Abstract
Immunity against new infections declines in the last quartile of life, as do numbers of naive T cells. Peripheral maintenance of naive T cells over the lifespan is necessary because their production drastically declines by puberty, a result of thymic involution. We report that this maintenance is not random in advanced aging. As numbers and diversity of naive CD8+ T cells declined with aging, surviving cells underwent faster rates of homeostatic proliferation, were selected for high T-cell receptor:pMHC avidity, and preferentially acquired “memory-like” phenotype. These high-avidity precursors preferentially responded to infection and exhibited strong antimicrobial function. Thus, T-cell receptor avidity for self-pMHC provides a proofreading mechanism to maintain some of the fittest T cells in the otherwise crumbling naive repertoire, providing a degree of compensation for numerical and diversity defects in old T cells.
Keywords: lymphocyte homeostasis, T-cell repertoire, CD8 T cells
Aging is accompanied by a decline in immune function (1, 2). That decline is believed to be the most important contributing factor to the known susceptibility of older adults to various infections and to their often suboptimal response to vaccination (3, 4). T cells have been shown to exhibit some of the most pronounced and consistent age-related immune defects (5–7) and improving T-cell function in aging often results in improved immunity (8, 9). However, the key factors that are responsible for these age-associated alterations to the T-cell pool and their direct mechanistic links to immune functionality remain poorly understood.
Both T-cell responsiveness and changes in the composition of the T-cell pool contribute to the decline in T-cell function with advancing age (6, 9). A large and diverse repertoire of naive T cells is required for protective immunity against newly encountered pathogens (10, 11), and that repertoire is generated by the production of new T cells by the thymus and maintained by the homeostatic mechanisms in secondary lymphoid organs, blood and lymph. As the thymic output dramatically declines with age because of involution of the thymus (1, 12), there is a shift from thymic T-cell production to peripheral T-cell maintenance during early adulthood to prevent lymphopenia and to maintain the size of the peripheral T-cell pool. Low levels of homeostatic cycling provide an important means to preserve T-cell diversity in adults. It is unclear to what extent these mechanisms maintain a balanced T-cell repertoire over the entire lifespan, as even slight differences in cycling and turnover rates have the potential to be amplified over time. Indeed, increased homeostatic cycling in the absence of infection has been linked to conversion of naive cells into memory cells, and there is some evidence that this process may increase with aging (reviewed in ref. 5).
Peripheral homeostatic signals required for survival and turnover of naive T cells include a T-cell receptor (TCR):self-pMHC contact and cytokine (IL-7 or IL-15) stimulation via the common-γ (Cγ) chain-containing receptor (13–15). It is unclear, however, whether these signals equally promulgate survival of all naive T cells. T cells expressing different TCR transgenes do not have equal ability to undergo expansion in response to lymphopenia (16–18); however, the significance of these observations to the lifelong maintenance of naive polyclonal T cells and to immune defense in aging remains unclear.
In the present study, we examined the naive antigen-specific CD8+ T-cell repertoire in unprimed adult and old mice and discovered that the naive precursors acquire more of memory-like phenotype and become increasingly biased toward the most homeostatically fit competitors with advancing age. The shedding of low-avidity clonotypes over the lifespan potentially further contributes to the age-related loss of TCR diversity already in progress because of thymic involution, but preserves the high-avidity clonotypes that are most useful for immune defense. These findings have important implications for improving the efficacy of vaccines for the elderly.
Results
Enumeration and Characterization of Naive gB-8p Precursors from Adult and Old Mice.
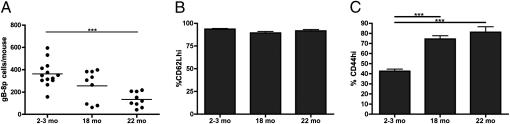
We used the tetramer enrichment protocol (19, 20) to isolate CD8+ T cells specific for the HSV-1 glycoprotein B498–505 (SSIEFARL, gB-8p in the text) bound to H-2Kb from the spleen and lymph nodes of individual unprimed 2- to 3-, 18-, and 22-mo-old C57BL/6 (B6, H-2b) mice (Fig. 1A). On average, we identified ∼400 gB-8p–specific CD8+ T cells in adult unprimed mice, similar to published results (21). However, the numbers of gB-8p precursors dropped with progressing age to ∼250 in 18-mo-old mice, and to ∼125 in 22-mo-old mice (Fig. 1A).
Fig. 1.
The size of the gB-8p precursor pool in unprimed mice declines with age and preferentially acquires a “memory-like” phenotype. (A) Total numbers of gB-8p–specific CD8+ T cells recovered from individual naive mice at 2 to 3, 18, and 22 mo of age. Phenotypic analysis of gB-8p precursors was also performed. The proportion of the naïve gB-8p pool that is CD62hi (B) or CD44hi (C) in different aged mice is shown. All data are pooled from three separate experiments. Results depict mean ± SEM, ***P < 0.001.
To gain insight into how the aging gB-8p pool is maintained, we examined the phenotype of gB-8p precursors obtained from adult and old mice. CD62L, a marker of naive cells capable of homing and remaining in the lymph nodes, did not exhibit age-related changes in expression (Fig. 1B). In contrast, the percentage of CD44hi cells increased from 30% to 40% at 2 to 3 mo, and to 70% to 80% at 18 mo of age (Fig. 1C). These data suggested that many naive CD8+ T-cell precursors gradually assume certain markers typically seen in virtual memory (VM) (21) CD8+ T cells with advancing age. VM cells have been shown to arise in the naive repertoire as a consequence of antigen-independent, cytokine (IL-7/15)-mediated proliferation, and can be delineated from naive CD8+ T cells by their ability to rapidly produce IFN-γ in response to IL-12 or IL-18 cytokine stimulation (21). Indeed, in parallel with the rise in CD44hi cells among the old gB-8p precursors, we found an accumulation of IFN-γ–producing cells with advancing age (Fig. S1). Overall, these findings suggest that the reduced size of the gB-8p pool with aging is accompanied by a proportional increase in VM cells.
Naive gB-8p Pool Becomes Dominated by CD44hi Vβ10+ Precursors with Aging.
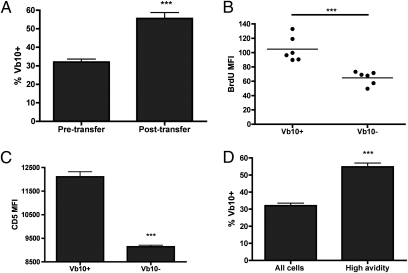
Following HSV-1 infection, ∼50% to 60% of responding gB-8p CD8+ T cells in H-2Kb mice use the TCR Vβ10 chain to make its TCR (22–24). With aging, this bias in Vβ10 use becomes even more prominent, making up >70% of antigen-driven gB-8p CD8+ T cells in old mice primed with vaccinia virus-gB (23). If all gB-8p precursors equally survive and are equally recruited and expanded following infection, we would expect the antigen-driven and unprimed pool to mirror each other. An analysis of the percentages of Vβ10 clonotypes revealed that the frequency of Vβ10 TCR use among gB-8p precursors in unprimed mice increased from ∼30% at 2 to 3 mo of age and to ∼50% at 18 mo of age (Fig. 2A), and that frequency further increased in the antigen-driven repertoire of both adult and old animals by another 20% or more (23). Therefore, the dominance of the Vβ10+ CD8+ T cells in the immune repertoire responding to gB-8p is at least in part because of the Vβ10 bias in the unprimed pool, which increases with aging.
Fig. 2.
The aging gB-8p precursor pool in unprimed mice becomes biased toward CD44hi Vβ10+ cells. (A) The proportion of the naive gB-8p pool that uses Vβ10 in different aged mice was assessed by flow cytometry. (B) The frequencies of CD44hi memory phenotype cells within the Vβ10+ and Vβ10− gB-8p populations are also shown. All data are pooled from at least three separate experiments. Results depict mean ± SEM from n > 13 mice/group. *P < 0.05, ***P < 0.001.
As shown in Fig. 1C, a significant proportion of gB-8p–specific CD8+ T precursors in unprimed mice are comprised of CD44hi VM precursors that more rapidly respond to antigens than naive phenotype precursors. Analysis of CD44 expression revealed that in 2- to 3-mo-old mice the Vβ10+ gB-8p precursors already contained significantly higher levels of VM cells (∼55–60%) compared with all other (Vβ10−) gB-8p precursors (∼25–30%) (Fig. 2B and Fig. S2), and that trend continued to increase with age, so that >80% of Vβ10+ cells expressed high levels of CD44 by 18 mo of age. Although the percentage of CD44hi cells also increased with age in the Vβ10− population, it remained significantly lower at all times compared with the corresponding Vβ10+ population. Collectively, these findings indicate that the accumulation of VM cells in the aging gB-8p pool preferentially occurred among the Vβ10+ gB-8p–specific precursors.
Vβ10+ gB-8p Precursors Exhibit Higher TCR Avidity and Undergo Faster Rates of Homeostatic Proliferation.
Increased conversion of the naive Vβ10+ gB-8p precursors into VM cells could be a result of faster rates of homeostatic proliferation; two lines of evidence supported this possibility. First, we adoptively transferred adult CD8+ T cells into adult RAG1−/− mice, forcing them into homeostatic proliferation in a lymphopenic environment. After only 30 d, the proportion of gB-8p precursors expressing Vβ10 significantly increased from ∼30% to ∼50% (Fig. 3A), similar to the effects of normal aging between 3 and 18 mo. Because it is known that some proliferation in RAG1−/− hosts is driven by antigens of the intestinal flora (25), we next sought evidence of increased in situ proliferation of Vβ10+ gB-8p–specific CD8+ naive cells over their Vβ10− counterparts in a normal adult host. Vβ10+gB-8p precursors incorporated almost twice as much BrdU as all other (Vβ10−) gB-8p precursors (Fig. 3B), and the Vβ10+ gB-8p precursors also exhibited significantly higher expression of phenotypic markers CD122, CD11a, and Ly6C associated with homeostatic proliferation (Fig. S3). In contrast, in preliminary experiments, none of the unprimed VM cells expressed elevated levels of inhibitory markers, such as PD-1 and 2B4 (Fig. S4). Therefore, naive Vβ10+ gB-8p–specific precursors underwent more intense homeostatic proliferation than Vβ10− counterparts under physiological conditions. We believe that this proliferation belies cytokine-driven proliferation, rather than gut flora antigen-driven proliferation based on the above and on the kinetics of the BrdU incorporation (Fig. 3A); however, our experiments did not formally exclude the latter possibility.
Fig. 3.
Vb10+ gB-8p precursors undergo faster rates of homeostatic proliferation caused by stronger interactions with peptide:MHC complexes. (A) “Untouched” old adult CD8+ T cells (2–3 mo old) were transferred into Rag1−/− mice and the percentage of Vb10+ gB-8p cells were determined 1 mo later. (B) Adult mice (2–3 mo old) were given BrdU in their drinking and the amounts of BrdU incorporated (mean fluouresence intensity, MFI) within Vβ10+ and Vβ10− gB-8p precursors was measured 1 mo later. (C) Expression levels of CD5 (MFI) within Vβ10+ and Vβ10− gB-8p precursors isolated from adult mice (2-3 mo old). (D) The tetramer dissociation assay was performed on naive adult gB-8p precursors (2–3 mo old). The percentage of Vβ10+ cells within the high-avidity fraction (or those that were still tetramer-bound at the end of the assay) was compared with the levels observed in the total gB-8p population. All data are pooled from at least two separate experiments, with n ≥ 3 mice per group per experiment. Results depict mean ± SEM, ***P < 0.001.
Prior studies suggested that the capacity of CD8+ T cells to undergo lymphopenia-induced proliferation may be linked to the affinity/avidity of interaction between their TCR and pMHC complexes. CD5 is a negative regulator of TCR signaling, and a surrogate marker whose expression is directly proportional to TCR affinity (26, 27). The levels of CD5 expression were significantly elevated in adult Vβ10+ gB-8p naive precursors compared with non-Vβ10 counterparts (Fig. 3C and Fig. S5). A direct assessment of TCR avidity, using the dissociation rate of the pMHC complex from the TCR, corroborated this finding. Tetramer dissociation assay in adult mice showed that the proportion of Vβ10+ cells significantly increased from ∼30% in the total population to ∼55% within the high avidity fraction (Fig. 3D). Therefore, higher TCR avidity correlated with superior homeostatic proliferation and preferential maintenance of Vβ10+ gB-8p precursors.
Composition of the Naive Vβ10+ gB-8p Cell Repertoire Is Dramatically Altered in Old Mice.
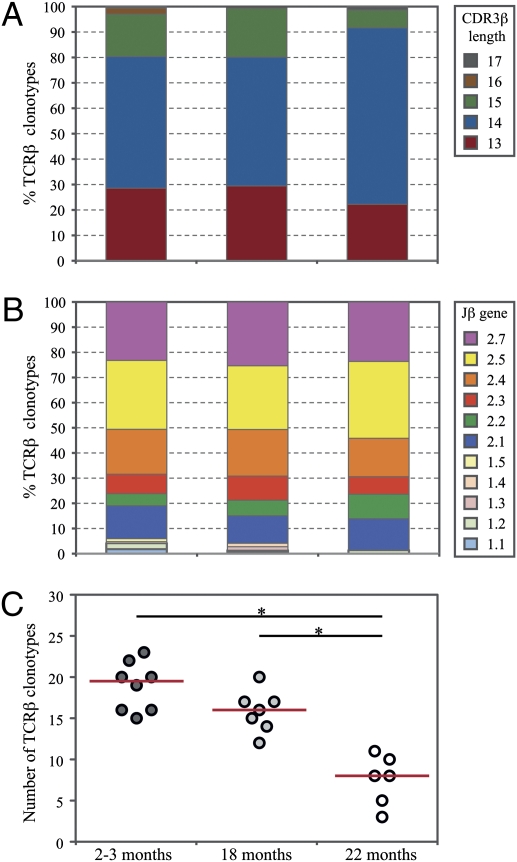
We next performed molecular analysis of clonotypes in the naive gB-8p–specific TCR repertoire across aging. For a total of 21 unprimed mice (6–8 mice per age group) we obtained an average of 40 Vβ10+ TCRβ sequences per mouse (range: 23–66; representative sequences from one mouse per age group shown in Table S1). The Vβ10+ TCRβ repertoires of unprimed gB-8p precursors exhibited similar distinctive features to those associated with CD8+ T-cell responses to HSV-1 infection, including: a prevalent CDR3β length of 14 amino acids (Fig. 4A), preferential use of the Jβ2 family of genes (Fig. 4B), and a prevalent CDR3β amino acid motif featuring a tryptophan-glycine doublet in CDR3β positions 6 and 7 [which correspond to CDR3 positions 3 and 4 using the Chothia definition (28)] (Fig. S6A). We recently showed that the immune CD8+ repertoire against gB-8p was accompanied by the narrowing of the gB-8p–specific Vβ10+ TCRβ repertoire with age (23). Here, we found this result to be caused by reduced diversity of the Vβ10+ gB-8p precursors in the unprimed pool with age. The number of different gB-8p–specific Vβ10+ TCRβ clonotypes was significantly lower in 22-mo-old mice compared with both 2- to 3-mo and 18-mo-old mice (Fig. 4C). This reduction in TCRβ clonotype diversity was accompanied by substantial skewing of the clonal dominance hierarchy, as measured by Simpson's diversity index (Fig. S6B). Although the gB-8p–specific Vβ10+ TCRβ clonotypes in 2- to 3-mo-old mice exhibited a relatively even distribution of clone sizes, the naive TCRβ repertoire in 22-mo-old mice tended to be largely dominated by one or a few individual TCRβ clonotypes (Fig. S6C).
Fig. 4.
Clonotypic composition of Vb10+ gB-8p precursors from unprimed mice at different ages. The percentages of unique TCRβ clonotypes pooled across all mice (n = 6–8) per age group that have a particular (A) CDR3β length and (B) Jβ gene use. The diversities of the TCRβ repertoires for individual mice were evaluated using (C) the number of different TCRβ clonotypes. A Mann–Whitney test was used for each pairwise comparison between age groups, with the statistical significance for each pairwise comparison determined at *P < 0.0167, using Bonferroni correction for multiple pairwise comparisons.
High-Avidity Vβ10+ gB-8p Cells Dominate the Immune Response in Old Mice.
The above data demonstrate that over the lifetime self-pMHC selects those naive clones with high TCR avidity that preferentially homeostatically cycle and survive as memory-phenotype naive cells. Is there an age-related benefit or penalty from undergoing extensive homeostatic proliferation? Natural selection of the best-fitting CD8+ T cells (16, 29) could be beneficial because only the higher-avidity T cells will turn over and replace themselves; however, excessive rates of homeostatic proliferation could also expand clonotypes that first become senescent in old age. Therefore, we assessed the functional ability of Vβ10+ and Vβ10− gB-8p precursors in 2-, 18-, and 22-mo-old mice to respond to bacterial challenge with a recombinant Listeria monocytogenes expressing gB-8p (LM-gB). At the peak of the response (day 7), there was a dramatic reduction in the total number of gB-8p–specific CD8+ T cells in spleens of old mice (Fig. 5A), accompanied by an increase in utilization of Vβ10 (∼50% in adults, ∼70% in old mice) (Fig. 5B), consistent with our previous results (23) and the finding that frequency of Vβ10 utilization was significantly higher than that observed in the naive pool (∼30% in adults, ∼50% in old mice) (Fig. 2). Furthermore, effector gB-8p CD8+ T cells responding to LM-gB in old mice showed significantly slower tetramer dissociation kinetics compared with adults (Fig. 5 C and D), and the age-associated increase in TCR avidity was most pronounced in Vβ10+ gB-8p cells (Fig. 5 C and D).
Fig. 5.
The reduced gB-8p response to LM-gB in old mice is dominated by high-avidity Vb10+ gB-8p cells. Different aged mice were infected with 5,000 CFU LM-gB. At 7 d postinfection, the total number (A) and Vb10 use (B) of gB-8p cells in the spleen were evaluated. TCR:pMHC off-rates (tetramer decay) determination in Vβ10+ and Vβ10− gB-8p cells (C and D) was also assessed at 7 d after LM-gB infection. All data are representative of at least two separate experiments with n ≥ 7 mice per group. Results depict mean ± SEM; *P < 0.05, **P < 0.01, ***P < 0.001.
Immune Functionality Is Selectively Preserved Within Old Vβ10+ gB-8p Precursors.
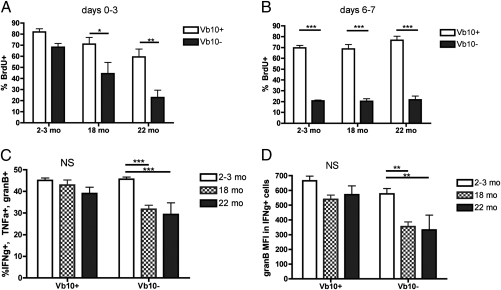
We next examined the impact of aging on the recruitment into proliferation of gB-8p precursors by administering BrdU in the drinking water of adult and old mice during infection. Vβ10+ gB-8p precursors showed strong early recruitment (day 3) into proliferation regardless of age, but other (Vβ10−) gB-8p precursors exhibited an age-related reduction in proliferation (Fig. 6A). At the peak of the response, the percentage of Vβ10+ gB-8p–specific cells in cycle (as measured by BrdU incorporation) was nearly three times higher in adult and old mice relative to Vβ10− gB-8p cells (Fig. 6B). Therefore, superior proliferation and increased precursor frequency of Vβ10+ gB-8p precursors provide a straightforward explanation for their predominance in adult and old mice following infection. Robust effector T-cell differentiation and the ability of a single effector T cell to secrete multiple effector cytokines have been correlated with superior immune protection against a range of pathogens (30) but were found to decline with age (6). Old Vβ10+ gB-8p precursors exhibited robust up-regulation of KLRG-1 and down-regulation of CD62L during effector differentiation, whereas old non-Vβ10 gB-8p precursors showed age-related impairment in regulation of both molecules (Fig. S7). Moreover, representation of polyfunctional gB-8p CD8+ T cells (able to simultaneously produce IFN-γ, TNF-α, and Granzyme B) and the intensity of Granzyme B expression in IFN-γ+ effectors were found to be similar in the Vβ10+ gB-8p cells between adult and old mice, but both features were significantly reduced in Vβ10− gB-8p cells with aging (Fig. 6 C and D).
Fig. 6.
Immune responsiveness is preferentially maintained within old Vb10+ gB-8p precursors. (A) Different aged mice were infected with 5,000 CFU LM-gB and given BrdU in their drinking water. At 3 d postinfection, gB-8p cells were isolated and the percentage of BrdU+ cells within the Vβ10+ and Vβ10− fractions were evaluated. (B) LM-gB infected mice were pulsed with 0.8 mg BrdU (i.p.) on day 6 and the percentage of BrdU+ cells was analyzed in splenic Vβ10+ and Vβ10− gB-8p cells on day 7 postinfection. (C) Polyfunctional CD8+ T cells or those that are capable of simultaneously producing IFN-γ, TNF-α, and Granzyme B following gB-8p peptide stimulation were calculated at 7 d postinfection. (D) The MFI of Granzyme B produced individually by Vβ10+ and Vβ10− gB-8p-specific CD8+ T cells is also shown. Data shown in A were pooled from two separate experiments, with n = 3 mice per group per experiment. Data shown in B to D are representative of at least two separate experiments with n = 5–8 mice per group. Results depict mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Our studies provide first direct quantitative evidence that the size and diversity of the naive antigen-specific pool contracts in unprimed mice with advancing age. More importantly, we show that the maintenance of this numerically reduced and constricted repertoire pool is not random, but is rather biased to favor VM cells that exhibit higher TCR avidity and undergo faster rates of homeostatic proliferation. In the gB-8p–specific precursor pool, many Vβ10+ gB-8p precursors exhibit higher TCR avidity for foreign, and also likely for self, (based on higher expression of CD5 and high proliferation upon transfer into lymphopenic hosts) pMHC, which enables them to undergo more homeostatic proliferation. Over time, the progeny of the most homeostatically fit Vβ10+ gB-8p clonotypes dominate the gB-8p pool, whereas many other clones are lost, resulting in a loss of TCR diversity. However, despite this loss in TCR diversity, the preferential responsiveness of Vβ10+ gB-8p cells to LM-gB remains intact, and in fact increases in old mice. Consequently, this form of homeostatic peripheral selection may be able to partially compensate for the age-associated defects by preserving the precursors with the best-fitting TCRs. The increased homeostatic turnover of Vβ10+ gB-8p cells also converts significantly more precursors into VM cells, which are more readily activated than naive phenotype cells. Because of these two mechanisms, Vβ10+ gB-8p precursors preferentially respond to infection with a relatively strong effector function, comparable to that elicited from adult precursors. This finding has the potential to at least partially compensate for the numerical and qualitative T-cell defects that accumulate with aging (5, 6).
Do all Vβ10+ CD8+ T cells exhibit longer lifespans than other Vβ family members? Recent work has shown that the CDR1 and CDR2 domains of TCRV-β elements are “hard-wired” to bind to MHC molecules (31), and it is left up to the CDR3 domain to interfere with this interaction just enough to prevent negative selection but still allow for positive selection. Thus, it is possible that the CDR1 and CDR2 domains of Vβ10 elements are intrinsically more reactive toward MHC than those of the other TCRV-β family members, and it is this interaction between the TCR and the H-2Kb that drives peripheral selection in these animals. However, because the percentage of Vβ10+ cells in the naive gB-8p pool levels out in old age (at least between 18 and 22 mo of age), although the clonal diversity of these cells continues to narrow, clonal competition is likely to operate between both different TCR Vβ families and individual epitope-specific clonotypes containing identical CDR1 and CDR2 domains.
The increase in TCR avidity and bias toward homeostatically fit precursors demonstrates that the strength of the interaction between the TCR and self-pMHC complexes critically shapes the peripheral repertoire in aging. Homeostatic proliferation in early life was postulated to play a beneficiary role by selecting higher-avidity clonotypes that may provide more efficient immune protection (29). This hypothesis and our findings are consistent with the paradoxical decrease in TCR diversity and simultaneous increase in certain autoimmune disease (e.g., rheumatoid arthritis, giant cell arteritis) observed in the elderly. A repertoire dominated by high-avidity VM cells would be more difficult to control by peripheral tolerance mechanisms, and thus more likely to respond to autoantigens. Conversely, clones that fail to undergo homeostatic proliferation will not survive as long and could leave behind “holes” in the naive repertoire of old animals, particularly if most clones specific for an epitope (32) (or even worse, for one pathogen) fall into this category.
In conclusion, our findings indicate that T-cell maintenance during aging is not random and chaotic, but is governed by TCR avidity and homeostatic fitness, which are proofread by self-pMHC complexes. This finding yields the repertoire that may be able to prolong immune defense longer than what would be expected if such selection did not exist. Efforts to elucidate structural TCR features that allow for long T-cell lifespans in the periphery should be of both theoretical and practical value, and the studies of autoimmunity and protective immunity in older adults should benefit from a better understanding of how the peripheral clonal selection process contributes to the immunological abnormalities seen in aged individuals.
Materials and Methods
Mice.
Old (18 and 22 mo) C57BL/6 (B6, H-2b) mice were obtained from the National Institute of Aging breeding colony (Harlan). Adult (2–3 mo) B6 and RAG1−/− mice were purchased from the Jackson Laboratory. Mice were maintained under specific pathogen-free conditions in the animal facility at the University of Arizona and experiments conducted under guidelines and the approval of the Institutional Animal Care and Use Committee of the University of Arizona.
Infections.
Recombinant L. monocytogenes expressing the MHC class I-restricted CTL epitope HSV gB498–505 (SSIEFARL, gB-8p in the text), designated LM-gB was generated by PCR amplifying the promoter and coding regions for gB498-505 peptide and truncated ovalbumin (OVA) from the pAM401 based expression construct (33), subcloning into the temperature sensitive plasmid pKSV7 (34), and electroporation into Lm-OVA (35) and selection for clones with homologous recombination.. Mice were injected intravenously with 5 × 103 colony forming units in 200 μL of PBS.
Reagents.
The gB-8p:Kb tetramer was obtained from the National Institutes of Health Tetramer Core Facility. Antibodies were purchased from eBioscience, BioLegend, or BD Biosciences.
Enrichment of gB-8p CD8+ T cells.
The tetramer-enrichment protocol was adapted from Obar et al. (20) with slight modifications. The spleen, inguinal, cervical, and axillary lymph nodes were harvested from individual mice. An additional collagenase-digestion step was used for isolating cells from mice infected with LM-gB, as this was previously shown to be necessary in antigen-challenged mice (36). In both cases, cells were then resuspended in 1 mL of isolation buffer (PBS with 0.2% NaN3, 0.5% BSA, and 2 mM EDTA) and stained with anti-CD8 (clone 53–6.7), PE- and APC-labeled gB-8p:Kb tetramers and Fc block for 1 h at room temperature. Cells were washed, resuspended in 500 μL of isolation buffer plus 50 μL anti-APC microbeads (Miltenyi Biotec), and slowly rocked for 30 min at 4 °C. Cells were then washed, resuspended in 500 μL of isolation buffer, and passed over a LS magnetic column (Miltenyi Biotec) according to the manufacturer's instructions. The columns were removed from the magnetic field, and bound cells were eluted by pushing 5 mL of isolation buffer through the column with a plunger. The resulting tetramer-enriched fractions were stained with a mixture of flurochrome-labeled antibodies for 30 min at 4 °C that either served as a “dump” gate (anti-CD19, anti-CD4, anti-MHC class II, anti-F4/80) or labeled other markers of interest (anti-CD62L, anti-CD44, anti-CD5, anti-Vβ10, anti-CD11a, anti-Ly6C, anti-CD122). Cells were washed and the entire sample was analyzed with a LSRII cytometer (Beckton Dickinson), with analysis performed using FloJo software (Treestar).
In Vitro Cytokine Stimulation Assay.
The ability of naive gB-8p precursors to produce IFN-γ in response to IL-12 plus IL-18 stimulation was examined exactly as described by Haluszczak et al. (21). Spleen and lymph node cells from adult and old mice were resuspended in 10 mL of RPMI complete media with either IL-2 alone (10 U/mL) or in combination with IL-12 (10 ng/mL) and IL-18 (10 ng/mL) for 18 h. During the last 4 h of culture, 2 μg/mL of brefeldin A was added. At the end of the culture period, gB-8p precursors were isolated via the tetramer enrichment method and stained with anti–IFN-γ according to the intracellular staining kit protocol (eBioscience).
In Vivo Homeostatic Proliferation Assays.
To measure homeostatic proliferation under lymphopenic conditions, untouched CD8+ T cells isolated from individual adult B6 mice (∼5–10 × 106 cells per mouse) were adoptively transferred intravenously into RAG1−/− recipient mice. One month later, gB-8p precursors were isolated from the spleen and lymph nodes via the tetramer enrichment method and assessed by flow cytometry. To measure homeostatic proliferation in naive adult B6 mice, BrdU was delivered to mice in their drinking water at a concentration of 0.8 mg/mL; 1 mo later, gB-8p precursors were isolated from the spleen and lymph nodes via the tetramer enrichment method and stained with anti-BrdU according to the BrdU flow kit protocol (BD Biosciences).
Single-Cell Sorting and RT-PCR.
The gB-8p precursors were isolated from naive mice as described above and sorted as single cells using the FACSAria cell sorter (BD Biosciences). cDNA synthesis, PCR amplification, and sequencing of individual Vβ10 transcripts were performed exactly as previously described (24).
TCRβ Clonotype Analysis.
Each gB-8p–specific CD8+ TCRβ sequence was sequentially aligned against the Vβ10 [TRBV4 in the International Immunogentics Information System (IMGT) nomenclature] gene and then the determined best-match Jβ gene, using the IMGT reference alleles for the Mus musculus TRβ genes (37). The CDR3β sequence was then identified between, and inclusive of, the conserved cysteine in the Vβ-region and the conserved phenylalanine in the Jβ-region.
TCR Avidity Analysis.
Following LM-gB infection, splenocytes were stained with anti-CD8 and gB-8p:Kb tetramer for 1 h at 4 °C. Cells were washed and incubated in the presence of saturating amounts of anti-Kb antibody (AF6) at room temperature to prevent rebinding. At various times, cells were removed, placed in fixation buffer, and the amount of gB-8p:Kb tetramer remaining on the surface was quantified by flow cytometry. These measurements were expressed as a percentage of cells that are tet+ relative to tetramer staining at t = 0. To examine TCR:pMHC interaction kinetics in naive adult gB-8p precursors, an anti-Kb antibody was added after tetramer staining and we considered the high avidity gB-8p precursors to be those still tetramer-bound 15 min later, which represented ∼25% of the initial starting population.
Statistical Analysis.
Normally distributed comparisons were performed using two-tailed unpaired t-test and ANOVA followed by Tukey's posttest comparison. The diversities of the gB-8p–specific CD8+ TCRβ repertoires were evaluated by the number of different TCRβ amino acid sequence clonotypes and Simpson's diversity index (38). To account for the differences in the TCRβ repertoire samples sizes obtained between mice, the diversity of each TCRβ repertoire was estimated as the median value of 10,000 random draws of subsamples of 23 TCRβ sequences from the total TCRβ repertoire obtained for each mouse (38). The diversity analysis was performed using Matlab. The features of the gB-8p–specific CD8+ TCRβ repertoires were compared between each pair of age groups using a Mann–Whitney test, with Bonferroni correction for multiple pairwise comparisons (i.e., each pairwise test was assessed at the significance level of α = 0.05/3 = 0.0167). All statistical analyses were performed using GraphPad Prism software.
Supplementary Material
Acknowledgments
We thank members of the J.N.-Ž. and M.P.D. laboratories for help and stimulating discussion, Paula Campbell for expert sorting assistance, and the National Institutes of Health (NIH) Tetramer Facility at Emory University for proficient tetramer production. This work was supported by NIH Grants AG20719 and AI066096 (to J.N.-Ž.) and also in part by the Australian Research Council (ARC). B.D.R. was supported in part by NIH K99 Award 1K99HD067290-01. V.V. is an ARC Future Fellow, M.P.D. is a National Health and Medical Research Council Senior Research Fellow, and J.N.-Ž. is Bowman Professor of Medical Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107594108/-/DCSupplemental.
References
- 1.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 2.Murasko DM, Jiang J. Response of aged mice to primary virus infections. Immunol Rev. 2005;205:285–296. doi: 10.1111/j.0105-2896.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 3.McElhaney JE. The unmet need in the elderly: Designing new influenza vaccines for older adults. Vaccine. 2005;23(Suppl 1):S10–S25. doi: 10.1016/j.vaccine.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Gardner EM, Gonzalez EW, Nogusa S, Murasko DM. Age-related changes in the immune response to influenza vaccination in a racially diverse, healthy elderly population. Vaccine. 2006;24:1609–1614. doi: 10.1016/j.vaccine.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 5.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maue AC, et al. T-cell immunosenescence: Lessons learned from mouse models of aging. Trends Immunol. 2009;30:301–305. doi: 10.1016/j.it.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messaoudi I, et al. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci USA. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messaoudi I, Guevara Patiño JA, Dyall R, LeMaoult J, Nikolich-Zugich J. Direct link between mhc polymorphism, T cell avidity, and diversity in immune defense. Science. 2002;298:1797–1800. doi: 10.1126/science.1076064. [DOI] [PubMed] [Google Scholar]
- 11.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4(2):123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 12.Montecino-Rodriquez E, Min H, Dorshkind K. Reevaluating current models of thymic involution. Semin Immunol. 2005;17:356–361. doi: 10.1016/j.smim.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11(2):173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 15.Goldrath AW, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J Exp Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha B, von Boehmer H. Peripheral selection of the T cell repertoire. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 17.Surh CD, Sprent J. Homeostatic T cell proliferation: How far can T cells be activated to self-ligands? J Exp Med. 2000;192(4):F9–F14. doi: 10.1084/jem.192.4.f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172(1):40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- 19.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obar JJ, Khanna KM, Lefrançois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haluszczak C, et al. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cose SC, Kelly JM, Carbone FR. Characterization of diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential V beta bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: Evidence for clonal homogenization of the old TCR repertoire. J Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudd BD, et al. Diversity of the CD8+ T cell repertoire elicited against an immunodominant epitope does not depend on the context of infection. J Immunol. 2010;184:2958–2965. doi: 10.4049/jimmunol.0903493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174:3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 26.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzam HS, et al. Fine tuning of TCR signaling by CD5. J Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 28.Chothia C, Boswell DR, Lesk AM. The outline structure of the T-cell alpha beta receptor. EMBO J. 1988;7:3745–3755. doi: 10.1002/j.1460-2075.1988.tb03258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowling MR, Hodgkin PD. Why does the thymus involute? A selection-based hypothesis. Trends Immunol. 2009;30:295–300. doi: 10.1016/j.it.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: Implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 31.Scott-Browne JP, White J, Kappler JW, Gapin L, Marrack P. Germline-encoded amino acids in the alphabeta T-cell receptor control thymic selection. Nature. 2009;458:1043–1046. doi: 10.1038/nature07812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yager EJ, et al. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orr MT, Orgun NN, Wilson CB, Way SS. Cutting edge: Recombinant Listeria monocytogenes expressing a single immune-dominant peptide confers protective immunity to herpes simplex virus-1 infection. J Immunol. 2007;178:4731–4735. doi: 10.4049/jimmunol.178.8.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith K, Youngman P. Use of a new integrational vector to investigate compartment-specific expression of the Bacillus subtilis spoIIM gene. Biochimie. 1992;74:705–711. doi: 10.1016/0300-9084(92)90143-3. [DOI] [PubMed] [Google Scholar]
- 35.Foulds KE, et al. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 36.Jabbari A, Legge KL, Harty JT. T cell conditioning explains early disappearance of the memory CD8 T cell response to infection. J Immunol. 2006;177:3012–3018. doi: 10.4049/jimmunol.177.5.3012. [DOI] [PubMed] [Google Scholar]
- 37.Lefranc MP, et al. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999;27:209–212. doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venturi V, Kedzierska K, Turner SJ, Doherty PC, Davenport MP. Methods for comparing the diversity of samples of the T cell receptor repertoire. J Immunol Methods. 2007;321(1–2):182–195. doi: 10.1016/j.jim.2007.01.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.