Abstract
B-1 B cells have been proposed to be preferentially generated from fetal progenitors, but this view is challenged by studies concluding that B-1 production is sustained throughout adult life. To address this controversy, we compared the efficiency with which hematopoietic stem cells (HSCs) and common lymphoid progenitors (CLPs) from neonates and adults generated B-1 cells in vivo and developed a clonal in vitro assay to quantify B-1 progenitor production from CLPs. Adult HSCs and CLPs generated fewer B-1 cells in vivo compared with their neonatal counterparts, a finding corroborated by the clonal studies that showed that the CLP compartment includes B-1– and B-2–specified subpopulations and that the former cells decrease in number after birth. Together, these data indicate that B-1 lymphopoiesis is not sustained at constant levels throughout life and define a heretofore unappreciated developmental heterogeneity within the CLP compartment.
Keywords: bone marrow, clonal analysis, hematopoiesis
Immune system development has been hypothesized to be a layered process in which lymphoid populations of increased complexity are produced in successive waves in the fetus, neonate, and adult. The initial wave of fetal lymphopoiesis has been proposed to generate lymphocytes involved in innate immunity, whereas waves appearing later produce cells involved in adaptive immune responses (1). A corollary of the layered immune system hypothesis is that, after peaking in the fetus/neonate, the initial wave(s) of lymphopoiesis wanes as the adult wave establishes.
The layered immune system hypothesis arose from studies aimed at defining the origin of two types of B lymphocytes, termed B-1 and B-2 cells (2). B-1 cells are innate effectors distinguished by their preferential localization in serous cavities and an unusual sIgMhigh CD11b+ CD5+ B-1a and sIgMhigh CD11b+ CD5− B-1b phenotype (3, 4). B-1a cells spontaneously secrete IgM natural antibodies, whereas production of Ig by B-1b cells can be induced by antigen exposure, and the latter cells exhibit immunologic memory (5, 6). Human B-1 cells with properties similar to those described in mice have recently been described (7). In contrast, conventional B cells, referred to as B-2 cells, are mediators of adaptive immune responses, predominate in the spleen and lymph nodes, and undergo somatic hypermutation after antigen encounter (8, 9).
Studies showing that cells from fetal liver are more efficient than cells from adult bone marrow at reconstituting B-1 cells in irradiated recipients initially suggested that innate B cells arise from progenitors that appear during a fetal wave of development (10, 11). CD5+ B-1a cells, in particular, were preferentially generated from fetal sources (2, 12, 13). These early findings are supported by more recent studies showing the existence of lineage negative (Lin−) CD45R−/low CD19+ B-1–specified progenitors that arise in the embryonic yolk sac, peak in number in the fetal liver, and then decline in the adult (14–17). Taken together with data showing that selected γδ T cells are produced more efficiently from fetal than adult progenitors (18) and recent reports that distinct types of B and T cells are generated during fetal and adult life in humans (19, 20), a model postulating that the adult immune system consists of lymphocytes, particularly those lymphocytes involved in innate immunity, that have emerged in distinct waves of development has evolved.
However, multiple studies have shown the generation of B-1 cells from adult bone marrow (21). Of particular note are recent reports showing the development of B-1 cells from adult hematopoietic stem cells (HSCs) and common lymphoid progenitors (CLPs) (22–25). The concomitant production of B-1 and B-2 cells from adult CLPs, defined as B lineage-specified progenitors from which surface IgM+ B cells derive (26), also links the development of these two B-cell populations. In view of these observations, it has been suggested that de novo B-1 production is sustained throughout life (22, 25), which challenges the premise that progenitors for B-1 cells are primarily generated during a fetal/neonatal wave of development.
To address this controversy, we compared both the frequency and total number of B-1 cells generated by neonatal and young adult HSCs and CLPs in vivo. In addition, we developed a clonal in vitro assay that allowed B-1 progenitor production from CLPs to be quantified. The results of the in vivo transplantation experiments revealed that the number of B-1 cells generated by adult HSCs and CLPs is reduced compared with their neonatal counterparts. These results were corroborated by the clonal analyses, which showed that the CLP compartment includes B-1– and B-2–specified subpopulations and that the former cells decrease in number between neonatal and adult life. Taken together, these data show that B-1 production is not sustained at constant levels after birth and define an unappreciated developmental heterogeneity in the common lymphoid progenitor population.
Results
Adult HSCs Have Attenuated B-1 Potential.
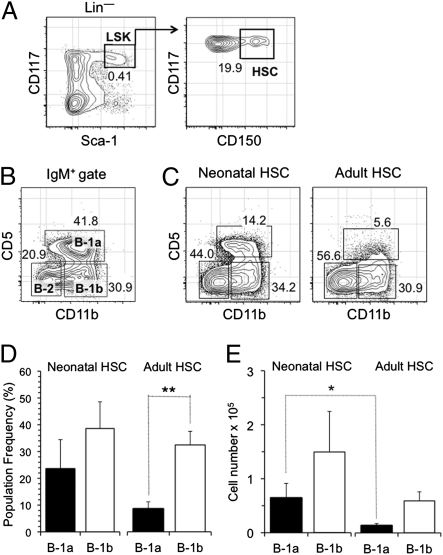
To determine if B-1 potential is sustained at a constant level in postnatal bone marrow, we isolated Lin− CD117 (c-kit)high Sca-1high CD150+ HSCs (27) from the bone marrow of 2.5-wk-old neonatal and 15-wk-old adult mice (Fig. 1A) and transplanted equivalent numbers into CD45.1 Rag2−/− recipients. The production of donor B-1 cells in the peritoneal cavity, which normally includes B-1a, B-1b, and B-2 cells (Fig. 1B), was examined 6 wk later based on preliminary studies indicating that this time was the earliest time that we could detect them in reliable numbers. Because B-1 cells can expand and mask initial input numbers (11, 12, 28), we did not examine reconstitution at later time points.
Fig. 1.
B-1 potential of neonatal and adult HSCs. HSCs (500–1,000) isolated from the bone marrow of neonatal (2.5 wk) and adult (15 wk) mice were injected i.v. in vivo, and the generation of donor (CD45.2) sIgMhigh CD11b+ CD5+ B-1a and sIgMhigh CD11b+ CD5− B-1b cells in the peritoneal cavity was evaluated 6 wk later. (A) Resolution of HSCs in bone marrow based on their Lin– CD117high Sca-1high CD150+ phenotype. The frequency of Lin– Sca-1+ c-kit+ cells in total bone marrow is indicated in Left. The Lin– Sca-1+ c-kit+ cells were also gated according to the phenotype shown on the plot in Right. (B) Distribution of B-1a, B-1b, and B-2 cells in the peritoneal cavity of a young (6 wk) WT B6 mouse. The frequency of the respective populations among total sIgM+ cells is indicated. (C) Representative FACS plots of donor B-1 reconstitution in the peritoneal cavity of recipients of neonatal and adult HSCs. The frequency of the respective populations among total sIgM+ cells is indicated. (D) Frequency of donor B-1a and B-1b cells in the peritoneal cavity of recipient mice 6 wk after reconstitution with neonatal and adult HSCs. (E) Total number of donor B-1a and B-1b cells in the peritoneal cavity of recipient mice 6 wk after reconstitution with neonatal and adult HSCs. Five recipients of neonatal and six recipients of adult HSCs were analyzed. *P ≤ 0.05; **P ≤ 0.01.
In agreement with previous reports indicating that adult HSCs can produce B-1 cells (22, 24, 25), both neonatal and adult HSCs generated B-1 cells in the peritoneal cavity of recipient mice, although the frequency with which adult stem cells produced B-1a cells was lower (Fig. 1 C and D). However, frequency alone does not provide insights into the efficiency with which the neonatal and young adult HSCs generated B-1 progeny. To obtain this information, we also determined the total number of B-1 cells produced. When this determination was done, it was apparent that adult HSCs produced B-1 cells and B-1a cells, in particular, less efficiently (Fig. 1E). Taken together, these data indicate that B-1 potential is not sustained at a constant level after birth.
Adult CLPs Have Attenuated B-1 Potential.
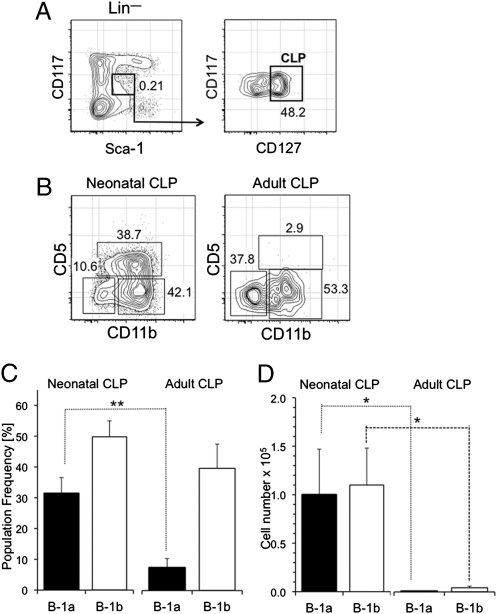
The data showing that adult HSCs exhibit attenuated B-1 potential would suggest that their downstream CLP progeny should do so as well. We thus isolated Lin− CD117low Sca-1low CD127+ CLPs from the bone marrow of 2.5- and 15-wk-old mice (Fig. 2A) and transplanted equivalent numbers into Rag2−/− recipients as described above. The peritoneal cavity of these mice was then examined 6 wk later for donor B-1 cells. As shown in Fig. 2 B and C, neonatal CLPs efficiently generated donor B-1a and B-1b cells, whereas B-1 production by adult CLPs was biased to the generation of CD5− B-1b cells. When the total number of B-1 cells generated by neonatal and adult CLPs is compared, it is evident that adult progenitors exhibit a significant deficiency in B-1 production (Fig. 2D).
Fig. 2.
B-1 potential of neonatal and adult CLPs. CLPs (5 × 103–1 × 104) isolated from the bone marrow of neonatal (2.5 wk) and adult (15 wk) mice were injected i.v. in vivo, and the generation of donor (CD45.2) sIgMhigh CD11b+ CD5+ B-1a and sIgMhigh CD11b+ CD5− B-1b cells in the peritoneal cavity was evaluated 6 wk later. (A) Resolution of CLPs in bone marrow based on their Lin– CD117low Sca-1low CD127 (IL-7R)+ phenotype. The frequency of Lin– Sca-1low c-kitlow cells in total bone marrow is indicated in Left. Those cells were also gated based on expression of CD127 (Right). (B) Representative FACS plots of donor B-1 reconstitution in the peritoneal cavity of recipients of neonatal and adult CLPs. The frequency of the respective populations among total sIgM+ cells is indicated. (C) Frequency of donor B-1a and B-1b cells in the peritoneal cavity of recipient mice 6 wk after reconstitution with neonatal and adult CLPs. (D) Total number of donor B-1a and B-1b cells in the peritoneal cavity of recipient mice 6 wk after reconstitution with neonatal and adult CLPs. Six recipients of neonatal and five recipients of adult CLPs were analyzed. *P ≤ 0.05; **P ≤ 0.01.
Development of a Clonal Assay to Quantify B-1 Progenitors.
The lower number of B-1 cells produced by adult HSCs and CLPs could be because of a decline in the ability of these precursors to generate B-1 progenitors and/or a reduced quality of the progenitors that are produced. Multiple issues such as engraftment efficiency and the ability of mature B-1 cells to self-renew (12, 28) make it difficult to distinguish between these possibilities in vivo. We thus developed a clonal in vitro assay that would allow us to quantify CD45R−/low CD19+ B-1 progenitor production by CLPs from neonatal and young adult bone marrow.
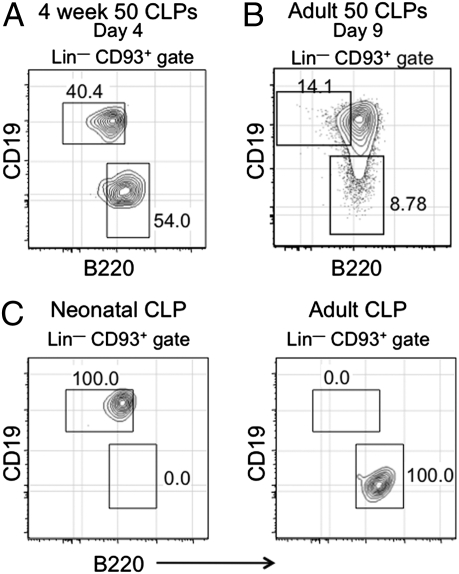
In a first proof of principle step, we isolated CLPs from 4-wk-old mice and seeded pools of 50 cells into wells in a cytokine mixture previously defined to support the growth and differentiation of B-lymphoid progenitors (29). Consistent with data showing that B-1 progenitors are CLP progeny (23), Lin− CD45R−/low CD19+ B-1 progenitors were easily detected in these cultures after 4 d (Fig. 3A). As expected, Lin– CD45R+ CD19– B-2 prepro-B cells, which are known to be CLP progeny (8, 9), were also observed. These data indicate that both B-1 and B-2 progenitors can simultaneously emerge in these cultures and that one type of progenitor does not suppress the growth of the other. To establish that this culture system detects progenitors with normal developmental potential, replicate wells seeded with 50 CLPs were analyzed at 9 d after initiation of the cultures. As shown in Fig. 3B, both CD19+ CD45Rlow/– B-1 and CD19− CD45R+ B-2 progenitors acquired a B220+ CD19+ phenotype.
Fig. 3.
Development of a clonal assay to quantify B-1 progenitor production. (A) Fifty CLPs from bone marrow of 4-wk-old mice were seeded into wells, and 4 d later, cells were harvested and phenotyped. The FACS plot shows that the culture conditions support the development of both B-1 and B-2 progenitors in the same well. The CD45R−/low CD19+ B-1 and CD45R+ CD19low/– B-2 progenitors harvested from the in vitro cultures 5 d later were resolved based on labeling of comparable populations in fresh bone marrow samples shown in Fig. 4C. (B) Replicate wells seeded with 50 CLPs were analyzed at 9 d after initiation of the cultures. CD19+ CD45Rlow/− B-1 progenitors matured into CD19+ CD45Rdim cells, and the CD19– CD45R+ B-2 progenitors acquired a B220+ CD19+ phenotype. (C) Single CLPs from bone marrow of neonatal (2.5 wk) or adult (15 wk) mice were seeded into wells, and the phenotype of the cells was determined 4–6 d later. Values on the plots indicate the frequency of the different progenitor cell populations in the cultures.
We then determined if the cultures would support the differentiation of single CLPs into B-1 and/or B-2 progenitors. We were able to show that this was the case and that CLPs from both neonatal and adult bone marrow could generate B-1 and B-2 progenitors after 4–5 d in culture (Fig. 3C). Over the course of four separate experiments in which over 1,000 individual CLPs were examined, B-1 and B-2 progenitors were never detected together in a single well. Instead, wells contained either B-1 or B-2 progenitors (Fig. 3C). This result indicates that B-1 vs. B-2 fate decisions are already made before the CLP stage of development and reveals an unappreciated developmental heterogeneity within this compartment.
B-1 Progenitor Production Is Attenuated in the Adult.
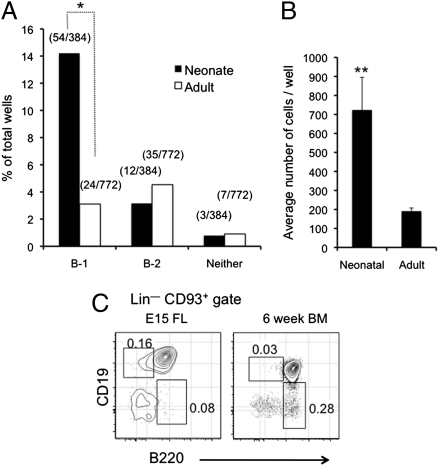
We next used the above clonal assay to quantify the number of B-1 and B-2 progenitors generated from single neonatal and adult CLPs. CLPs from neonatal and adult bone marrow generated B-2 progenitors at similar levels (Fig. 4A), reflecting the fact that B-2 development, which initiates in the fetus, is sustained throughout young adult life. However, this finding was not the case with B-1 progenitors. Phenotypic analysis of cells harvested 4–6 d after initiation of cultures with a single CLP showed a clear differentiation bias of neonatal CLPs to the B-1 lineage; of 384 neonatal CLPs tested, 54 (14%) generated B-1 progenitors (Fig. 4A). In contrast, adult CLPs showed a significant reduction in their capacity to differentiate into B-1 cells; of 772 adult CLPs tested, 24 (3%) produced B-1 progenitors (Fig. 4A).
Fig. 4.
Clonal analysis of CLP developmental potential. (A) Frequency of wells seeded with single neonatal or adult CLPs in which B-1 and B-2 progenitors were observed. The number of positive wells over the number of wells seeded is indicated. (B) Single CLPs from bone marrow of 2.5- and 15-wk-old mice were seeded into wells. The number of B-1 progenitors per well produced from the different aged CLPs was determined 6 d later. (C) Frequency of Lin– CD45R−/low CD19+ B-1 progenitors among Lin– CD93+ cells in E15 fetal liver and bone marrow of 6-wk-old WT mice. *P ≤ 0.05; **P ≤ 0.01.
In addition to the lower efficiency with which they were produced, the quality of the B-1–specified CLPs in the adult was also reduced, because neonatal CLPs generated a higher number of B-1 progenitors per clone compared with their adult counterparts (Fig. 4B). Thus, in addition to the reduced efficiency with which adult CLPs generate B-1 progenitors, the progenitors that are produced exhibit diminished proliferative potential. Together, these declines likely account for the reduction in the frequency of B-1 progenitors in adult compared with fetal bone marrow (Fig. 4C).
Discussion
The results of this study show that B-1 developmental potential is not sustained at a constant level after birth. Instead, the data clearly show that, by young adulthood, the potential of HSCs and CLPs to generate B-1 cells is significantly attenuated. These findings are in agreement with a report by Lam and Stall (30), which showed that fetal B-cell developmental programs, although detectable in postnatal bone marrow, are highly reduced, and a recent study from Ghosn et al. (16), which reported that B-1 progenitors, phenotypically defined as described herein, are present at high numbers in neonatal spleen and significantly decline in number in the adult. This reduced B-1 potential of adult HSCs and CLPs is not part of the general decline in B lymphopoiesis that accompanies aging (31–33); B-2 development was normal in the adult 15-wk-old mice analyzed, and age-related reductions in B lymphopoiesis are not observed at that age in the strains that we used (32).
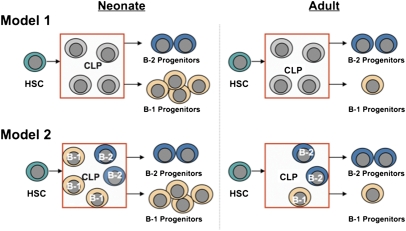
In addition to providing quantitative data showing that B-1 progenitor production is significantly attenuated in the adult, the in vitro clonal assay system allowed us to distinguish between two alternative explanations for this occurrence (Fig. 5). One possibility was that CLPs are bipotential cells that can generate both B-1 and B-2 progenitors, but their B-1 potential declines after neonatal life (Fig. 5, model 1). Alternatively, the CLP compartment could have included both B-1– and B-2–specified progenitors, with the number of the former cells declining in the young adult (Fig. 5, model 2). The data support the latter view. Regardless of age, an individual CLP generated either B-1 or B-2 progenitors, indicating that B-1 vs. B-2 fate decisions are made before the CLP stage of development, thus providing additional support for the hypothesis that B-1 and B-2 cells represent distinct lineages (28).
Fig. 5.
Two models to explain the attenuated potential of adult CLPs to generate B-1 progenitors. In model 1, both neonatal and adult HSCs generate CLPs that can differentiate into B-1 or B-2 progenitors, but B-1 potential declines within the first few weeks after birth. In model 2, neonatal and adult HSCs generate B-1– or B-2–specified CLPs, with the production of the B-1–specified CLPs predominating during neonatal life and declining in the adult.
To determine whether B-1– and B-2–specified HSCs or CLPs could be isolated by phenotype, we examined specific cell surface markers expressed on these populations. Fetal/neonatal HSCs express CD11b (34). In view of the preferential generation of B-1 progenitors from young stem cells, we tested the possibility that CD11b+ HSCs would generate B-1 cells more efficiently than CD11b− HSCs. However, this was not the case (Fig. S1). We also examined the CLP compartment for determinants that would segregate B-1–specified CLPs, like CD138 (17), CD11b (Mac-1) (35), and MHC class II antigens (17, 36). However, neither CD138 nor CD11b selectively identified B-1– or B-2–specified CLPs (Figs. S2 and S3), and MHC class II antigens were not expressed on CLPs (Fig. S4). The stage of development at which B-1 vs. B-2 commitment takes place also remains undetermined. Attempts to determine whether B-1 and B-2 biased HSCs existed were made using the clonal assay system, but these efforts were not successful.
In addition to the reduced production of B-1 progenitors in the adult, the number of B-1 progenitors produced by a single neonatal CLP was higher than the number generated from a single adult CLP. This observation is consistent with reports that neonatal HSCs proliferate more vigorously than their adult counterparts (37, 38). Thus, the reduced ability of adult bone marrow to generate B-1 cells together with the reduced proliferative potential of the progenitors that are produced likely combine and account for the attenuated B-1 potential of young adult bone marrow stem and progenitor cells.
The degree to which B-1 production occurs in the adult has remained a lingering issue. Our data are in agreement with multiple studies showing that adult bone marrow stem and progenitor cells can produce B-1 cells. However, they also clearly show that, when production efficiency is taken into consideration, B-1 lymphopoiesis declines significantly by young adulthood. These results, combined with our previous data showing that the production of B-1 progenitors is most robust in the fetus (14), are consistent with the tenets of the layered immune system hypothesis (1).
Experimental Procedures
Mice.
C57BL/6J (CD45.2) mice were obtained from Jackson Laboratory. 129S6/SvEvTac-Rag2tm1Fwa (Rag2−/−; CD45.1) mice were obtained from Taconic Farms. Mice were housed in the Division of Laboratory Animal Medicine, and experiments were conducted according to University of California at Los Angeles Institutional Animal Care and Use Committee guidelines.
Transplantation.
Donor CD45.2 HSCs (500–1,000) or CLPs (5 × 103–5 × 104) isolated from the bone marrow of 2.5-wk-old neonates and 15-wk-old young adults were injected i.v. into 4- to 8-wk-old CD45.1 Rag2-−/− mice (39) that were preconditioned with 500 R from a 137Cs irradiator (120 R/min, Mark I-68A; JL Shepperd and Associates) 24 h earlier. Donor (CD45.2) levels of reconstitution in the peritoneal cavity of recipients were assessed at 6 wk posttransplantation.
Flow Cytometry.
Isolation and analysis of HSCs, CLPs, B-1 and B-2 progenitors from bone marrow, and B-1a, B-1b, and B-2 cells from the peritoneal cavity were performed on a FACSaria or LSRII (Becton Dickenson), respectively, using previously described protocols (14, 40). The MHC class II antibody (clone AF6-120.1) was obtained from eBiosciences.
Cell Culture and Clonal Analysis.
Single CLPs from the bone marrow of 2.5- or 15-wk-old mice were deposited into wells of 96-well microtiter plates by the automatic cell deposition unit on a FACSaria (Becton-Dickinson). Cells were cultured in RPMI 1640, 10% FCS, 5 × 10−5 M 2-β-mercaptoethanol, 1 mM l-glutamine, 100 U/mL streptomycin, 100 μg/mL penicillin, 50 μg/mL gentamycin, 20 ng/mL IL-3, 20 ng/mL IL-6, 20 ng/mL Stem Cell Factor (SCF) 20 ng/mL Flt-3 ligand, and 20 ng/mL IL-7 (Biosource International) for 4–6 d in a humidified incubator at 37 °C and 5% CO2/air. Cells were then harvested and tested for their B-1 and B-2 progenitor phenotype by immunostaining (14) and analysis with the high-throughput sampler 96-well plate adapter for the BD LSR II flow cytometer. Only wells in which 20 or more cells had been generated were considered for analysis.
Statistics.
Data are expressed as mean ± SEM. Differences between groups were tested by two-tailed, unpaired Student t tests (α = 0.05). Comparisons of absolute cell numbers were tested by one-tailed, unpaired Student t tests (α = 0.05).
Supplementary Material
Acknowledgments
We thank Jesse Oakes for assistance in single cell sorting. This work was supported by National Institutes of Health Grant AI021256 (to K.D.). The University of California at Los Angeles Flow Cytometry Core is supported by National Cancer Institute Grant CA16042.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107172108/-/DCSupplemental.
References
- 1.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 2.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–2754. doi: 10.4049/jimmunol.177.5.2749. [DOI] [PubMed] [Google Scholar]
- 4.Herzenberg LA, Tung JW. B cell lineages: Documented at last! Nat Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- 5.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 6.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70- J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Hardy RR, Hayakawa K. CD5 B cells, a fetal B cell lineage. Adv Immunol. 1994;55:297–339. doi: 10.1016/s0065-2776(08)60512-x. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol. 1995;7:55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 13.Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lièvre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- 14.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikuta K, et al. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- 19.Sanz E, et al. Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proc Natl Acad Sci USA. 2010;107:5925–5930. doi: 10.1073/pnas.0907942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi K, Kondo M. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci USA. 2006;103:17852–17857. doi: 10.1073/pnas.0603368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inlay MA, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Düber S, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 26.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 27.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 29.Montecino-Rodriguez E, Dorshkind K. Stromal cell-dependent growth of B-1 B cell progenitors in the absence of direct contact. Nat Protoc. 2006;1:1140–1144. doi: 10.1038/nprot.2006.163. [DOI] [PubMed] [Google Scholar]
- 30.Lam KP, Stall AM. Major histocompatibility complex class II expression distinguishes two distinct B cell developmental pathways during ontogeny. J Exp Med. 1994;180:507–516. doi: 10.1084/jem.180.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Put E, Sherwood EM, Blomberg BB, Riley RL. Aged mice exhibit distinct B cell precursor phenotypes differing in activation, proliferation and apoptosis. Exp Gerontol. 2003;38:1137–1147. doi: 10.1016/j.exger.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Miller JP, Allman D. The decline in B lymphopoiesis in aged mice reflects loss of very early B-lineage precursors. J Immunol. 2003;171:2326–2330. doi: 10.4049/jimmunol.171.5.2326. [DOI] [PubMed] [Google Scholar]
- 33.Min H, Montecino-Rodriguez E, Dorshkind K. Effects of aging on early B- and T-cell development. Immunol Rev. 2005;205:7–17. doi: 10.1111/j.0105-2896.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- 34.Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci USA. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghosn EE, Yang Y, Tung J, Herzenberg LA, Herzenberg LA. CD11b expression distinguishes sequential stages of peritoneal B-1 development. Proc Natl Acad Sci USA. 2008;105:5195–5200. doi: 10.1073/pnas.0712350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hayakawa K, Tarlinton D, Hardy RR. Absence of MHC class II expression distinguishes fetal from adult B lymphopoiesis in mice. J Immunol. 1994;152:4801–4807. [PubMed] [Google Scholar]
- 37.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bowie MB, et al. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci USA. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Signer RAJ, Montecino-Rodriguez E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16Ink4a and Arf. Genes Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.