Abstract
The goal of the present studies was to investigate the role of changes in hydrogen sulfide (H2S) homeostasis in the pathogenesis of hyperglycemic endothelial dysfunction. Exposure of bEnd3 microvascular endothelial cells to elevated extracellular glucose (in vitro “hyperglycemia”) induced the mitochondrial formation of reactive oxygen species (ROS), which resulted in an increased consumption of endogenous and exogenous H2S. Replacement of H2S or overexpression of the H2S-producing enzyme cystathionine-γ-lyase (CSE) attenuated the hyperglycemia-induced enhancement of ROS formation, attenuated nuclear DNA injury, reduced the activation of the nuclear enzyme poly(ADP-ribose) polymerase, and improved cellular viability. In vitro hyperglycemia resulted in a switch from oxidative phosphorylation to glycolysis, an effect that was partially corrected by H2S supplementation. Exposure of isolated vascular rings to high glucose in vitro induced an impairment of endothelium-dependent relaxations, which was prevented by CSE overexpression or H2S supplementation. siRNA silencing of CSE exacerbated ROS production in hyperglycemic endothelial cells. Vascular rings from CSE−/− mice exhibited an accelerated impairment of endothelium-dependent relaxations in response to in vitro hyperglycemia, compared with wild-type controls. Streptozotocin-induced diabetes in rats resulted in a decrease in the circulating level of H2S; replacement of H2S protected from the development of endothelial dysfunction ex vivo. In conclusion, endogenously produced H2S protects against the development of hyperglycemia-induced endothelial dysfunction. We hypothesize that, in hyperglycemic endothelial cells, mitochondrial ROS production and increased H2S catabolism form a positive feed-forward cycle. H2S replacement protects against these alterations, resulting in reduced ROS formation, improved endothelial metabolic state, and maintenance of normal endothelial function.
Hydrogen sulfide (H2S) is an endogenously produced labile diffusible mediator with multiple roles in the cardiovascular system in health and disease (1–4). Cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase are key enzymes involved in its production of H2S (3–6). CSE is primarily responsible for most of the H2S production in the vasculature (2, 3). The roles of H2S in the cardiovascular system include vasodilatation (7, 8) and stimulation of angiogenesis (9, 10).
Endothelial dysfunction (the inability of the vascular endothelial cells to produce vasorelaxant mediators such as nitric oxide) plays a key role in the pathogenesis of various diabetic complications (11, 12). Overproduction of mitochondrial reactive oxygen species (ROS) is a principal contributor to the pathogenesis of hyperglycemic endothelial dysfunction (11–14). Because H2S production and degradation is a dynamic process in biological systems, and ROS can enhance the degradation of H2S (15–17), here we tested whether hyperglycemia results in a H2S-deficient state and examined whether H2S replacement affects the development of hyperglycemic endothelial dysfunction in vitro and in vivo.
Results
In Vitro Hyperglycemia Is Associated with Increased H2S Degradation Caused by Mitochondrial ROS Overproduction.
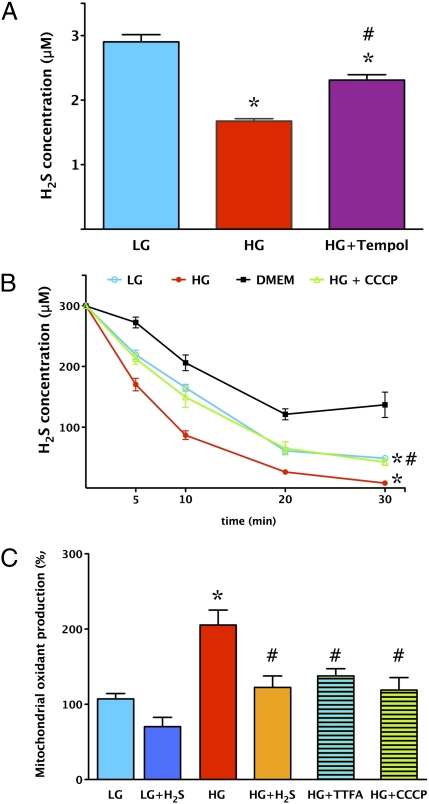
Exposure of endothelial cells to elevated glucose for 7 d resulted in a significant suppression of the H2S concentration in the supernatant (Fig. 1A). Incubation of the cells with the ROS scavenger Tempol increased the H2S concentration in the medium (Fig. 1A), suggesting that the reduced H2S level in hyperglycemia may be attributable to increased consumption of H2S by ROS. Administration of H2S to culture medium (without cells) resulted in a decline of ambient H2S concentrations because of the reaction of H2S with oxygen and culture media constituents. The consumption of H2S was increased in the presence of normoglycemic cells and was further accelerated in the presence of hyperglycemic cells (Fig. 1B). Addition of the mitochondrial uncoupling agent carbonyl cyanide 3-chlorophenylhydrazone (CCCP) to hyperglycemic cells resulted in a slower consumption rate of H2S (Fig. 1B), consistent with the hypothesis that mitochondrially derived ROS production contributes to the increase in H2S consumption in hyperglycemic cells. The increased mitochondrial ROS production in hyperglycemic cells was demonstrated by the redox-sensitive dye MitoSOX red; ROS production was reduced by the uncoupling agents CCCP and thenoyltrifluoroacetone (TTFA) (Fig. 1C), confirming previous studies (11–14) showing that mitochondria represent a major source of ROS.
Fig. 1.
H2S levels are decreased in endothelial cells placed in high extracellular glucose because of enhanced consumption by mitochondrially derived reactive species. (A) Extracellular concentration of H2S in endothelial cells cultured in low (5.5 mM, LG) or high (40 mM, HG) glucose was determined by the amperometric H2S sensor method at 7 d. H2S levels in high glucose were significantly suppressed (*P < 0.05 compared with low glucose), an effect that was attenuated by the antioxidant Tempol (100 μM) (#P < 0.05; n = 5). (B) The rate of consumption of exogenous H2S was measured by the amperometric H2S sensor. Cells were incubated for 7 d in low and high glucose, respectively, at which point a single concentration of H2S (300 mM) was added to the medium. There was a significant (*P < 0.05) increase in the rate of decline in H2S concentrations in cells incubated with high glucose, an effect that was attenuated (#P < 0.05) by treatment of the hyperglycemic cells with the uncoupling agent CCCP (0.5 μM) (n = 5). (C) MitoSOX red oxidation was increased in cells exposed to high glucose (*P < 0.05), and this effect was significantly inhibited by H2S (300 μM) (#P < 0.05) or by the uncoupling agents TTFA (10 μM) and CCCP (0.5 μM) (#P < 0.05; n = 5).
H2S Replacement Exerts Cytoprotective Effects in Hyperglycemic Endothelial Cells in Vitro.
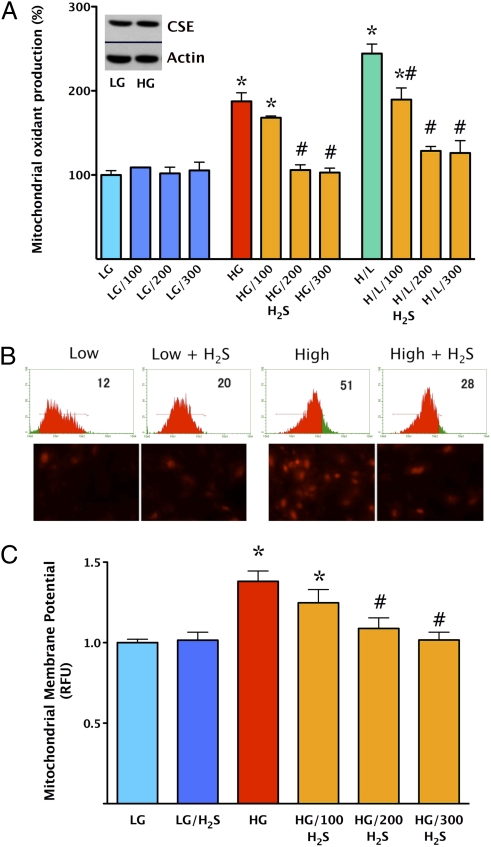
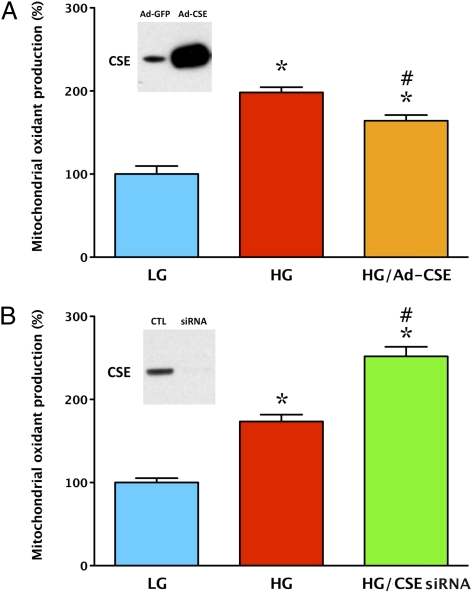
Addition of H2S (100–300 μΜ) for the last 4 d of the 7 d of the hyperglycemic period provided a concentration-dependent protection against cellular ROS production (Fig. 2 A and B) and attenuated mitochondrial membrane depolarization as measured by the fluoroprobe 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzamidazol-carboncyanine (JC-1) (Fig. 2C). Exposure of the cells to intermittent high-/low-glucose conditions is known to exacerbate hyperglycemic endothelial dysfunction (18–20). Accordingly, intermittent high/low glucose induced a more pronounced increase in ROS production compared with a steady elevation of glucose, and H2S continued to attenuate this response (Fig. 2A). H2S administration also reduced DNA injury (Fig. S1A) and the activation of the nuclear enzyme poly(ADP-ribose) polymerase (PARP) (Fig. S1B), which are known downstream consequences of mitochondrial ROS formation in hyperglycemic endothelial cells (11–14). Overexpression of CSE in endothelial cells elevated extracellular H2S levels in normoglycemic cells (by 32 ± 5%), but only to a smaller degree in hyperglycemic cells (by 10 ± 3%), consistently with the increased consumption of H2S during hyperglycemia, as demonstrated above. Overexpression of CSE attenuated the hyperglycemia-induced increase in ROS production (Fig. 3A). Both pharmacological replacement of H2S and overexpression of CSE protected against the hyperglycemia-induced decline in cellular viability. For instance, hyperglycemia decreased cell viability by 18 ± 2% (P < 0.05), whereas in the endothelial cells overexpressing CSE, cell viability increased by 6 ± 3%, compared with normoglycemic controls (n = 4).
Fig. 2.
Replacement of H2S normalizes mitochondrial oxidant production and protects against mitochondrial depolarization in endothelial cells placed in high extracellular glucose. Mitochondrial ROS production was measured in low (5.5 mM, LG) or high (40 mM, HG) glucose conditions at 7 d by using the MitoSOX red method. A shows the responses to H2S (100–300 μM) in no glucose, high glucose, and alternating high/low extracellular glucose conditions (H/L). H2S afforded a concentration-dependent and significant suppression of MitoSOX oxidation (#P < 0.05). The Western blot Inset in A shows that the expression of the H2S-generating enzyme CSE was not suppressed by high glucose. B shows representative flow cytometric and fluorescent microscopic images for the four respective groups (low and high glucose with and without 300 μM H2S). C shows the oxidation of JC-1, a dye used to detect mitochondrial membrane depolarization. In cells placed in high glucose, there was an increase in mitochondrial depolarization (*P < 0.05), which was attenuated by H2S (P < 0.05) (n = 5).
Fig. 3.
Overexpression of CSE attenuates mitochondrial oxidant production in endothelial cells placed in high extracellular glucose, whereas silencing of CSE exacerbates this response. Mitochondrial ROS production was measured in low (LG) or high (HG) glucose at 7 d by using the MitoSOX red method. In response to elevated extracellular glucose, an increase in MitoSOX red oxidation was observed (*P < 0.05), which was attenuated when cells were overexpressing CSE (#P < 0.05; A) and enhanced when CSE was silenced with siRNA (#P < 0.05; B) (n = 4). The Western blot Insets confirm efficient CSE overexpression and knockdown, respectively.
Mechanisms of the Cytoprotective Effect of H2S in Hyperglycemic Endothelial Cells.
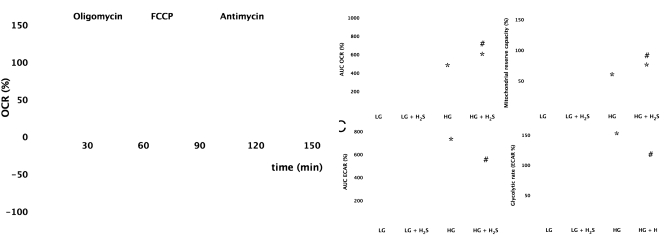
Analysis of the cellular metabolic status of the cells showed that high glucose induces a shift away from the mitochondrial oxidative phosphorylation toward glycolysis: the basal respiratory capacity and the respiratory reserve capacity response to carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) were reduced in hyperglycemic cells, compared with the normoglycemic cells (Fig. 4). Treatment of the cells with H2S resulted in an improvement of mitochondrial respiration, whereas the hyperglycemia-induced increase in the glycolytic activity of the cells was normalized (Fig. 4). There were no marked alterations in the cellular ATP levels in hyperglycemic cells, likely because of the compensatory effect of increased glycolysis (ATP levels decreased to 88 ± 1% in hyperglycemic endothelial cells vs. normoglycemic endothelial cells; n = 3, P < 0.05). H2S prevented the hyperglycemia-induced suppression of cellular ATP levels (104 ± 2% of control normoglycemic cells, n = 3). Thus, H2S treatment produced a partial reversal of the hyperglycemia-induced metabolic switch and normalized the energetic status of the cells. As opposed to the effects of H2S administration for 4 d (see above), a short period of H2S administration (the last 60 min of the hyperglycemic period) failed to affect the mitochondrial ROS overproduction (Fig. S2). Thus, although high concentrations of H2S are known to inhibit mitochondrial cytochrome c oxidase, resulting in a suppression of mitochondrial oxidative phosphorylation (21–23), the present effects of H2S in decreasing mitochondrial ROS production in hyperglycemic endothelial cells are not mediated by an acute suppression of mitochondrial function.
Fig. 4.
H2S reduces the degree of the bioenergetic derangements in endothelial cells placed in high extracellular glucose. Bioenergetic analysis of the cells placed in low (LG) or high (HG) glucose for 7 d in the absence or presence of H2S treatment (300 μM) was performed by using the Seahorse Biosciences XF24 Analyzer system. In A, a time course for measurement of oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) is shown under basal conditions, followed by the sequential addition of oligomycin (1 μg/mL), FCCP (0.3 μM), and antimycin A (2 μg/mL). B shows oxygen consumption rate and mitochondrial reserve capacity, and C shows extracellular acidification rate and glycolytic rate values, representing oxidative phosphorylation and glycolysis, respectively. Hyperglycemia induced a suppression of oxidative phosphorylation and an increase in glycolysis (*P < 0.05), and H2S attenuated these changes [#P < 0.05; n = 15 wells (mean ± SEM) from n = 3 experiments performed on different experimental days].
The expression of CSE (a principal H2S-producing enzyme in vascular tissues) was not affected by exposure of the endothelial cells to elevated glucose for 7 d (Fig. 2A). Furthermore, the effects of H2S in hyperglycemic endothelial cells did not depend on the activation of the ATP-sensitive potassium (KATP) channel because the KATP channel blocker glibenclamide failed to reverse the protective effect of H2S (Fig. S3).
H2S Replacement Therapy Improves Endothelial Function in Hyperglycemic Endothelial Cells and in Diabetic Rats.
Overexpression of CSE in rat aortic rings produced a significant protection against the development of endothelial dysfunction induced by elevated extracellular glucose (Fig. 5). Pharmacological supplementation of H2S yielded a similar protective effect (Fig. S4). Streptozotocin-diabetic rats exhibited a decrease in their blood H2S concentrations (Fig. 6A) without any change in the tissue expression of CSE or CBS (Fig. S5). H2S replacement therapy did not affect circulating glucose levels in the diabetic animals (Fig. 6B) but protected against the development of diabetes-induced endothelial dysfunction ex vivo (Fig. 6C).
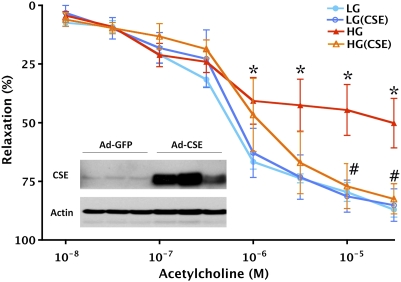
Fig. 5.
CSE overexpression protects against the development of endothelial dysfunction in thoracic aortic rings placed in elevated extracellular glucose. Rat aortic rings were incubated in low (LG) or high (HG) glucose for 48 h. High glucose suppressed endothelium-dependent relaxant responses (*P < 0.05), an effect that was attenuated in rings overexpressing CSE (#P < 0.05; n = 4). (Inset) Representative Western blots for CSE in rings exposed to adenovirus expressing GFP or CSE, respectively.
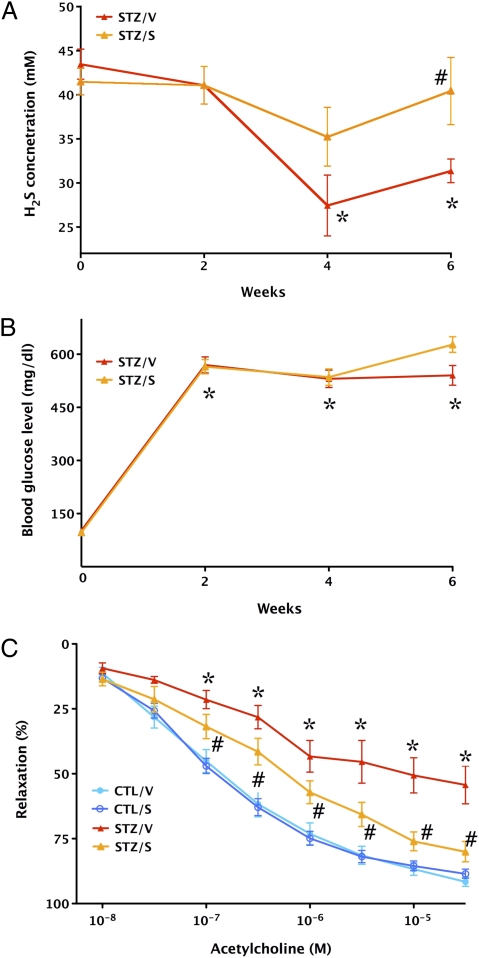
Fig. 6.
Improvement of endothelial function by H2S in diabetic rats ex vivo. (A) Streptozotocin-diabetic vehicle-treated rats (STZ/V) exhibit reduced blood H2S levels (*P < 0.05), an effect that is normalized by supplementation of H2S using the H2S-releasing minipumps (STZ/S) (#P < 0.05). (B) The hyperglycemic response is unaffected by H2S-releasing minipumps: *P < 0.05 shows significant and comparable degree of hyperglycemia in STZ rats treated with vehicle or H2S-releasing pumps. (C) The thoracic aortas of streptozotocin-diabetic rats (STZ/V) exhibit reduced endothelium-dependent relaxant function in response to acetylcholine (1 nM to 30 μM) (*P < 0.05); supplementation of H2S using the H2S-releasing minipumps (STZ/S) attenuated the degree of this endothelial dysfunction (#P < 0.05; n = 4–6).
Absence of CSE Exacerbates Hyperglycemic Endothelial Cell Dysfunction.
In endothelial cells where endogenous H2S production was suppressed by siRNA knockdown of CSE, high glucose induced a more pronounced degree of ROS production than in the corresponding control cells (Fig. 3B). Moreover, in thoracic aortic rings from CSE−/− mice, incubation in extracellular glucose for 24 h caused a more pronounced impairment of endothelium-dependent relaxations than in corresponding rings from wild-type mice (Fig. 7). These results point to the protective role of endogenously produced H2S against hyperglycemic endothelial dysfunction.
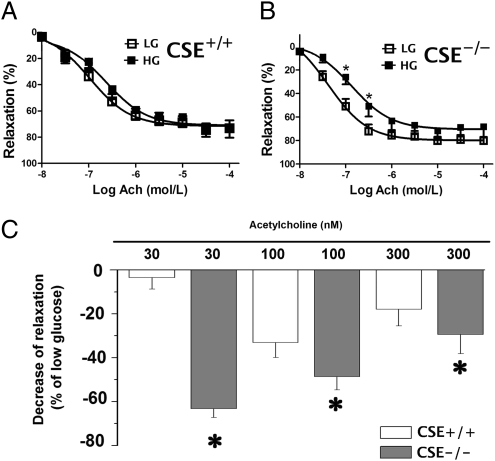
Fig. 7.
CSE deficiency exacerbates the development of endothelial dysfunction in thoracic aortic rings placed in elevated extracellular glucose. ACh-induced relaxations in aortic rings from wild-type (CSE+/+; A) or CSE−/−(B) mice placed in low (LG) or high (HG) glucose for 24 h. *P < 0.05 shows significant inhibition of relaxations in CSE−/− rings. C shows a comparison of the percentage decrease of the relaxation by high glucose between CSE+/+ and CSE−/− mice; *P < 0.05 shows a higher degree of impairment of the relaxations in the CSE−/− rings than in wild-type rings (n = 6–12).
Discussion
The formation of ROS from endothelial cells is a key factor in the pathogenesis of diabetic complications (11, 12). In addition, increased ROS formation and endothelial dysfunction has been linked to various forms of critical illness, to postoperative conditions, as well as to impaired glucose tolerance conditions and postprandial hyperglycemia (11, 12, 24–31). Mitochondrial electron transport is recognized as a key source of ROS in hyperglycemic endothelial cells (11, 12). ROS, on their own and by combining with endothelial nitric oxide to form the reactive oxidant peroxynitrite, can induce DNA damage and activation of suicidal pathways governed by the nuclear enzyme PARP (32).
The biosynthesis of H2S, as well as the biological degradation (consumption) of H2S, is a dynamic process (3, 5, 15). The current results point to the existence of a crucial interplay between endothelial H2S formation and ROS production in maintaining mitochondrial function: elevated glucose perturbs this balance. Our data demonstrate that the consumption of H2S is accelerated in endothelial cells placed in elevated glucose, an effect that depends on mitochondrial ROS formation (because it can be attenuated by mitochondrial uncoupling). It is conceivable that this accelerated H2S consumption is responsible for the lower baseline levels of H2S detected in the medium of cells placed in elevated extracellular glucose and for the decreased H2S levels measured in the circulation of streptozotocin-diabetic rats. On the other hand, down-regulation of CSE does not occur in hyperglycemia and diabetes under our experimental conditions and, therefore, is not responsible for the reduced H2S levels.
H2S, as a reducing agent and an antioxidant molecule, has been previously shown to protect various cell types from oxidative injury (33–36). Based on the current results, we hypothesize that H2S provides a reducing/antioxidant intracellular environment that contributes to the maintenance of normal mitochondrial function. This balance is perturbed when mitochondrial ROS production is stimulated by high concentrations of glucose. We hypothesize that the ROS from hyperglycemic mitochondria directly reacts with and consumes the intracellular H2S, which then creates additional mitochondrial dysfunction, possibly by oxidative modification to mitochondrial proteins. Such a positive feed-forward cycle may then culminate in a dysfunctional mitochondrial state where molecular oxygen is used to produce ROS (as opposed to ATP) and where mitochondrial efficacy is diminished. These events will lead to a loss of mitochondrial membrane potential and, finally, a spillage of ROS to the cytosolic and nuclear compartments.
Previous studies have demonstrated that antioxidant depletion is a hallmark of hyperglycemia in endothelial cells (37–39). It has also been demonstrated previously that endothelial ROS overproduction leads to oxidative and nitrosative protein modifications, DNA injury, and activation of secondary deleterious cellular cycles of injury, such as the one governed by the activation of PARP (11–14, 18–20, 32). The beneficial effects of antioxidants on the endothelial function in hyperglycemia may be, at least in part, related to the preservation of the endothelial H2S homeostasis.
The current bioenergetic findings, in agreement with a recent analysis of bioenergetic alterations in rat retinal endothelial cells placed in high extracellular glucose (40), demonstrate that bEnd3 endothelial cells placed in high extracellular glucose exhibit a reduced oxygen consumption rate (i.e., reduced mitochondrial oxidative phosphorylation), an effect that is partially counterbalanced by an up-regulation of glycolysis. Our results also demonstrate that H2S replacement therapy protects against this pathophysiological switch between oxidative phosphorylation and glycolysis. We conclude that restoration of oxidative phosphorylation, coupled with an improvement of cellular ATP levels, mitochondrial depolarization, and mitochondrial ROS production are the key intracellular events through which H2S replacement is able to restore normal cellular function in hyperglycemic endothelial cells and prevent the development of endothelial dysfunction.
The results of the current study demonstrate that replacement of the H2S, either by supplementation into the culture medium or by overexpression of the H2S-producing enzyme CSE, is able to protect from the deleterious consequences of hyperglycemia. On the other hand, siRNA silencing of CSE, or the deletion of the CSE gene, results in conditions where hyperglycemia induces an exacerbated endothelial response (more ROS production and more severe loss of endothelium-dependent relaxant function), consistent with the hypothesis that endogenous H2S plays a protective role against the deleterious consequences of hyperglycemia in endothelial cells.
Our in vivo/ex vivo observations, showing that supplementation of H2S to streptozotocin-diabetic rats improves the endothelium-dependent relaxant function of vascular rings, are consistent with our in vitro findings in endothelial cells. Also, previous studies have demonstrated that pharmacological interventions (e.g., ROS scavenging, peroxynitrite neutralization, PARP inhibition) that protect hyperglycemic endothelial cells are also able to improve endothelium-dependent relaxations in diabetic rodents (11, 12, 29–32), and, therefore, prevention of the activation of these downstream pathways is likely to contribute to the effects of H2S in the current experimental system. The translational value of the current findings is enhanced by the observation that circulating H2S levels are lowered not only in streptozotocin-diabetic and in NOD mice (a model of type 1 diabetes) but also in patients with diabetes (41–43). We conclude that hyperglycemia produces a H2S-deficient state in endothelial cells: H2S replacement therapy in hyperglycemic conditions may be of therapeutic potential.
Methods
Cell Culture.
The bEnd3 microvascular endothelial cell line was purchased from the American Type Culture Collection, cultured at 37 °C at 5% CO2, in a humidified chamber, with 5.5. mM glucose containing DMEM with 10% FBS, 2 mM glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 1% nonessential amino acids. Cells were plated in six-well plates at 1 × 105 cells per well in a final volume of 2 mL with 5.5 mM DMEM. On the following day, cells were placed in DMEM containing 5.5 mM (low-glucose control) or 40 mM glucose (high glucose). The culture medium was changed every day. After 3 d, 100–300 μM NaHS was added to the wells and reapplied every 8 h until the completion of the experiments. Treatment with 10 μM TTFA, 0.5 μM CCCP, or 10 μM glibenclamide was applied for 3 d. Cells were incubated at 37 °C at 5% CO2, in a humidified chamber for 7 d, followed by analysis. In another experimental series, the effect of 100–300 μM NaHS was tested on ROS production induced by alternating (12-h cycle) high-/low-glucose conditions. To overexpress CSE in endothelial cells, cells were transfected with an adenoviral plasmid as previously described (44). For CSE silencing, Silencer Select siRNA for CSE and nonsense control siRNA were obtained from Ambion and were transfected with Lipofectamine 2000.
Measurement of H2S Levels.
Amperometric H2S sensors (WPI) were used for the real-time measurement of dissolved H2S concentration in the medium (15). The amperometric H2S sensor was calibrated before each experiment with freshly prepared (anoxic) NaHS stock solution (0–300 μM).
Measurement of Mitochondrial ROS Production.
MitoSOX red (Invitrogen), a mitochondrion-specific hydroethidine-derivative fluorescent dye, was used to assess mitochondrial O2− production in situ (45).
Western Blotting.
Whole-cell lysate were made by using RIPA buffer with EDTA with a protein protease inhibitor mixture. Equal amounts of protein lysate were separated with 8–12% SDS/PAGE gels, transferred to a 0.45-μm nitrocellulose. The membrane was blocked with 5% low-fat milk in PBS or Tris-buffered saline containing 0.05% Tween-20 and incubated with the primary antibody overnight at 4 °C. Primary antibodies for CSE, CBS, and PARP were from Santa Cruz Biotechnology, and for actin, from Sigma.
Measurement of Membrane Potential by the Fluorescent Dye JC-1.
We assessed mitochondrial membrane potential by using JC-1 as described (39).
Quantitation of DNA Strand Breaks.
DNA strand breaks were detected with a single-cell gel electrophoresis assay (14). DNA strand breaks were quantitated by examining the fixed and stained cells under a fluorescence microscope. The mean length of the DNA tail was determined by measuring 20 cells for each condition.
Cell Viability Assay.
The mitochondrial-dependent reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was used to measure mitochondrial respiration, as an indicator of cell viability (46).
Bioenergetic Analysis.
The XF24 Analyzer (Seahorse Biosciences) was used to measure bioenergetic function in intact bEnd3 cells. The XF24 creates a transient 7-μL chamber in specialized microplates that allows for oxygen consumption rate and extracellular acidification rate or proton production rate to be monitored in real time (40, 47). To measure indices of mitochondrial function, oligomycin, FCCP, and antimycin A were injected sequentially at the final concentrations of 1 μg/mL, 0.3 μM, and 2 μg/mL, respectively. Using these agents, we determined the basal level of oxygen consumption, the amount of oxygen consumption linked to ATP production, the level of non-ATP–linked oxygen consumption (proton leak), the maximal respiration capacity, and the nonmitochondrial oxygen consumption. Cellular ATP content was measured by a luminescent assay (48).
Vascular Studies of in Vitro Hyperglycemia.
Thoracic aortic rings from Sprague-Dawley rats were incubated for 48 h under normoglycemic or hyperglycemic conditions in DMEM as described above, in the presence or absence of 200 μM NaHS, applied every 8 h, followed by the determination of endothelium-dependent relaxations (29). Adenoviral CSE overexpression in vascular rings was performed as described for endothelial cells above, followed by incubation of the rings for 48 h under normoglycemic or hyperglycemic conditions in DMEM. Vascular studies from thoracic aortae wild-type and CSE−/− mice were performed as described (8); rings were incubated either in normoglycemic or hyperglycemic conditions in DMEM for 24 h, followed by the determination of acetylcholine-induced relaxations.
Vascular Studies in Diabetic Rats.
Diabetes in male Sprague-Dawley rats was induced with a single streptozotocin injection of 60 mg/kg of body wt i.p. prepared in citrate buffer (pH 4.5). On day 14, animals were implanted with osmotic pumps (Alzet) filled with NaHS (releasing a dose of 16 μg/kg per min) or vehicle. Rats were divided into groups as follows: control group (CTL/V, n = 11, nondiabetic rats treated with vehicle), control with H2S (CTL/S, n = 12; nondiabetic rats treated with H2S), streptozotocin-induced diabetes group (STZ/V, n = 7; diabetic rats treated with vehicle), and streptozotocin-induced diabetes group (STZ/S, n = 9; diabetic rats treated with H2S). Minipumps were replaced at 2 wk. H2S or vehicle treatment lasted for 28 d. Blood glucose and blood H2S levels were measured with an Accu-Chek Advantage (Roche) and the amperometric H2S sensors.
Statistical Analysis.
Data are expressed as means ± SEM. Statistical analysis was performed by ANOVA.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institutes of Health (R01GM060915), the Shriners Burns Hospitals (#8661), and the Juvenile Diabetes Foundation (17-2010-542) (all to C.S.), from the Thorax Foundation (to Z.Z.), and from the Canadian Institutes of Health Research (to L.W. and R.W.).
Footnotes
Conflict of interest statement: C.S. is a stockholder in Ikaria Inc., a for-profit organization involved in the development of H2S-based therapeutics.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105121108/-/DCSupplemental.
References
- 1.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Moore PK. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 4.Wang R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 5.Szabo C. Gaseotransmitters: New frontiers for translational science. Sci Transl Med. 2010;2:59ps54. doi: 10.1126/scitranslmed.3000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem. 2009;146:623–626. doi: 10.1093/jb/mvp111. [DOI] [PubMed] [Google Scholar]
- 7.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001;20:6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G, et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papapetropoulos A, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci USA. 2009;106:21972–21977. doi: 10.1073/pnas.0908047106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabó C, Papapetropoulos A. Hydrogen sulfide and angiogenesis: Mechanisms and applications. Br J Pharmacol. December 30, 2010 doi: 10.1111/j.1476-5381.2010.01191.x. 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szabo C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br J Pharmacol. 2009;156:713–727. doi: 10.1111/j.1476-5381.2008.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishikawa T, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 14.Du X, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–1057. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doeller JE, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Geng B, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun. 2004;318:756–763. doi: 10.1016/j.bbrc.2004.04.094. [DOI] [PubMed] [Google Scholar]
- 17.Carballal S, et al. Reactivity of hydrogen sulfide with peroxynitrite and other oxidants of biological interest. Free Radic Biol Med. 2011;50:196–205. doi: 10.1016/j.freeradbiomed.2010.10.705. [DOI] [PubMed] [Google Scholar]
- 18.Piconi L, et al. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: The role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2:1453–1459. doi: 10.1111/j.1538-7836.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- 19.Piconi L, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- 20.Horváth EM, et al. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52:952–961. doi: 10.1007/s00125-009-1304-0. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls P, Kim JK. Sulphide as an inhibitor and electron donor for the cytochrome c oxidase system. Can J Biochem. 1982;60:613–623. doi: 10.1139/o82-076. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson RA, et al. Inhibition of respiratory and bioenergetic mechanisms by hydrogen sulfide in mammalian brain. J Toxicol Environ Health A. 1998;54:491–507. doi: 10.1080/009841098158773. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RW, Valentine HL, Valentine WM. Cytotoxic mechanisms of hydrosulfide anion and cyanide anion in primary rat hepatocyte cultures. Toxicology. 2003;188:149–159. doi: 10.1016/s0300-483x(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 24.Piconi L, Quagliaro L, Ceriello A. Oxidative stress in diabetes. Clin Chem Lab Med. 2003;41:1144–1149. doi: 10.1515/CCLM.2003.177. [DOI] [PubMed] [Google Scholar]
- 25.Horváth EM, Benko R, Gero D, Kiss L, Szabó C. Treatment with insulin inhibits poly(ADP-ribose)polymerase activation in a rat model of endotoxemia. Life Sci. 2008;82:205–209. doi: 10.1016/j.lfs.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehr HA, et al. Consensus meeting on “Relevance of parenteral vitamin C in acute endothelial dependent pathophysiological conditions (EDPC)”. Eur J Med Res. 2006;11:516–526. [PubMed] [Google Scholar]
- 27.Wallace JP, Johnson B, Padilla J, Mather K. Postprandial lipaemia, oxidative stress and endothelial function: A review. Int J Clin Pract. 2010;64:389–403. doi: 10.1111/j.1742-1241.2009.02146.x. [DOI] [PubMed] [Google Scholar]
- 28.Standl E, Schnell O, Ceriello A. Postprandial hyperglycemia and glycemic variability: Should we care? Diabetes Care. 2011;34(Suppl 2):S120–S127. doi: 10.2337/dc11-s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabó C, et al. Part I: Pathogenetic role of peroxynitrite in the development of diabetes and diabetic vascular complications: Studies with FP15, a novel potent peroxynitrite decomposition catalyst. Mol Med. 2002;8:571–580. [PMC free article] [PubMed] [Google Scholar]
- 30.Pacher P, Szabó C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr Opin Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivitz WI, Yorek MA. Mitochondrial dysfunction in diabetes: From molecular mechanisms to functional significance and therapeutic opportunities. Antioxid Redox Signal. 2010;12:537–577. doi: 10.1089/ars.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia Soriano F, et al. Diabetic endothelial dysfunction: The role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 33.Yin WL, He JQ, Hu B, Jiang ZS, Tang XQ. Hydrogen sulfide inhibits MPP+-induced apoptosis in PC12 cells. Life Sci. 2009;85:269–275. doi: 10.1016/j.lfs.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 34.Tyagi N, et al. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang XQ, et al. Hydrogen sulfide antagonizes homocysteine-induced neurotoxicity in PC12 cells. Neurosci Res. 2010;68:241–249. doi: 10.1016/j.neures.2010.07.2039. [DOI] [PubMed] [Google Scholar]
- 36.Hu LF, Lu M, Wu ZY, Wong PT, Bian JS. Hydrogen sulfide inhibits rotenone-induced apoptosis via preservation of mitochondrial function. Mol Pharmacol. 2009;75:27–34. doi: 10.1124/mol.108.047985. [DOI] [PubMed] [Google Scholar]
- 37.Price KD, Price CS, Reynolds RD. Hyperglycemia-induced ascorbic acid deficiency promotes endothelial dysfunction and the development of atherosclerosis. Atherosclerosis. 2001;158:1–12. doi: 10.1016/s0021-9150(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 38.Weidig P, McMaster D, Bayraktutan U. High glucose mediates pro-oxidant and antioxidant enzyme activities in coronary endothelial cells. Diabetes Obes Metab. 2004;6:432–441. doi: 10.1111/j.1462-8902.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 39.Ungvari Z, et al. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am J Physiol Heart Circ Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: Implications for diabetic retinopathy. Am J Pathol. 2010;177:447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brancaleone V, et al. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br J Pharmacol. 2008;155:673–680. doi: 10.1038/bjp.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jain SK, et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal. 2010;12:1333–1337. doi: 10.1089/ars.2009.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whiteman M, et al. Adiposity is a major determinant of plasma levels of the novel vasodilator hydrogen sulphide. Diabetologia. 2010;53:1722–1726. doi: 10.1007/s00125-010-1761-5. [DOI] [PubMed] [Google Scholar]
- 44.Bucci M, et al. Hydrogen sulfide is an endogenous inhibitor of phosphodiesterase activity. Arterioscler Thromb Vasc Biol. 2010;30:1998–2004. doi: 10.1161/ATVBAHA.110.209783. [DOI] [PubMed] [Google Scholar]
- 45.Mukhopadhyay P, Rajesh M, Yoshihiro K, Haskó G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun. 2007;358:203–208. doi: 10.1016/j.bbrc.2007.04.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virág L, Salzman AL, Szabó C. Poly(ADP-ribose) synthetase activation mediates mitochondrial injury during oxidant-induced cell death. J Immunol. 1998;161:3753–3759. [PubMed] [Google Scholar]
- 47.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Módis K, et al. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol. 2009;158:1565–1578. doi: 10.1111/j.1476-5381.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.