Abstract
We identified 20 global key conservation sites for all marine (123) and freshwater (6) mammal species based on their geographic ranges. We created geographic range maps for all 129 species and a Geographic Information System database for a 46,184 1° x 1° grid-cells, ∼10,000-km2. Patterns of species richness, endemism, and risk were variable among all species and species groups. Interestingly, marine mammal species richness was correlated strongly with areas of human impact across the oceans. Key conservation sites in the global geographic grid were determined either by their species richness or by their irreplaceability or uniqueness, because of the presence of endemic species. Nine key conservation sites, comprising the 2.5% of the grid cells with the highest species richness, were found, mostly in temperate latitudes, and hold 84% of marine mammal species. In addition, we identified 11 irreplaceable key conservation sites, six of which were found in freshwater bodies and five in marine regions. These key conservation sites represent critical areas of conservation value at a global level and can serve as a first step for adopting global strategies with explicit geographic conservation targets for Marine Protected Areas.
Keywords: biodiversity, conservation priorities, political endemism
The current loss of biological diversity is one of the most severe global environmental problems and probably is the only one that is truly irreversible. Recent studies show that anthropogenic factors are causing increasing rates of extinctions of both populations and species (1–3). Despite their immense value, marine ecosystems are deteriorating rapidly, especially because of habitat degradation, overexploitation, introduction of exotic species, pollution (including noise), acidification, and climate disruption (4, 5), in part because roughly 60% of the world's human population lives within 100 km of a coast, and 20% of ecosystems adjacent to oceans have been highly modified (6, 7). Because of those anthropogenic environmental changes, many species of marine animals have undergone local, regional, or global extinctions (8). Marine mammals provide some of the best-known cases of population and species extinction through overexploitation. Many species have experienced severe population depletion, and at least three [Caribbean monk seal (Monachus tropicalis), Atlantic gray whale (Eschrichtius robustus), and the Steller's sea cow (Hydrodamalis gigas)] became extinct because of hunting for their fur, blubber, and meat during the 19th and 20th centuries. The most recent extinction, caused by several human activities including illegal hunting for meat and body parts used in traditional medicine, is the baiji (Lipotes vexillifer) from the Yangtze River in China, which was declared extinct in 2008 (9).
Understanding geographical variation in species richness and other large-scale patterns can be especially valuable for the establishment of global conservation priorities (10–13). Those patterns, for example, allow assessment of what would be required to preserve all species in a given taxon and to determine critical sites for their conservation (14–16). Given that the distribution patterns of species richness usually are not closely related to those of endemism and extinction risk, conservation actions to minimize global species extinction necessarily involve a combined evaluation of patterns of richness, endemism, and endangerment (17, 18). Global distribution patterns have been determined for different vertebrate groups such as birds, amphibians, fish, and terrestrial mammals (19–22), but such large-scale analyses are lacking for marine/freshwater mammals (23).
Here we present a global analysis of distribution patterns for 129 marine mammals, focusing on the following goals: (i) describing their geographic ranges; (ii) assessing patterns of species richness and composition; and (iii) determining key conservation sites as a basis for understanding global conservation needs. We created a database with the geographic distribution of all 129 species of pinnipeds, cetaceans, sirenians, two species of otters, and the polar bear (24). We followed Reeves et al. (24) and Wilson and Reeder (25) for the basic taxonomic arrangement (SI Appendix). It is important to emphasize, however, that the taxonomy of many marine mammals is still confused. The oceans are the last remaining places where large, charismatic species doubtless remain to be described; new species have been found in the last 20 y. For example, Mesoplodon perrini (a 4-m beaked whale) (26) and Orcaella heinsohni (the 2-m Australian snubfin dolphin) (27) were scientifically described recently. The taxonomic position of many species is controversial and likely to change radically in the future when more data are available. For example, recent studies suggest that there are several species of orcas (28, 29), Bryde's whales (30), and Blue whales (31, 32). The taxonomy of dolphins also is complex. For example, some consider the Amazonian Tucuxi dolphin (Sotalia fluviatilis) to be two species (33, 34). Obviously, as taxonomic knowledge improves, one would expect changes in the overall distribution patterns we describe. We defined endemic species as those whose distribution is limited to a single country (political endemism), and the conservation status of all species follows that given by the International Union for the Conservation of Nature (SI Appendix) (35).
The lack of better distributional data precludes more sophisticated analysis, such as modeling standard habitat suitability, to predict ranges of the majority of marine mammal species on very large scales (36). Any comprehensive consideration of the distribution of cetaceans is hampered by the uneven sighting effort; range maps therefore must be interpreted with caution. To date, descriptive statistical techniques have been used to explore cetacean–habitat relationships for selected species in specific areas. There are fewer studies that examine patterns of species richness and geographic ranges using computationally intensive statistic modeling techniques. The development of models to test specific hypotheses about the ecological processes determining cetacean distributions has just begun (37). Marine spatial planning is clearly a way forward, particularly for the high seas, where nonspatial monitoring is difficult and where data gaps obstruct conventional management approaches (38).
To make the data from different species as compatible as possible, we used the same source of distribution information for all species. Despite the limitations in present knowledge, it is imperative to evaluate and implement conservation measures in ways that attempt to compensate for the uncertainties. Spatial modeling incorporates data on the environment to generate a spatial prediction of relative density based on the preference for habitats defined by combinations of environmental covariates. The areas identified for the candidate Marine Protected Areas (MPAs) thus provide a good description of distribution available, as informed by features of the habitat that are shown to be important (39). Terrestrial mammal conservation faces similar uncertainties (40, 41), but significant progress has been made in identifying conservation sites critical for species richness, endemism, and endangerment, using data similar to those used in our study. Such knowledge has contributed to the steps that have been taken to protect many species (2, 15–18).
Results and Discussion
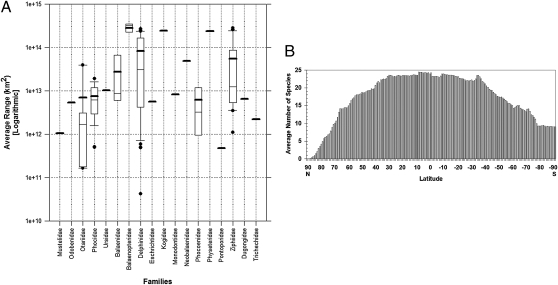
Marine mammals are a polyphyletic group that comprises 129 species grouped in three orders, Cetacea, Sirenia, and Carnivora (Table 1). The smallest marine mammal is the sea otter (1.15 m, 4.5 kg), and the biggest is the blue whale (30 m, 190 tons). Marine mammals show very complex, heterogeneous distributions throughout the oceans and also are found in a few freshwater lakes and rivers. The average geographic range for all species is 52 million km2 (Fig. 1A). The most widely distributed species, with ranges exceeding 350 million km2, are Bryde's (Balaenoptera edeni) and humpback (Megaptera novaeangliae) baleen whales. The marine species with the most restricted range is the vaquita (42) (Phocoena sinus), a porpoise species endemic to 4 000 km2 in the upper northern Gulf of California, Mexico. However, most of the species with very restricted ranges, such as the Baikal seal (Pusa sibirica), are freshwater species endemic to lakes. They probably have relict distributions, remnants of much larger ranges in geologic times (24). Both endemic and restricted-range species have high priority for conservation because they usually are more vulnerable to anthropogenic impacts (2, 10).
Table 1.
Variation in the number of cells and the area covered by different targets to select top-priority cells for marine mammal conservation
| % Grid cells | # Grid cells evaluated | Extension (km2) | Total species richness |
| 1 | 462 | 4,620,000 | 91 (71%) |
| 2.5 | 1,155 | 11,550,000 | 108 (84%) |
| 5 | 2,309 | 23,090,000 | 127 (98%) |
| 7.5 | 3,464 | 38,104,000 | 129 (100%) |
Fig. 1.
Geographic distribution of marine mammals of the world. (A) Box plot with the conservative estimates of geographic range sizes by family. The thick black horizontal lines represent the average family range; the thin line inside the box marks the median family range; the top and bottom edges of the box are the first quartile (bottom edge) and the third quartile (upper edge) of the family range; bars derived from the box represent the maximum range value (upper bar) and the minimum range value (lower bar). Black dots represent outlier species. (B) Latitudinal trends in marine mammal species richness. Note that, as with terrestrial mammals, species richness is greater with decreasing latitude. However, in marine mammals the number of species is relatively similar from 30° N to 40° south, very different from the distribution of land mammal species.
In terms of richness, the analysis of our 46,184-cell, ∼10,000-km2 global geographic quadrant grid (Methods) showed that the number of species per cell varied from 1 to 38, with an average of 17 species, across vast regions of the oceans. Interestingly, latitudinal gradients of species richness of marine and land mammals are very different. Marine mammals have undergone considerable anatomical modifications during their evolution. The unique characteristics of the marine ecosystems have resulted in the many different physiological and ecological responses that marine mammals have experienced. These modifications undoubtedly have resulted in energetic constraints. One of several complex structures of the marine environment is a more-or-less unpredictable, patchy distribution of food over large spatial and temporal scales; this patchy distribution almost certainly has contributed to the evolution of marine mammal energetics, especially through its effect upon energy storage and expenditure strategies. Species richness of land mammals increases sharply from temperate latitudes toward the equator. In contrast, species richness in marine mammals has a more northerly temperate component, showing a higher concentration of species (24 species average) between 30° N and 40° S (Figs. 1B and 2A). Other factors contributing to this pattern in marine mammals remain to be evaluated; nevertheless, the results of our richness-distribution patterns are consistent with other approaches analyzing marine mammal distribution patterns (36, 43).
Fig. 2.
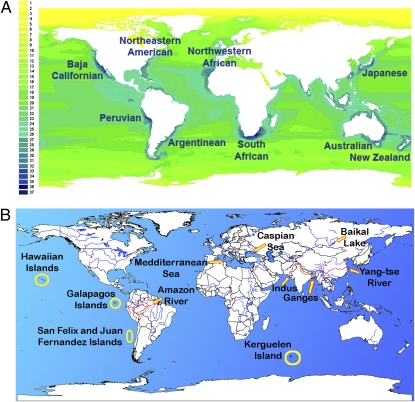
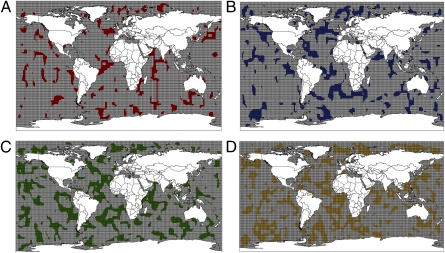
Patterns of geographic distribution and key conservation sites for marine mammals. (A) The distribution of marine species richness is very heterogeneous. The most diverse 10,000-km2 cells have 37 species. The number of species in each cell is shown in the column on the left. The map shows the nine key conservation sites selected as being among the top 2.5% of cells in species richness. These areas include strictly marine species exclusively. (B) Irreplaceable key conservation sites were selected so that all marine mammals are represented in a conservation network.
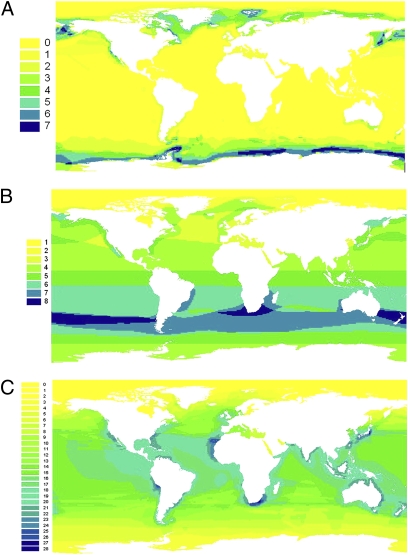
Regions especially rich in marine species (Fig. 2A) were found along the coasts of North and South America, Africa, Asia, and Australia. Such patterns apparently are correlated with ocean currents and their dynamics, especially with nutrient flows connected to upwellings. For example, along the Pacific coast of the American continent, the highest species richness was found along the California, Baja California, and Peruvian coasts, where large upwelling systems maintain very productive fish communities (44). Interestingly, among higher taxa, patterns of species distribution in marine mammals differed strongly (Fig. 3 A–C). Pinniped (seal and sea lion) species richness was concentrated at the poles, especially near Antarctica, whereas Mysticetes (baleen whales) exhibited high species richness at 30° S latitude, and Odontocetes (toothed whales) were concentrated near tropical coasts. There also was variation in distribution at the family level within and among orders; for example, the two families in Sirenia had contrasting distributions: The Trichechidae (manatees) were found exclusively in the North and South Atlantic, whereas the Dugongidae (dugong) were restricted to the North Pacific and Indo-Pacific.
Fig. 3.
Patterns of geographic distribution of species richness in different orders of marine mammals. (A) Pinnipeds (e.g., sea lions). (B) Mysticetes (e.g., blue whale). (C). Odontocetes (e.g., dolphins). Note the highly contrasting patterns and the higher species richness in Odontocetes. The number of species in each cell is shown in the column on the left.
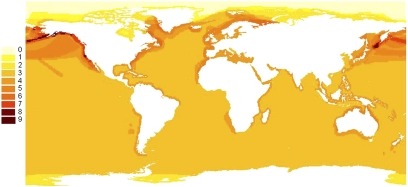
Political endemic species [i.e., species found in only one country, a restriction that may increase their vulnerability (2)] included seven species; the Baikal seal (Pusa sibirica), the Australian sea lion (Neophoca cinerea), the Galapagos fur seal (Arctocephalus galapagoensis), the Galapagos sea lion (Zalophus wollebaeki), the New Zealand dolphin (Cephalorhynchus hectori), the Hawaiian monk seal (Monachus schauinslandi), and the vaquita (Phocoena sinus) (45). Seven species, among them the New Zealand sea lion (Phocarctos hookeri) and the Australian Snubfin dolphin (Orcaella heinsohni), had restricted ranges. In terms of extinction risk, 10% of all marine mammals are considered vulnerable, 11% endangered, and 3% critically endangered (SI Appendix). Species at risk were found throughout the oceans but were concentrated at higher latitudes, especially near the Aleutian Islands and the Kamchatka Peninsula, where extensive exploitation of whales and seals occurred in the past (Fig. 4).
Fig. 4.
Patterns of geographic distribution of marine mammal species that are at risk for extinction. The species included are those considered vulnerable, endangered, or critically endangered by the International Union for the Conservation of Nature (25). The number of species in each cell is shown in the column on the left.
To assess the conservation challenges to marine mammals, we determined the area (i.e., the number of cells) required to incorporate different percentages (i.e., 10%, 15%, 20%, and 25%) of the geographic ranges of all species, using the Marxan optimization algorithm (Methods). Conserving at least 10% of all of the species’ geographic range required ca. 45 million km2 (5,700 grid cells), roughly equivalent to 12% of the world's ocean area (e.g., two times the extent of the Southern Ocean). This study provides grounds for future assessment of an area-explicit conservation parameter for marine mammals. The “target” of 10% was used so this work would be comparable to our previous papers on terrestrial mammals (15, 46); it also is one of the targets suggested by the Convention on Biological Diversity (47). This Convention has called for networks of protected areas, which, in addition to other conservation measures, are necessary components of sustainable use (39). Targeting 15%, 20%, and 25% of each marine mammal's distribution range considerably increased the area required to meet the targets (Fig. 5). Clearly, protecting larger targets must incorporate, by necessity, other conservation mechanisms in addition to reserves or MPA's (48, 49).
Fig. 5.
Conservation targets covering (A) 10%, (B) 15%, (C) 20%, and (D) 25% of the marine mammal distributions using the Marxan optimization algorithm to optimize the number of grid cells and its geographic location.
Our next step was to identify key conservation sites representing all marine mammal species in a geographically explicit way. We selected those sites using the grid cells with the greatest diversity followed by “irreplaceable” cells (i.e., cells with species represented nowhere else), using the Marxan optimization algorithm (Methods). We evaluated the representation of all marine mammal species in 1%, 2.5%, 5%, 7.5%, and 10% of the grid cells (Table 1). We chose 2.5% because these grid cells included 108 (84%) of all species; the missing species were all the 10 endemic species and 11 additional species that have restricted distributions, so they were dispersed in a very large area. Selecting the top 5% of the grid cells would include an additional 19 species but would require more than twice the area required in the 2.5% scenario. Therefore we used Marxan to select in a more effective way the cells containing all missing species, optimizing the area required so that all marine mammals would be represented in our network of key conservation sites. Also, the 2.5% cutoff has been used in studies with land mammals, so using that cutoff allows comparison of terrestrial and marine conservation issues (17, 18).
We identified 20 key conservation sites (Fig. 2 A and B). These key sites can be the basis for identifying a comprehensive conservation strategy with MPAs representing all marine mammals, their ecological roles, and some threats (39, 50). The nine key conservation sites selected because of their species richness were along the coasts of Baja California, Northeastern America, Peru, Argentina, Northwestern Africa, South Africa, Japan, Australia, and New Zealand. These sites represent 108 species (84% of all marine mammal species), including five endemic species (Fig. 2). They are located in all continental waters except Europe and are mostly in temperate latitudes; only the key conservation site off Peru is located in tropical waters. They occur in five of the seven ocean regions (24), being absent from the polar regions, and include 11 (25%) of the 44 marine ecoregions (25). Not surprisingly, these key sites seem to be located in upwelling oceanic areas, where there is a confluence of cold and warm currents. These oceanographic circumstances favor zones of high primary production, which are good feeding areas for marine mammals (43). As expected, the areas of concentration of specific orders vary strongly across space (Fig. 3 A–C). The 11 key conservation sites that were deemed irreplaceable because the presence of endemic species were the Hawaiian Islands, Galapagos Islands, Amazon River, San Felix and Juan Fernández Islands, Mediterranean Sea, Caspian Sea, Lake Baikal, Yang-Tze River, Indus River, Ganges River, and the Kerguelen Islands (Fig. 2B). These sites had unique species, such as the Galapagos fur seal (A. galapagoensis) and the Mediterranean monk seal (Monachus monachus). Interestingly, six irreplaceable sites were continental (rivers and lakes), and five were marine.
We understand that grid cells are not all equally important for conservation aside from their species richness or endemic species (51, 52). In marine mammals, breeding and feeding grounds and migratory routes are especially important for conservation. Therefore, to identify the key conservation sites, special weight was given in the Marxan optimization algorithm (Methods) to grid cells found in calving/breeding/feeding grounds and to known migratory routes of several species. For example, the locations of the breeding grounds for humpback and right whales are well known and often are relatively concentrated, as are all or part of the migratory corridors for some populations. However, such information is not available for many species. Giving more weight to breeding/feeding areas of migratory routes is very important for marine mammals that are highly mobile.
We analyzed the relationship of three human impacts—climate disruption, ocean-based pollution, and commercial shipping (53)—with grid-cell species richness, using a Spearman rank correlation. As we expected, the three impacts have a significant correlation with species richness (rs = 0.693, n = 46,164, P < 0.01 for climate disruption; rs = 0.666, n = 46,164, P < 0.01 for pollution; and rs =0.678, n = 46,164, P < 0.01 for shipping). Our results indicate the widespread impact of human activities on marine ecosystems and their potential for negatively impacting key marine mammal conservation sites. Around 70% of the highest values for the three impacts were located within or near one of our key conservation sites. Adding other human impacts such as commercial fishing probably will show even stronger impacts of human activities on marine mammal conservation.
Areas of overlap between fisheries and marine mammal groups are concentrated mostly in the Northern Hemisphere and appear to occur primarily between pinnipeds and fisheries. Partly because of the comparatively low total food intake of dolphins, the overlap between dolphins and fisheries is quite low and, again, is concentrated mostly in the Northern Hemisphere. Not surprisingly, the lowest overlap occurs between fisheries and deep-diving large-toothed whales, whose diets consist primarily of large squid species and mesopelagic fish not currently exploited by fisheries (54). Narrow coastal fringes are the location of nine of our key conservation sites identified by their species richness. The Japanese and Peruvian richness sites are located within the Northwest and Southeastern Pacific zones, respectively; these two zones have the highest fisheries catch of the major fishing areas in the world (55). The Australian key conservation site, the one with the highest species richness, is in the East Indian Ocean and the Southwest Pacific zones, which are ranked sixth and 18th, respectively, by catch intake (55). The Japanese richness site also is located within Chinese waters (China is top fish-harvesting nation in the world), where 17 million tons of fish are captured annually and where at least 30 marine mammal species live (55). In addition, at least five of the key conservation sites overlap with highly impacted ocean areas where high bycatch fishing occurs (53).
Many marine species and populations [e.g., North Atlantic right whale (Eubalaena glacialis) and the Sei whale (Balaenoptera borealis)] are at the brink of extinction from overharvesting, pollution, bycatch, and exhaustion of prey-species populations (24, 25, 56–58), and their long-term survival depends on sound management that addresses the factors causing their decline. The baiji dolphin, once endemic to the Yang-Tze River in China, is a disturbing example of the plight of marine mammals impacted by human activities (9). The next candidate to become extinct if no solid conservation and management strategies are implemented is the Mexican vaquita. Endemic to the Gulf of Baja California, the species has been declining sharply for at least 2 decades; one fifth of the population is killed in gillnets every year, and there now are only an estimated 150–300 individuals (59). Indeed, more than 650,000 marine mammals die from entanglement in fishing nets each year (60), making bycatch the single largest cause of mortality for small cetaceans and pushing several species to the verge of extinction.
Conservation strategies also should take into account the possible impacts of anthropogenic climate disruption (61, 62) on the distribution of these mammals and its repercussions on the establishment of connective corridor systems between protected areas (61) and on management plans. Finally, management interventions must be evaluated critically with regard to ecological viability and benefits vs. costs (61).
By selecting the smallest area of reserves using an optimization algorithm, the opportunity conservation cost would be generally lower, but this approach will depend on the distribution of other potential economic activities (63). For instance, an evaluation of fisheries values could provide a feasible first cut at calculating those costs. Given the distribution patterns of marine mammals, the increasing pressures of human activities in the oceans, and the threat of climate disruption, the conservation of marine mammals is a daunting problem. Saving one or two populations of most species will not be enough (2) because of the role that such charismatic mammals play in the ecological dynamics of marine and freshwater ecosystems and in the provision of ecosystem services. As many scientists have emphasized in other forums, especially in connection with whaling (64), the complexity and scale of the problem requires an unprecedented international effort with the development of both new attitudes and institutions (65). The main objectives of selection criteria for MPAs are to identify potential MPAs for highly mobile and temporally variable pelagic species, including high-density areas, feeding or breeding grounds, and migratory routes; to provide a transparent and systematic approach to selection; and to help determine priorities for action (39).
Uncertainty will always be a factor in research on pelagic organisms and their environment. Empirical data point to dramatic declines and changes in marine systems, and ongoing research continues to provide techniques to incorporate and contend with uncertainty. The challenge is to produce timely and scientifically defensible research based on available data to address this conservation crisis now (56). The future of marine mammals in particular and biodiversity in general will depend on the actions we take.
Methods
We compiled and digitized the geographic range maps from published sources for all 129 species and created a Geographic Information System database for 46,184 1° x 1° grid-cells, ∼10,000-km2. We then conducted a presence/absence analysis to determine the number of species in each grid cell and the number of cells in which each species was recorded. We created maps of global species richness, irreplaceable sites, endemism, and threatened species. Key conservation sites for species richness were determined either as the 2.5% of the cells with the highest species richness or as irreplaceable sites, defined as regions containing species not represented in any other part of the world (17, 18). Additionally, we used optimization algorithms, i.e., ResNet (66) and Marxan (67, 68 69), to determine the number of cells required to cover 10%, 15%, 20%, and 25% of the geographic ranges of all species and the area of the ocean covered by each percentage.
Marxan is software that delivers decision support for reserve system design intended to solve a particular class of reserve design problem in which the goal is to achieve some minimum representation of biodiversity features for the smallest possible cost. Given reasonably comprehensive data on species, habitats, and/or other relevant biodiversity features, Marxan aims to identify the reserve system (a combination of planning units) that will meet user-defined biodiversity targets for the minimum cost. In this particular case, Marxan selected planning units (here, grid cells) to meet the targets (10%, 15%, 20%, and 25% of the geographic ranges of all 129 species) and also considered the following factors. Each grid cell is assigned a “cost” depending on the target (e.g., area, number of species, threat), and Marxan minimizes the combined grid-cell cost of the conservation network, still selecting expensive grid cells if they are needed to meet the targets. This cost can be a measure of any aspect of the planning unit (25, 69, 70); in this case, it was species richness plus cells weighted for breeding/feeding ground or migratory route. We set Marxan to select adjacent planning units preferentially rather than a series of unconnected units, which would be less ecologically viable and more difficult to manage. Then Marxan identified a set of grid cells each time it was run: 100 runs generated 100 different grid-cell networks (67). Units that appeared in every network were considered irreplaceable, because they always would be needed to meet the targets, whereas other units could be swapped with similar units, and the targets still would be met. Fig. 5 was achieved using these methods.
Supplementary Material
Acknowledgments
We thank Gretchen C. Daily, Rurik List, Stuart Pimm, and Rob Pringle for insightful discussion on this topic and Irma Salazar, Antonio Iturbe, Jesús Sajama, and Pablo Ortega for help with the data analyses. This study was supported by grants from Dirección General de Asuntos del Personal Académico (Universidad Nacional Autónoma de México), Ecociencia Sociedad Civil, the National Council for Science and Technology (Mexico), and the Cetacean Society International.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. P.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101525108/-/DCSupplemental.
References
- 1.Ehrlich PR, Ehrlich A. Extinction: The Causes and Consequences of the Disappearance of Species. New York: Random House Inc.; 1981. [Google Scholar]
- 2.Ceballos G, Ehrlich PR. Mammal population losses and the extinction crisis. Science. 2002;296:904–907. doi: 10.1126/science.1069349. [DOI] [PubMed] [Google Scholar]
- 3.Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GA, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 4.Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA. Global patterns of predator diversity in the open oceans. Science. 2005;309:1365–1369. doi: 10.1126/science.1113399. [DOI] [PubMed] [Google Scholar]
- 5.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 6.Burke L, et al. Pilot Assessment of Global Ecosystems: Coastal Ecosystems. Washington, D.C.: World Resources Institute; 2000. [Google Scholar]
- 7.Steadman DW. Extinction and biogeography of tropical Pacific birds. Chicago: Univ of Chicago Press; 2006. [Google Scholar]
- 8.Dulvy N, Sadovy Y, Reynolds J. Extinction vulnerability in marine populations. Fish Fish. 2003;4:25–64. [Google Scholar]
- 9.Turvey ST, et al. First human-caused extinction of a cetacean species? Biol Lett. 2007;3:537–540. doi: 10.1098/rsbl.2007.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaston K. The Structure and Dynamics of Geographic Ranges. Oxford: Oxford Univ. Press; 2003. [Google Scholar]
- 11.Graves GR, Rahbek C. Source pool geometry and the assembly of continental avifaunas. Proc Natl Acad Sci USA. 2005;102:7871–7876. doi: 10.1073/pnas.0500424102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rapoport EH. Areography: Geographical Strategies of Species. Oxford: Pergamon; 1982. [Google Scholar]
- 13.Brown JH. Macroecology. Chicago: Univ of Chicgo Press; 1989. [Google Scholar]
- 14.Margules CR, Pressey RL. Systematic conservation planning. Nature. 2000;405:243–253. doi: 10.1038/35012251. [DOI] [PubMed] [Google Scholar]
- 15.Ceballos G, Ehrlich PR, Soberón J, Salazar I, Fay JP. Global mammal conservation: What must we manage? Science. 2005;309:603–607. doi: 10.1126/science.1114015. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues AS, et al. Effectiveness of the global protected area network in representing species diversity. Nature. 2004;428:640–643. doi: 10.1038/nature02422. [DOI] [PubMed] [Google Scholar]
- 17.Orme CD, et al. Global patterns of geographic range size in birds. PLoS Biol. 2006;4:e208. doi: 10.1371/journal.pbio.0040208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceballos G, Ehrlich PR. Global mammal distributions, biodiversity hotspots, and conservation. Proc Natl Acad Sci USA. 2006;103:19374–19379. doi: 10.1073/pnas.0609334103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colwell RK, Hurtt GC. Nonbiological gradients in species richness and a spurious Rapoport effect. Am Nat. 1994;144:570–595. [Google Scholar]
- 20.Fischer AG. Latitudinal variations in organic diversity. Evolution. 1960;14:64–81. [Google Scholar]
- 21.Simpson GG. Species Density of North American Recent Mammals. Syst Zool. 1964;13:57–73. [Google Scholar]
- 22.International Union for the Conservation of Nature, Conservation International, NatureServe. Global Amphibian Assessment. 2006. [Google Scholar]
- 23.Douvere F. The importance of marine spatial planning in advancing ecosystem-based sea use management. Mar Policy. 2008;32:762–771. [Google Scholar]
- 24.Reeves R, Stewart B, Clapham P, Powell J. Guide to Marine Mammals of the World. Nat. Audubon Soc. New York: Alfred A. Knopf; 2002. [Google Scholar]
- 25.Wilson DE, Reeder M. Mammal Species of the World. A Taxonomic and Geographic Reference. Baltimore: Johns Hopkins Univ Press; 2005. 3rd Ed. [Google Scholar]
- 26.Dalebout M, et al. A new species of beaked whale Mesoplodon perrini sp. n. (Cetacea: Ziphiidae) discovered through phylogenetic analyses of mitochondrial DNA sequences. Mar Mamm Sci. 2002;18:577–608. [Google Scholar]
- 27.Beasley I, et al. Description of a new dolphin, the Australian snubfin dolphin Orcaella heinsohni sp. n. (Cetacea, Delphinidae) Mar Mamm Sci. 2005;21:365–400. [Google Scholar]
- 28.Perrin W. Perrin WF, editor. World Cetacea Database. 1982. Available at http://marinespecies.org/aphia.php?p=taxdetails&id=380525; accessed June 8, 2010.
- 29.Pitman R, et al. A dwarf form of killer whale in Antarctica. J Mammal. 2007;88:43–48. [Google Scholar]
- 30.Kanda N, et al. Population genetic structure of Brydes Whale (Balaenoptera brydei) at the inter-oceanic and trans-equatorial levels. Cons. Gen. 2007;8:853–864. [Google Scholar]
- 31.Garrigue C, Clua E, Breitenstein D. Identification of a juvenile pygmy blue whale (Balaenoptera musculus brevicauda) in New Caledonia, South-West Pacific. 2003. SC/55/SH4. Available at http://www.operationcetaces.nc/uploads/IWC%20papers/20-SC-55-SH4%20Garrigue%20et%20al%20Blue%20whale.pdf.
- 32.Ichihara T. In: Norris KS, editor. 1996. Whales, Dolphins and PorpoisesUniv of California Press Los Ángeles79–113. [Google Scholar]
- 33.Cunha HA, et al. Riverine and marine ecotypes of Sotalia dolphins are different species. Mar Biol. 2005;148:449–457. [Google Scholar]
- 34.Caballero S, et al. Molecular systematics of South American dolphins Sotalia: Sister taxa determination and phylogenetic relationships, with insights into a multi-locus phylogeny of the Delphinidae. Mol Phylogenet Evol. 2008;23:358–386. doi: 10.1016/j.ympev.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 35.International Union for the Conservation of Nature. The IUCN Red List of Threatened Species, Version 2010.4. 2011. (IUCN, Gland, Switzerland) Available at http://www.iucnredlist.org/
- 36.Kaschner K, Watson R, Trites AW, Pauly D. Mapping worldwide distributions of marine mammals using a Relative Environmental Suitability (RES) model. Mar Ecol Prog Ser. 2006;316:285–310. [Google Scholar]
- 37.Redfern JV, et al. Techniques for cetacean-habitat modeling. Mar Ecol Prog Ser. 2006;310:271–295. [Google Scholar]
- 38.Ardron J, Gjerde K, Pullen S, Tilot V. Marine spatial planning in the high seas. Marine Policy. 2008;32(5):832–839. [Google Scholar]
- 39.Evans P. Proceedings of the ECS/ASCOBANS/ACCOBAMS. Workshop: Selection criteria for marine protected areas for cetaceans. 2008 European Cetacean Society's 21st Annual Conference, The Aquarium, San Sebastian, Spain, April 22, 2007. p 108. [Google Scholar]
- 40.Ceballos G, Ehrlich PR. Discoveries of new mammal species and their implications for conservation and ecosystem services. Proc Natl Acad Sci USA. 2009;106:3841–3846. doi: 10.1073/pnas.0812419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balmford A, Green R, Jenkins M. Measuring the changing state of nature. Trends Ecol Evol. 2003;18:326–330. [Google Scholar]
- 42.Lundmark C. Science Sings the Blues: Other Words for Nothin' Left to Lose. BioScience. BioBriefs. 2007;59 10.1641/B570218. [Google Scholar]
- 43.Kaschner K, Tittensor DP, Ready J, Gerrodette T, Worm B. Current and future patterns of global marine mammal biodiversity. PLoS ONE. 2011;6:e19653. doi: 10.1371/journal.pone.0019653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauly D, Christensen V. Primary production required to sustain global fisheries. Nature. 1995;374:255–257. [Google Scholar]
- 45.Nogueira C, et al. Restricted-range fishes and the conservation of Brazilian freshwaters. PLoS ONE. 2010;5:e11390. doi: 10.1371/journal.pone.0011390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carwardine J, et al. Conservation planning when costs are uncertain. Conservation Biology. 2010;24:1529–1537. doi: 10.1111/j.1523-1739.2010.01535.x. [DOI] [PubMed] [Google Scholar]
- 47.Convention on Biological Diversity (CBD) Programmes and Issues; 2010 Biodiversity Target; Assessing; Goals and Sub-targets. 2010. Available at http://www.cbd.int/2010-target/goals-targets.shtml.
- 48.UNEP (United Nations Environmental Programme)/CBD (Convention on Biological Diversity)/SBSTTA (Subsidiary body on Scientific, Technical, and Technological Advice) Indicators for assessing progress towards the 2010 target: Connectivity/fragmentation of ecosystems. 2005 10th Meeting of the UNEP/CBD/SBSTTA. Bangkok, February 7–11, 2005. p 9. [Google Scholar]
- 49.Kelleher G. Guidelines for Marine Protected Areas. Gland, Switzerland: IUCN; 1999. p. 107. [Google Scholar]
- 50.Crowder L, Norse E. Essential ecological insights for marine ecosystem-based management and marine spatial planning. Mar Policy. 2008;32:772–778. [Google Scholar]
- 51.Gerber L, Heppell S, Ballantyne F, Sala E. The role of dispersal and demography in determining the efficacy of marine reserves. Can J Fish Aquat Sci. 2005;62:863–871. [Google Scholar]
- 52.Gerber L, Heppell S. The use of demographic sensitivity analysis in marine species conservation planning. Biol Conserv. 2004;120:121–128. [Google Scholar]
- 53.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 54.Pauly D, Kaschner K. Competition between Marine Mammals and Fisheries: FOOD FOR THOUGHT. 2004. Available at: http://www.hsi.org.au/editor/assets/admin/Daniel_Pauly_Report.pdf.
- 55.Food and Agriculture Organization of the United Nations (FAO) The state of world fisheries and aquaculture. 2008. available at: ftp://ftp.fao.org/docrep/fao/011/i0250e/i0250e.pdf.
- 56.Lewison R, Crowder L, Read A, Freeman S. Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol Evol. 2004;19:598–604. [Google Scholar]
- 57.Roberts CM, et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science. 2002;295:1280–1284. doi: 10.1126/science.1067728. [DOI] [PubMed] [Google Scholar]
- 58.DeMaster DP, et al. The sequential megafaunal collapse hypothesis: Testing with existing data. Prog Oceanogr. 2006;68:329–342. [Google Scholar]
- 59.Rojas-Bracho L, Reeves R, Jaramillo A. Conservation of the vaquita Phocoena sinus. Mammal Rev. 2006;36:179–216. [Google Scholar]
- 60.Crowder LB, et al. The impacts of fisheries on marine ecosystems and the transition to ecosystem-based management. Annu Rev Ecol Evol Syst. 2008;39:259–278. [Google Scholar]
- 61.Halpin PN. Global climate change and natural-area protection: Management responses and research directions. Ecol Appl. 1997;7:828–843. [Google Scholar]
- 62.MacLeod C. Oceanic climate change, range changes and implications for the conservation of marine cetaceans: A review and synthesis. Endanger Species Res. 2009;7:125–136. [Google Scholar]
- 63.Stewart R, et al. Opportunity cost of ad hoc marine reserve design decisions: An example from South Australia. Mar Ecol Prog Ser. 2003;253:25–38. [Google Scholar]
- 64.Springer AM, et al. Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proc Natl Acad Sci USA. 2003;100:12223–12228. doi: 10.1073/pnas.1635156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ehrlich PR, Ehrlich AH. One with Nineveh: Politics, Consumption, and the Human Future. Washington, DC: Island; 2004. [Google Scholar]
- 66.Garson J, Aggarwal A, Sarkar S. 2007. ResNet Manual. Versión 1.2. http://uts.cc.utexas.edu/∼consbio/Cons/program.html.
- 67.Ball I, Possingham H. MARXAN (Marine reservedesign using spatially explicit annealing) Australia: Univ of Queensland; 2000. [Google Scholar]
- 68.Ball I, Possingham H. Marxan (v1.8.2). Marine Reserve Design using Spatially Explicit Annealing. Brisbane, Australia: Univ of Queensland; 2000. [Google Scholar]
- 69.Richardson EA, Kaiser MJ, Edwards-Jones G, Possingham HP. Sensitivity of marine-reserve design to the spatial resolution of socioeconomic data. Conserv Biol. 2006;20:1191–1202. doi: 10.1111/j.1523-1739.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 70.Wilson K, et al. Measuring and incorporating vulnerability into conservation planning. Environ Manage. 2005;35:527–543. doi: 10.1007/s00267-004-0095-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.