal and aneurogenic limb bud showed labeled nuclei in the mesenchyme and epidermis (Fig. 3 O and P). In both cases, ∼30% of the nuclei in the epidermis were labeled, raising the possibility that there is considerable cell generation at this stage. It is possible that cells could be lost by apoptosis, but TUNEL staining of the epidermis at relevant stages revealed no examples of apoptosis in either normal or aneurogenic cases, except for a few examples of secretory gland cells possibly at the end of their holocrine cycle (Fig. 3 Q and R and Fig. S1). Apoptosis was so rarely observed that we reject the hypothesis that it plays any role in the difference in mAG expression between normal and aneurogenic epidermis.
Interestingly, the down-regulation of AG expression after innervation is not seen in limb development of the anuran Xenopus (Fig. S2). It is unlikely that this relates to the failure of limb regeneration in Xenopus, which occurs in a proximal-to-distal direction beginning at stage 53 (39). We observed that the down-regulation of AG expression does not begin, even in proximal regions, until the prometamorphic stages 58–59 (Fig. S2 A–C).
Down-Regulation of mAG After Innervation of the Aneurogenic Limb.
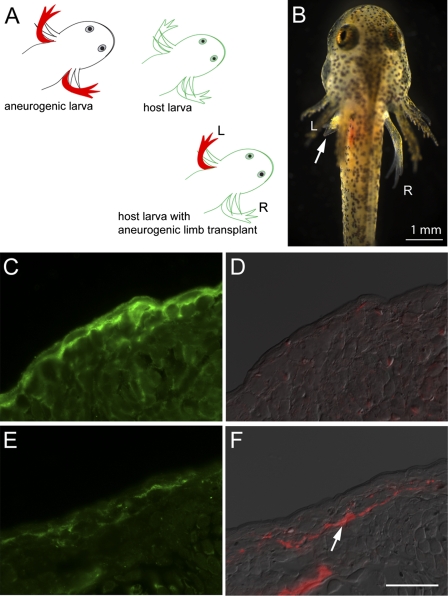
It was shown previously that transplantation of an aneurogenic limb to the flank of a normal larva leads to functional innervation by the host and establishment of nerve dependence for regeneration (25). To investigate the effect of innervation on mAG expression, a group of bilateral aneurogenic larvae was derived as described earlier (Fig. 1B). The left ANL was transplanted at stage 45 (when mAG expression in the epidermis is high) to the flanks of host larvae in place of the host left limb (Fig. 4 A and B). The level of mAG expression in the epidermis and the degree of innervation were analyzed at 17 or 30 d after transplantation, and in each case were compared with the nontransplanted right limb from the donor aneurogenic larva. In this pairwise comparison of nine samples, seven of the transplanted limbs showed clear down-regulation of mAG in the epidermis. A representative comparison from the 17-d series is shown in Fig. 4 C–F. The right limb shows strong expression of mAG in the epidermis (Fig. 4C) with no detectable nerve staining (Fig. 4D), whereas its transplanted counterpart shows diminished mAG expression (Fig. 4E) and clear evidence of innervation (Fig. 4F). Down-regulation of mAG was essentially complete by 30 d after transplantation, particularly in locations of high-density innervation (Fig. S3 A–D). We conclude that the expression of mAG in the aneurogenic epidermis can be reversed by innervation. This would be consistent with the correlation of nerve-dependent regeneration with a state of diminished epidermal expression.
Fig. 4.
Transplantation of aneurogenic limb. (A) Schematic representation of the transplantation experiment. The left limb from an aneurogenic larva at stage 45 was transplanted to the left side of a normal host larva after removal of its corresponding limb. The larval salamander was maintained for 17 d after transplantation of the limb. The right reference limb from the aneurogenic larva was removed at the time of transplantation and processed for immunolabeling with mAG antibody. (B) A host larva with the aneurogenic limb transplant (Left; arrow). (C) The right reference limb of the aneurogenic larva showing expression of mAG in the larval skin. (D) Staining with AAT antibody showing the absence of innervation. (E) Transplanted aneurogenic limb showing down-regulation of mAG protein (compare with C) after reinnervation of the aneurogenic limb from the host larva. (F) Staining with AAT shows reinnervation of the aneurogenic limb from the host larva. Both D and F are composite overlays of AAT antibody (red) with DIC. [Scale bar, 50 μm (C–F).]
Regeneration of Normal and Aneurogenic Limbs.
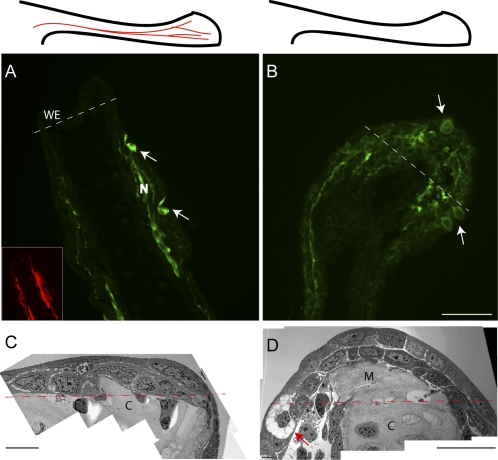
At early stages of regeneration, the aneurogenic blastema presents a different appearance from the normal blastema. This distinction is illustrated by staining sections of a normal (Fig. 5A) and an aneurogenic blastema (Fig. 5B) at 3 d postamputation. The normal limb is negative for expression in the wound epithelium and normal epidermis, with the exception of two glands (arrows) proximal to the amputation plane. Two branches of peripheral nerve are identified with antitubulin staining (Fig. 5A Inset) and are positive for mAG expression as reported for nerves in the early newt blastema (26, 36). The ANL has a positively stained epidermis and a strongly positive wound epithelium with gland cells (arrows). The wound epidermis and the mesenchymal blastema also appear different in transmission electron micrographs of normal (Fig. 5C) and aneurogenic blastemas (Fig. 5D). This analysis was also performed on normal and aneurogenic blastemas from parabiotic pairs, and an example is shown in Fig. S4. The normal and aneurogenic blastemas converge in their appearance during regeneration such that after 10 d postamputation they are essentially indistinguishable.
Fig. 5.
Regeneration of normal and aneurogenic limbs. The upper panels are schematic representations of the limbs before amputation (A, normal with nerves; B, aneurogenic). The images of the limb blastemas are at 3 d after amputation of the limb. The amputation plane is shown with a white dotted line. (A) Normal limb blastema. The gland cells (arrows) are proximal to the amputation plane and express mAG, whereas the wound epithelium of the regenerate does not show detectable reactivity. The nerve sheath in the stump tissue shows strong reactivity with mAG protein. (Inset) AAT staining of the nerve in the limb coinciding with the sheath staining. (B) An aneurogenic limb blastema. The wound epithelium shows strong reactivity with mAG protein. Gland cells are present within the wound epithelium (arrows) as well as proximal to the amputation plane. (C and D) Electron micrograph showing the morphology of the 3-d limb blastemas. The amputation plane is represented with a red dotted line. (C) The wound epithelium in a control limb regenerate has two layers of epithelial cells that migrate over the cartilage of the stump tissue. (D) The aneurogenic limb blastema has a well-formed wound epithelium. A gland cell (arrow) is visible at the amputation plane. Note the difference in the extent of the limb blastemas in C and D. C, cartilage; M, mesenchyme; N, nerve; WE, wound epidermis. [Scale bars, 100 μm (A, B, and D); and 20 μm (C).]
Discussion
The mAG protein has comparable activity to nAG when assayed on cultured newt blastemal cells and, given its orthology to nAG and expression during regeneration, we conclude that it is able to mediate nerve dependence. The expression of mAG in the limb epidermis is markedly down-regulated by innervation. This switch is detectable both during normal development of the A. maculatum limb bud and after transplantation of an ANL to a normal larva. During development, this is accompanied by a fivefold decrease in the incidence of gland cells in the epidermis. In view of the demonstrated ability of nAG to induce glands in the denervated newt blastema (26), as well as the earlier work on induction of the Xenopus cement gland (35), it is likely that the effect on the glands is a secondary consequence of the drop in mAG expression induced by the nerve rather than a direct effect of innervation on the glands. The consequence for the salamander limb is that the normal level in the epidermis, and in the wound epithelium derived from it, is sufficiently low that regeneration depends on axons to prime AG expression in the Schwann cells and then, by a relay mechanism, in the gland cells underneath or in the wound epithelium (36). It is noteworthy that nerves are able to up-regulate AG in Schwann cells during regeneration in newt (26), axolotl (36), and A. maculatum but down-regulate AG in the epidermal end-organ cells during development. In the ANL epidermis and wound epithelium the expression is sufficiently high that regeneration proceeds in the absence of the nerve, and is different in appearance from regeneration in a normal limb at early stages (Fig. 5), an observation that is absent from the earlier work on the ANL. This view provides an explanation for the finding that transplantation of normal but not aneurogenic epidermis to an ANL prevents regeneration (24). It is not possible for technical reasons to test the reciprocal combination of an aneurogenic epidermis on a normal denervated larval limb. It is interesting that limb development proceeds normally with either relatively high levels of AG in the ANL epidermis or with lower levels in the innervated case. By contrast, limb regeneration is dependent on an elevated level of AG to sustain proliferation. These considerations might suggest that the regulation of cell division is different in limb bud cells and limb blastemal cells.
The down-regulation of mAG by innervation appears to fulfill the dual role of abrogating the mechanism seen in the ANL, as well as establishing nerve dependence. Although positive effects of nerves on their targets are more familiar, there are well-established negative effects on gene expression, such as the effect of motor neurons on AChR gene expression in skeletal muscle (40) as well as effects of postmitotic neurons on the proliferation of neuronal precursor cells (41). Neuronal modulation of gene expression is generally reversible, and hence it is noteworthy that the effect on mAG expression in the epidermis is not reversed by denervation (Fig. 3B). This reflects the action of the nerve in “imprinting” the limb to a permanent state of nerve dependence for regeneration. In view of the demonstrated cellular turnover of the epidermis, it seems possible that this effect is exerted on the basal stem cell compartment rather than just the differentiated keratinocytes. The effect of the nerve is apparently not mediated by changing either S-phase labeling or apoptosis in the epidermis. We favor the hypothesis that the nerve inhibits differentiation of the AG-expressing lineage. It remains to be determined whether the action of the nerve depends on contact with the epidermal and gland cells or on the release of a secreted molecule.
Our results on Xenopus limb development might suggest that the down-regulation of mAG expression seen at stages 39–41 of A. maculatum is not a general feature of vertebrate limb development but evolved in relation to the establishment of nerve dependence, although more examples need to be analyzed before this can be concluded. We have argued elsewhere from evidence of molecular phylogeny that limb regeneration is not a purely ancestral mechanism and that certain aspects such as nerve dependence may have evolved in salamanders (42–44). The ANL raises a more general issue about causal inference in the analysis of cell–cell interactions. If the ANL had been analyzed without reference to the consequences of denervation, it would have been concluded that limb regeneration is independent of the nerve. The possibility of developmental or long-term modulatory effects by one cell type that are required to establish cellular interdependence with another should always be considered.
Materials and Methods
Animals.
Embryos of the spotted salamander (Ambystoma maculatum) were used in this study. The animal maintenance and microsurgical methods are described in SI Materials and Methods.
For information on histological methods, immunoelectron microscopy, immunofluorescence, RT-PCR, cell-cycle reentry assay, TUNEL assay, and blastemal cell assay, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank D. Lo, M. Yun, P. Martin, and P. Driscoll for comments, A. Janmohamed for help with Western blot, and the European Xenopus Resource Centre for tadpoles. This work was supported by a Medical Research Council Program grant and research professorship (to J.P.B.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. HQ676606).
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1108472108/-/DCSupplemental.
References
- 1.Hall SM. Mechanisms of repair after traumatic injury. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. 4th Ed. Philadelphia: Elsevier; 2005. pp. 1403–1434. [Google Scholar]
- 2.Parrinello S, et al. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- 4.Goldhamer DJ, Tomlinson BL, Tassava RA. Ganglia implantation as a means of supplying neurotrophic stimulation to the newt regeneration blastema: Cell-cycle effects in innervated and denervated limbs. J Exp Zool. 1992;262:71–80. doi: 10.1002/jez.1402620110. [DOI] [PubMed] [Google Scholar]
- 5.Kintner CR, Brockes JP. Monoclonal antibodies to the cells of a regenerating limb. J Embryol Exp Morphol. 1985;89:37–55. [PubMed] [Google Scholar]
- 6.Maden M. Neurotrophic control of the cell cycle during amphibian limb regeneration. J Embryol Exp Morphol. 1978;48:169–175. [PubMed] [Google Scholar]
- 7.Egar MW. Accessory limb production by nerve-induced cell proliferation. Anat Rec. 1988;221:550–564. doi: 10.1002/ar.1092210111. [DOI] [PubMed] [Google Scholar]
- 8.Endo T, Bryant SV, Gardiner DM. A stepwise model system for limb regeneration. Dev Biol. 2004;270:135–145. doi: 10.1016/j.ydbio.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Mescher AL. Effects on adult newt limb regeneration of partial and complete skin flaps over the amputation surface. J Exp Zool. 1976;195:117–128. doi: 10.1002/jez.1401950111. [DOI] [PubMed] [Google Scholar]
- 10.Stocum DL, Cameron JA. Looking proximally and distally: 100 years of limb regeneration and beyond. Dev Dyn. 2011;240:943–968. doi: 10.1002/dvdy.22553. [DOI] [PubMed] [Google Scholar]
- 11.Sidman RL, Singer M. Stimulation of forelimb regeneration in the newt, Triturus viridescens, by a sensory nerve supply isolated from the central nervous system. Am J Physiol. 1951;165:257–260. doi: 10.1152/ajplegacy.1951.165.1.257. [DOI] [PubMed] [Google Scholar]
- 12.Drachman DB, Singer M. Regeneration in botulinum-poisoned forelimbs of the newt, Triturus. Exp Neurol. 1971;32:1–11. doi: 10.1016/0014-4886(71)90159-2. [DOI] [PubMed] [Google Scholar]
- 13.Goss RJ. An experimental analysis of taste barbel regeneration in the catfish. J Exp Zool. 1956;131:27–49. [Google Scholar]
- 14.Candia-Carnevali M. Regeneration in echinoderms: Repair, regrowth, cloning. Invertebrate Surviv J. 2006;3:64–76. [Google Scholar]
- 15.Boilly-Marer Y. Role of the parapodial nervous system in the induction of supernumerary parapods by heterologus engraftment. Nereis pelagica CR Acad Sci. 1971;272:261–264. (in French) [Google Scholar]
- 16.Brockes JP, Kumar A. Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol. 2008;24:525–549. doi: 10.1146/annurev.cellbio.24.110707.175336. [DOI] [PubMed] [Google Scholar]
- 17.Harrison RG. Experiments in transplanting limbs and their bearing upon the problems of the development of nerves. J Exp Zool. 1907;4:239–281. [Google Scholar]
- 18.Popiela H. In vivo limb tissue development in the absence of nerves: A quantitative study. Exp Neurol. 1976;53:214–226. doi: 10.1016/0014-4886(76)90293-4. [DOI] [PubMed] [Google Scholar]
- 19.Tweedle CD. Ultrastructure of lateral line organs in aneurogenic amphibian larvae (Ambystoma) Cell Tissue Res. 1977;185:191–197. doi: 10.1007/BF00220664. [DOI] [PubMed] [Google Scholar]
- 20.Tweedle CD. Ultrastructure of Merkel cell development in aneurogenic and control amphibian larvae (Ambystoma) Neuroscience. 1978;3:481–486. doi: 10.1016/0306-4522(78)90052-0. [DOI] [PubMed] [Google Scholar]
- 21.Yntema CL. Regeneration in sparsely innervated and aneurogenic forelimbs of Amblystoma larvae. J Exp Zool. 1959;140:101–123. doi: 10.1002/jez.1401400106. [DOI] [PubMed] [Google Scholar]
- 22.Fekete DM, Brockes JP. Evidence that the nerve controls molecular identity of progenitor cells for limb regeneration. Development. 1988;103:567–573. doi: 10.1242/dev.103.3.567. [DOI] [PubMed] [Google Scholar]
- 23.Tassava RA, Olsen-Winner CL. Responses to amputation of denervated Ambystoma limbs containing aneurogenic limb grafts. J Exp Zoolog A Comp Exp Biol. 2003;297:64–79. doi: 10.1002/jez.a.10263. [DOI] [PubMed] [Google Scholar]
- 24.Steen TP, Thornton CS. Tissue interaction in amputated aneurogenic limbs of Ambystoma larvae. J Exp Zool. 1963;154:207–221. doi: 10.1002/jez.1401540208. [DOI] [PubMed] [Google Scholar]
- 25.Thornton CS, Thornton MT. Recuperation of regeneration in denervated limbs of Ambystoma larvae. J Exp Zool. 1970;173:293–301. [Google Scholar]
- 26.Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science. 2007;318:772–777. doi: 10.1126/science.1147710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Silva SM, Gates PB, Brockes JP. The newt ortholog of CD59 is implicated in proximodistal identity during amphibian limb regeneration. Dev Cell. 2002;3:547–555. doi: 10.1016/s1534-5807(02)00288-5. [DOI] [PubMed] [Google Scholar]
- 28.Park SW, et al. The protein disulfide isomerase AGR2 is essential for production of intestinal mucus. Proc Natl Acad Sci USA. 2009;106:6950–6955. doi: 10.1073/pnas.0808722106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao F, et al. Disruption of Paneth and goblet cell homeostasis and increased endoplasmic reticulum stress in Agr2−/− mice. Dev Biol. 2010;338:270–279. doi: 10.1016/j.ydbio.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fletcher GC, et al. hAG-2 and hAG-3, human homologues of genes involved in differentiation, are associated with oestrogen receptor-positive breast tumours and interact with metastasis gene C4.4a and dystroglycan. Br J Cancer. 2003;88:579–585. doi: 10.1038/sj.bjc.6600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson DA, Weigel RJ. hAG-2, the human homologue of the Xenopus laevis cement gland gene XAG-2, is coexpressed with estrogen receptor in breast cancer cell lines. Biochem Biophys Res Commun. 1998;251:111–116. doi: 10.1006/bbrc.1998.9440. [DOI] [PubMed] [Google Scholar]
- 32.Brychtova V, Vojtesek B, Hrstka R. Anterior gradient 2: A novel player in tumor cell biology. Cancer Lett. 2011;304:1–7. doi: 10.1016/j.canlet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301–18310. doi: 10.1074/jbc.M110.215707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sive HL, Hattori K, Weintraub H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell. 1989;58:171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- 35.Aberger F, Weidinger G, Grunz H, Richter K. Anterior specification of embryonic ectoderm: The role of the Xenopus cement gland-specific gene XAG-2. Mech Dev. 1998;72:115–130. doi: 10.1016/s0925-4773(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Nevill G, Brockes JP, Forge A. A comparative study of gland cells implicated in the nerve dependence of salamander limb regeneration. J Anat. 2010;217:16–25. doi: 10.1111/j.1469-7580.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jarial MS. Fine structure of the epidermal Leydig cells in the axolotl Ambystoma mexicanum in relation to their function. J Anat. 1989;167:95–102. [PMC free article] [PubMed] [Google Scholar]
- 38.Ohmura H, Wakahara M. Transformation of skin from larval to adult types in normally metamorphosing and metamorphosis-arrested salamander, Hynobius retardatus. Differentiation. 1998;63:238–246. [PubMed] [Google Scholar]
- 39.Dent JN. Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. J Morphol. 1962;110:61–77. doi: 10.1002/jmor.1051100105. [DOI] [PubMed] [Google Scholar]
- 40.Fambrough DM. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979;59:165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- 41.Mumm JS, Shou J, Calof AL. Colony-forming progenitors from mouse olfactory epithelium: Evidence for feedback regulation of neuron production. Proc Natl Acad Sci USA. 1996;93:11167–11172. doi: 10.1073/pnas.93.20.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blassberg RA, Garza-Garcia A, Janmohamed A, Gates PB, Brockes JP. Functional convergence of signalling by GPI-anchored and anchorless forms of a salamander protein implicated in limb regeneration. J Cell Sci. 2011;124:47–56. doi: 10.1242/jcs.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garza-Garcia AA, Driscoll PC, Brockes JP. Evidence for the local evolution of mechanisms underlying limb regeneration in salamanders. Integr Comp Biol. 2010;50:528–535. doi: 10.1093/icb/icq022. [DOI] [PubMed] [Google Scholar]
- 44.Garza-Garcia A, Harris R, Esposito D, Gates PB, Driscoll PC. Solution structure and phylogenetics of Prod1, a member of the three-finger protein superfamily implicated in salamander limb regeneration. PLoS One. 2009;4:e7123. doi: 10.1371/journal.pone.0007123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.