Abstract
The study of human and primate altruism faces an evolutionary anomaly: There is ample evidence for altruistic preferences in our own species and growing evidence in monkeys, but one of our closest relatives, the chimpanzee (Pan troglodytes), is viewed as a reluctant altruist, acting only in response to pressure and solicitation. Although chimpanzee prosocial behavior has been reported both in observational captive studies and in the wild, thus far Prosocial Choice Tests have failed to produce evidence. However, methodologies of previous Prosocial Choice Tests may have handicapped the apes unintentionally. Here we present findings of a paradigm in which chimpanzees chose between two differently colored tokens: one “selfish” token resulting in a reward for the actor only (1/0), and the other “prosocial” token rewarding both the actor and a partner (1/1). Seven female chimpanzees, each tested with three different partners, showed a significant bias for the prosocial option. Prosocial choices occurred both in response to solicitation by the partner and spontaneously without solicitation. However, directed requests and pressure by the partner reduced the actor's prosocial tendency. These results draw into question previous conclusions indicating that chimpanzees have a limited sensitivity to the needs of others and behave prosocially only in response to significant prompting.
Keywords: other-regarding, fairness, great ape
Humans routinely help others, even in situations in which they derive no direct benefit themselves (1, 2). However, the extent to which this behavior is unique to our species remains controversial (3, 4). Comparative studies with chimpanzees (Pan troglodytes) are of particular relevance to this question, given our shared evolutionary history and recent common ancestry (5). However, studies of chimpanzee behavior have not yielded consistent results. Disagreements stem from discrepancies between observational studies (indicating that chimpanzees share food, console distressed individuals, and show empathy in a variety of contexts) (6–8) and controlled experiments, which have not found consistent evidence for the prosocial tendencies thought to underlie these behaviors. Experimental studies can be divided into two main categories: Giving Assistance Tests (GAT) and Prosocial Choice Tests (PCT), the first of which has yielded more positive data.
In GAT, participants have a choice between providing instrumental help to another or doing nothing. Warneken et al. (9, 10) showed that young chimpanzees provided appropriate assistance to both humans and conspecifics by retrieving an out-of-reach object. Similarly, chimpanzees were able to provide a conspecific with a needed tool (11) or access to a chain that was used to pull in food (12). In each case, assistance was provided more readily when the partner indicated their need by reaching toward the desired object with an outstretched hand. When the chimpanzees’ congener, the bonobo (Pan paniscus), was tested on the GAT, it showed impressive generosity (13). Collectively, these results suggest that the genus Pan has well-developed helping tendencies, often enhanced by the partner's solicitation.
The critical role of communication in prosocial interaction among chimpanzees has been used to suggest limited sensitivity to the needs of others (14–16), but young children, too, fail to act prosocially toward a silent partner. By the age of 25 mo, children behave prosocially only if their partner vocally announces interest (17), indicating that with age, children develop a greater empathic sensitivity to the emotional needs of others (18). The same sensitivity is thought to underlie chimpanzee altruism (6).
Unlike the GAT, which offers a choice between action and inaction, the second paradigm used to study prosociality, the PCT, offers a choice between two actions that are equal in every regard except for their effect on a partner. First developed for macaques (19), participants select between a “prosocial” option that rewards both the actor and a partner (1/1) and a “selfish” option that rewards only the actor (1/0). In all four PCTs conducted to date, however, chimpanzees have failed to show systematic prosocial preferences and did not change their behavior depending on whether or not a partner was present (20–23). These negative outcomes, which have been interpreted to mean that chimpanzees “are indifferent to the welfare” of others (20), are especially puzzling given the positive results of PCTs conducted on brown capuchin monkeys (Cebus apella) (24–25), common marmosets (Callithrix jacchus) (15), and cotton-top tamarins (Saguinus oedipus) (26).
Several methodological factors have been proposed to explain the negative findings of previous chimpanzee PCT studies. These factors include the complexity of the apparatus used to deliver rewards, the actors’ preoccupation with visible reward options, limited communication between actors and participants, and competitive attitudes by actors toward the partners (4, 9, 12, 27). Here we present positive findings from a PCT paradigm specifically designed to avoid all of these issues.
To avoid a complex apparatus that may not be intuitive, we modified a token-exchange paradigm with which the chimpanzees already were familiar (28–30) and that had worked well with capuchin monkeys (24). Actors received a bucket of 30 tokens randomly jumbled together that they could exchange with an experimenter: 15 tokens of one color that resulted in a selfish outcome (1/0) and 15 tokens of another color that resulted in a prosocial outcome (1/1). The number of tokens in the bucket was always kept constant (Materials and Methods). This methodology was chosen to prevent the location biases that primates are known to have and that also were reported for the chimpanzees in previous PCTs (21, 22). Location biases may produce random performance if dyadic choice locations are randomized, as they are in most studies.
Once the actor had chosen a token from the bucket, it was placed on a platform, clearly visible to both actor and partner (Fig. 1). The platform also held two identical food rewards wrapped in paper. If the actor selected a selfish token, the experimenter held up only one reward and gave it to the actor. If a prosocial token had been selected, the experimenter held up both rewards and first handed one to the actor, followed immediately by one for the partner. The rewards were wrapped in paper to reduce the probability that actors were distracted by visible food (31) and to ensure audible food consumption (unwrapping the paper produced loud noise), making the receipt of a reward by the partner both visible and audible to the actor.
Fig. 1.
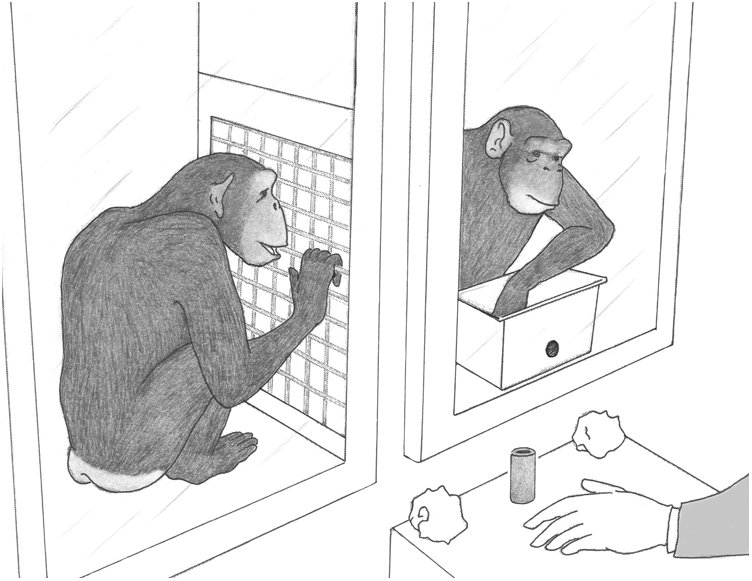
Schematic of two chimpanzees in the test setting. While her partner (Left) watches through a mesh partition, the actor (Right) reaches into a bucket with 30 tokens, 15 of each color, to select one and hand it to the experimenter. The token then is placed in full view, after which, depending on the token choice, one or two paper-wrapped rewards are held up in the air. A reward is handed either to the actor or to both chimpanzees. Drawing by J.D.C. from a video still.
We also sought to facilitate communication between actors and partners by having them sit close together, able to interact through a 72 × 52 cm window of 4-cm2 wire mesh. We achieved this proximity by positioning the token bucket next to the window and delivering rewards close to it, so that the two chimpanzees typically sat side-by-side less than 1 m apart.
Previous studies have sought to familiarize actors with the contingencies of their apparatus by allowing them to visit and receive rewards from the partner’s room (21, 23). Although there is no evidence that the chimpanzees can generalize this knowledge to understanding how choices affect a partner, it may foster competitive attitudes if the actor comes to expect both rewards. We avoided this possibility by never allowing actors to receive more than one reward.
Participants were seven adult female chimpanzees who were members of a larger group housed outdoors at Yerkes National Primate Research Center’s Field Station in Atlanta. Actors were tested with three different partners; a different set of tokens was used for each pairing. Actors and partners switched roles in most sessions so that the actor in the first session became the partner in the second session on the next possible day. No actor was paired with the same partner more than once.
Results
Prosocial Choice.
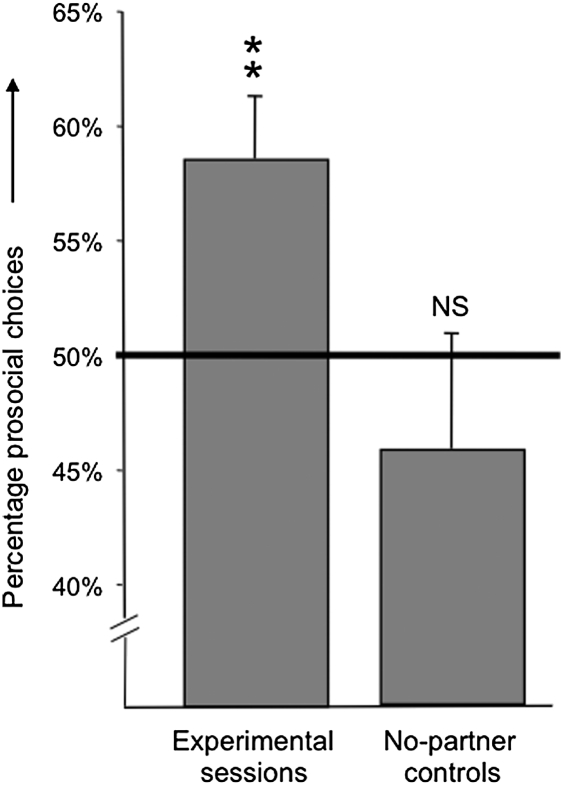
Prosocial vs. selfish token choices were combined for each actor across her three pairings. A heterogeneity G-test on individual data against a chance level of 0.5 showed nonsignificant heterogeneity (Gh = 9.55, df = 6, P = 0.145) and a significant pooled G value indicating a bias for the prosocial option (Gp = 19.22, df = 1, P = 0.000012). The prosocial tendency per subject ranged between 52.9% and 66.7%. When choices in the no-partner controls were analyzed in the same way, again a nonsignificant heterogeneity was found (Gh = 7.85, df = 5, P = 0.165) but the pooled G value also was nonsignificant (Gp = 1.09, df = 1, P = 0.296), indicating that the apes were choosing randomly (Fig. 2). During no-partner controls, the prosocial tendency per subject ranged between 26.7% and 56.7%.
Fig. 2.
Mean (+SEM) percentage of prosocial choices by actors in experimental and no-partner control conditions. Asterisks refer to the outcome of a heterogeneity G-test on token choices by the individual subject (n = 7) against a 50% expectation (**P < 0.01). NS, not significant.
Social Determinants of Choice.
We investigated reciprocity in nine pairs in which individuals participated as both actor and partner (Materials and Methods). There was no correlation between the prosocial tendency of an actor toward a partner and the choices made by that partner when the roles were reversed (Spearman ρ = 0.109, n = 9, P = 0.780). It was hypothesized further that subordinate females might make more prosocial choices out of fear of repercussions. However, the correlation between individual dominance rank and prosocial tendency was nonsignificantly negative (Spearman ρ = −0.62, n = 7, P = 0.139); that is, high-ranking individuals tended to be more prosocial than low-ranking ones.
Outcomes per pair were analyzed to determine the role of kinship. When the 21 pairs were ranked from high to low prosociality, the six kin-related pairs occupied ranks number 10 and below. However, even though kin pairs tended to be less prosocial, we found no significant difference between kin and nonkin pairs (Mann–Whitney test, N1 = 6, N2 = 15, U = 23, P = 0.095).
Finally, the prosociality score of a pair did not correlate with the level of mutual affiliation calculated from grooming and contact-sitting during daily group observations (Spearman ρ = −0.26, n = 21, P = 0.255).
Actor–Partner Interactions.
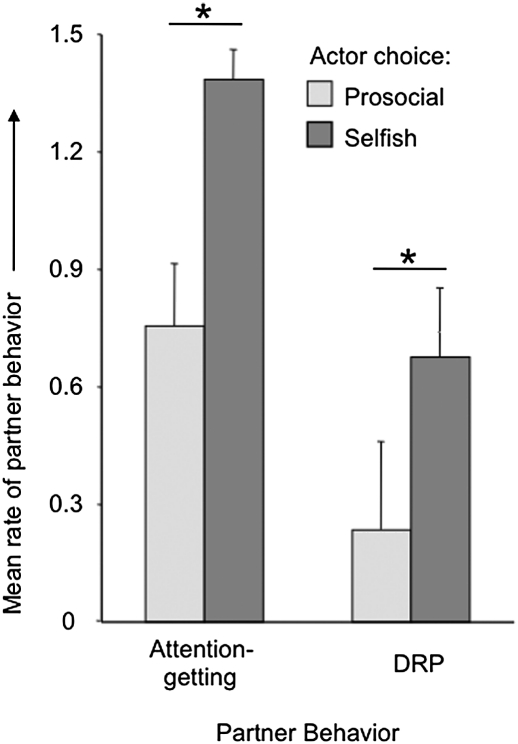
Previous PCT studies reported limited interaction between actors and partners (21, 22), perhaps reflecting the greater physical distance between the two chimpanzees and/or lack of understanding of the actor's role in outcomes. In the present study, in contrast, the chimpanzees interacted frequently. The behavior of partners following every token choice was categorized as (i) neutral (no reaction), (ii) attention-getting, or (iii) directed requests and pressure (DRP). Attention-getting was defined as behavior that attracted attention to the partner, such as self-scratching, noise, food-grunts, or hitting the caging, but not directed specifically toward the actor in the adjacent room. DRP was defined as behavior aimed at the actor on the other side of the mesh, such as poking paper (from the rewards) toward the actor, begging with an open hand, staring at the bucket with tokens, or aimed displaying with pilo-erection and hooting. Attention-getting was considered of lower intensity because it was not directed specifically at the actor but merely made the partner’s presence known. Fig. 3 shows the mean rate of attention-getting and DRP by partners following either a prosocial or selfish token choice by the actor. Partners produced both behaviors significantly more following selfish choices (attention-getting: Wilcoxon test, T = 1, n = 7, P < 0.05; DRP: T = 0, n = 7, P = 0.02), indicating that the partners were not passive food recipients but understood the difference between selfish and prosocial token choices.
Fig. 3.
Response by the partner dependent on the actor's token choice: mean (+SEM) rate of attention-getting or directed requests and pressure (DRP) following either a prosocial or a selfish choice by the actor. Both response types increased significantly following a selfish choice (*P < 0.05).
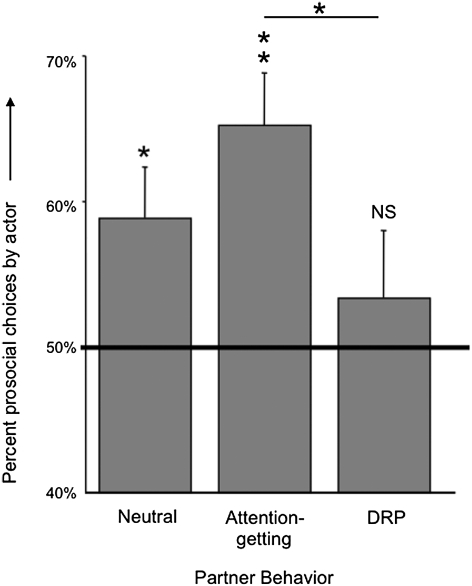
The next question was whether partner reactions influenced subsequent choices by the actors. Fig. 4 shows the mean percentage of prosocial choices that were preceded by each type of partner behavior (neutral, attention-getting, and DRP). Tested as before with a heterogeneity G-test, actors were significantly prosocial toward neutral partners (with Gh nonsignificant, Gp = 4.52, df = 1, P = 0.0336) and were even more prosocial toward attention-getting partners (with Gh nonsignificant, Gp = 27.32, df = 1, P = 0.0000002) but did not choose differently from chance after DRP by the partner (with Gh nonsignificant, Gp = 0.024, df = 1, P = 0.877). Attention-getting was associated with significantly more prosocial choice than DRP (Wilcoxon test: n = 7, T = 0, P = 0.02).
Fig. 4.
Mean (+SEM) percentage of prosocial choices by the actor following each type of partner communication (no communication, attention-getting, or DRP). Asterisks refer to the outcome of a heterogeneity G-test comparing the actor's token choice with a 50% expectation (*P < 0.05 and **P < 0.01). Attention-getting was followed by significantly more prosocial choice than was DRP. NS, not significant.
Discussion
Offered a free choice between a prosocial and selfish option, chimpanzees overwhelmingly favored the former to the advantage of their partner. Their prosocial tendency was not constrained significantly by kinship, dominance rank, affiliation, or reciprocity. Although this finding conflicts with previous PCTs on the same species, it fits with what is known about spontaneous chimpanzee behavior in both captivity and the field (8, 32). It also corresponds with the results of a different experimental paradigm, the GAT, according to which chimpanzees provide instrumental help to others pursuing a recognizable goal (9–12).
To understand why our results differ from previous ones, the first item to consider is physical separation: In some other studies the apes sat an estimated 3 m apart and/or faced each other separated by two barriers (20–22). Furthermore, some studies reported location biases for choices (20, 21), which seriously confound effect-based choice, or let actors retrieve food from the partner's side during familiarization, thus potentially inducing competition (21, 23). Also, the two choices were not exactly equivalent in all studies, such as one in which the selfish option meant pulling food toward oneself, but the prosocial option required pushing it away (22). Our methodology, in contrast, was designed to avoid a complex apparatus, eliminate location biases, ensure close proximity (<1 m) without glass barriers, enhance communication, avoid competitive attitudes, and make food consumption both visible and audible. We explicitly ensured that both actors and partners could see how choices were made and how these choices affected them. Our positive results confirm the critical importance of sometimes minor methodological variations (16, 33) and undermine claims that chimpanzees constitute an evolutionary anomaly marked by indifference to the welfare of others.
However, our data do raise their own puzzles. For example, unlike a similar PCT with capuchin monkeys (24), chimpanzees were equally prosocial toward all partners, including kin and unrelated group-mates with whom they had lived all their lives. We found no correlation between prosocial choice and kinship, affiliation, or rank. This result draws into question suggestions that nonhuman primate cooperation is largely kin-based (2, 4), a suggestion also countered by a comparison between DNA profiles and cooperation among wild chimpanzees (34, 35).
We found no evidence of reciprocity after role reversal between actors and partners. However, bonobos, a close relative of both chimpanzees and humans, recently have been shown to act prosocially, sharing food with unrelated out-group conspecifics with whom they have no possibility of reciprocity (13). Nevertheless, we cannot rule out the possibility that chimpanzees in our study were influenced by reciprocal exchanges outside the experimental setting, such as food sharing, increased grooming, or agonistic support. There is good evidence that chimpanzees remember and return past favors (36–39). Future studies therefore should try to relate test outcomes to social interactions within the group. It should be noted also that all actors in this study were female, and many of the species' cooperative behaviors, such as group hunting, border patrols, and coalitionary support, are more typical of males (7, 40–43). Consolation of distressed parties, however, is more common in females (44).
Unlike previous PCT studies on chimpanzees, we observed extensive communication between actors and partners. Communication levels also were higher than those reported for PCTs in monkeys, suggesting that chimpanzees may be more active negotiators of cooperation. The observed communication indicated a full understanding in both actors and partners of how the choices affected them, an understanding that may be greater in apes than monkeys. After selfish choices by the actor, their partners significantly increased both attention-getting behavior and DRP. Actors, in turn, showed increased prosociality after their partner’s attention-getting behavior but a significant drop after DRP. Spitting water (although rare), begging, whining, and intimidation behavior evidently did not help the partner's cause, thus contradicting suggestions in the literature that chimpanzees share only under pressure (14, 45). In fact, we found significant levels of prosocial choice under neutral behavioral conditions, when partners refrained from overt communication, thus suggesting that chimpanzees, like the monkeys tested thus far, are proactively prosocial.
Materials and Methods
Participants.
The Yerkes National Primate Research Center is fully accredited by the American Association for Accreditation for Laboratory Animal Care.
The study was conducted with seven adult female chimpanzees (age range: 15–46 y) who volunteered to participate and were willing to exchange tokens with an experimenter. Housed at the Yerkes National Primate Research Center’s Field Station, near Atlanta, these chimpanzees were members of the same long-established group of 12 adult individuals (1 male, 11 females) housed in a spacious grass outdoor enclosure (711 m2) with climbing structures and two indoor buildings: one with sleeping quarters, and the other a cognitive research facility. Control tests were conducted at the end of the study (see below), but unfortunately by this time one of the oldest participants had died of natural causes, resulting in an experimental group of six chimpanzees.
Actors were tested with three different adult partners. To ensure that actor–partner pairings were comparable for all participants, observational data from daily 1-h observations of the entire group (cf. ref. 46) were used to calculate a proximity index of affiliative tendencies (based on contact-sitting and grooming) for every potential pair. We used these data to select three partners for each actor: one with whom she had a significantly affiliative relationship, one with a significantly negative relationship, and one neutral pairing. Three pairings involved the only male in the group, who figured only as a partner, not as an actor. Actors used a different set of tokens with each partner and never were paired with the same partner more than once. In nine pairs, actors and partners switched roles so that the actor in one session became the partner in the next session conducted on the next possible day. Once they had performed both roles, individuals moved on to their next pairing with a different individual and a different set of tokens. This process was repeated so that the seven actors each experienced three different partners and three different token sets. No chimpanzee was tested more than once per day.
Statistics.
All statistics in this paper are nonparametric, and all reported P values two-tailed. For the heterogeneity G-test (a goodness-of-fit test), see ref. 47.
Experimental Procedure.
The study was conducted in two adjacent rooms of the cognitive research building, each with reinforced glass fronts with a surface of 1.7 × 1.7 m. Placed in immediately adjacent rooms, two chimpanzees were able to see, hear, and interact with each other through a 72 × 52 cm window of 4-cm2 wire mesh.
Actors received a choice of two differently colored tokens that they could exchange with the experimenter for food (cf. ref. 24). Tokens were PVC pipes 5 cm long and 3.5 cm in diameter. To reduce location biases, 30 tokens (15 of each color) were jumbled together in a large bin attached inside the actor’s room. The experimenter kept the total number of tokens of each color in the bin constant throughout test sessions by manually adding tokens through a hole in the bottom and jumbling them after each trial. The study used three sets of tokens so that actors used a different set with each partner. The three sets were purple/green, red/blue, and yellow/black. Before the study, color preference tests were conducted with each set. Based on a previous methodology (24), a token set would have been changed if more than two chimpanzees showed a significant bias for a particular color. However, this precaution was not necessary for any of the sets. Moreover, for each token set, the token least preferred by a particular chimpanzee was designated the prosocial token in the contingency training (see below) for that chimpanzee, so that preexisting biases could not explain prosocial tendencies.
Contingency Training.
Previous PCT studies have sought to familiarize participants with the outcome of selfish and prosocial choices by allowing them to visit both locations where rewards are delivered. However, we demonstrated the outcome of selfish and prosocial choices in the same environment as experimental sessions, i.e., actors always received only one reward, and partners always received the “extra” reward resulting from a prosocial choice. Actors never were permitted to visit the partner’s room in relation to rewards.
On a separate day from the preference test, two participants were called into the research building from the outdoor enclosure. One was designated as the actor and the other as the partner. If a chimpanzee declined to participate that day, her test was rescheduled for another day. The actor received 10 tokens (five of each color) to be returned to the experimenter. Tokens were provided to the actor by loading tokens into the empty bin in random order, one at a time, and requesting them back through an open-hand gesture. From this point on, the two token colors were assigned different outcomes: One selfish token resulted in a reward for the actor only; the other prosocial token resulted in rewards for both individuals. The actor always was rewarded 1–2 s before the partner so that the latency between returning a token and receiving a reward remained the same for the actor for both choices. By the end of the training, actors had returned five tokens of each color, and therefore both the actor and partner had experienced five selfish and five prosocial outcomes.
Prosocial Choice Test (PCT).
Immediately following the contingency training, actors were allowed to select tokens from a full bin with 30 tokens. Each color resulted in the same prosocial or selfish outcome that had been demonstrated in the contingency training. Consistent with the procedure for the contingency training, actors handed each token choice to the experimenter, who immediately reloaded the bin with the same color token (see above) before placing the selected token on a small platform clearly visible to both chimpanzees (Fig. 1). The platform also displayed two food rewards before each trial, thus eliminating association of one or the other token with different numbers of visible rewards. Rewards were a 1-cm slice of banana wrapped in butcher paper so that the chimpanzees were not distracted by visible food. Depending on the actor’s choice, the experimenter would hold up one or both rewards before handing them out. Unwrapping the paper made a loud noise (like eating bonbons), so that actors did not need to rely on vision alone to know whether the partner had been rewarded. Once the actor had finished eating, a second experimenter removed the token from the platform and placed two fresh rewards on the platform. The first experimenter then requested a second token from the actor. This procedure was repeated 30 times.
No-Partner Controls.
Control tests investigated whether possible prosocial tendencies resulted from the presence of the partner or from some unrelated artifact. Control trials were conducted with a different set of tokens (pink/gray), using the procedure described above, including preference tests, contingency training, and PCT. The only difference was the absence of a partner in the adjacent room. Actors could see the empty room through the mesh window. If a prosocial token was selected, the experimenter rewarded the actor as before and then pretended to reward an imaginary partner. Instead of pushing the reward through the mesh at the location where a partner normally would sit, the experimenter held the reward against the mesh while covertly pushing it under her sleeve out of sight of the actor. Her movements therefore were the same as before, except that there was no partner, and rewards did not build up in the empty room where they would be unavailable to the actor and might confuse her. No-partner controls were conducted post hoc to prevent inadvertent training that all tokens had the same outcome.
Behavioral Data.
Videotaped behavioral data were analyzed to determine the partner’s reaction immediately following each token choice by the actor. The next token chosen by the actor then was compared with the partner reaction. Each partner’s behavior was coded as neutral, attention-getting or DRP, defined as directed requests (e.g., begging, poking the actor through the mesh, staring) and pressure (e.g., intimidation displays, hooting, water-spitting). Videotaped behavioral data were coded by V.H. and by a second coder uninformed about the study's purpose. Interobserver reliability was calculated for three randomly selected trials per test (i.e., 15% of all data). There was 100% agreement on the color of token chosen by each chimpanzee and 85% agreement on the categorization of the partner’s behavior following every trial as neutral, attention-getting, or DRP. When discrepancies arose, both coders reviewed the video tapes together to decide upon an agreed coding. These recoded data were used for the analysis.
Acknowledgments
We thank Timothy Eppley and Matthew Campbell for assistance with the study; Katie Hall for providing data on social dominance; Morgan Mingle for assistance with interobserver reliability testing; Christophe Boesch and Felix Warneken for constructive feedback on the manuscript; and the animal care and veterinary staff at the Yerkes National Primate Research Center (YNPRC) for maintaining the health and well-being of the chimpanzees. This work was supported by the Living Links Center, Emory's College of Arts and Sciences, and by Base Grant RR-00165 from the National Institutes of Health to the YNPRC.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fehr E, Fischbacher U. The nature of human altruism. Nature. 2003;425:785–791. doi: 10.1038/nature02043. [DOI] [PubMed] [Google Scholar]
- 2.Richerson PJ, Boyd R. Not by Genes Alone. Chicago: Univ of Chicago Press; 2005. [Google Scholar]
- 3.Henrich J, et al. “Economic man” in cross-cultural perspective: Behavioral experiments in 15 small-scale societies. Behav Brain Sci. 2005;28:795–815. doi: 10.1017/S0140525X05000142. discussion 815–855. [DOI] [PubMed] [Google Scholar]
- 4.Silk JB, House BR. Colloquium paper: Evolutionary foundations of human prosocial sentiments. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10910–10917. doi: 10.1073/pnas.1100305108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varki A, Altheide TK. Comparing the human and chimpanzee genomes: Searching for needles in a haystack. Genome Res. 2005;15:1746–1758. doi: 10.1101/gr.3737405. [DOI] [PubMed] [Google Scholar]
- 6.de Waal FBM. Putting the altruism back into altruism: The evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 7.Boesch C, Boesch-Achermann H. The Chimpanzees of the Taï Forest. Oxford: Oxford Univ Press; 2002. [Google Scholar]
- 8.Boesch C, Bolé C, Eckhardt N, Boesch H. Altruism in forest chimpanzees: The case of adoption. PLoS ONE. 2010;5:e8901. doi: 10.1371/journal.pone.0008901. 10.1371/journal.pone.0008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warneken F, Tomasello M. Altruistic helping in human infants and young chimpanzees. Science. 2006;311:1301–1303. doi: 10.1126/science.1121448. [DOI] [PubMed] [Google Scholar]
- 10.Warneken F, Hare B, Melis AP, Hanus D, Tomasello M. Spontaneous altruism by chimpanzees and young children. PLoS Biol. 2007;5:e184. doi: 10.1371/journal.pbio.0050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto S, Humle T, Tanaka M. Chimpanzees help each other upon request. PLoS ONE. 2009;4:e7416. doi: 10.1371/journal.pone.0007416. 10.1371/journal.pone.0007416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melis AP, et al. Chimpanzees help conspecifics obtain food and non-food items. Proc Biol Sci. 2011;278:1405–1413. doi: 10.1098/rspb.2010.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hare B, Kwetuenda S. Bonobos voluntarily share their own food with others. Curr Biol. 2010;20:R230–R231. doi: 10.1016/j.cub.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Stevens JR. The selfish nature of generosity: Harassment and food sharing in primates. Proc Biol Sci. 2004;271:451–456. doi: 10.1098/rspb.2003.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkart JM, Fehr E, Efferson C, van Schaik CP. Other-regarding preferences in a non-human primate: Common marmosets provision food altruistically. Proc Natl Acad Sci USA. 2007;104:19762–19766. doi: 10.1073/pnas.0710310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jaeggi AV, Burkart JM, Van Schaik CP. On the psychology of cooperation in humans and other primates: Combining the natural history and experimental evidence of prosociality. Philos Trans R Soc Lond B Biol Sci. 2010;365:2723–2735. doi: 10.1098/rstb.2010.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownell CA, Svetlova M, Nichols S. To share or not to share: When do toddlers respond to another’s needs? Infancy. 2009;14:117–130. doi: 10.1080/15250000802569868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svetlova M, Nichols SR, Brownell CA. Toddlers’ prosocial behavior: From instrumental to empathic to altruistic helping. Child Dev. 2010;81:1814–1827. doi: 10.1111/j.1467-8624.2010.01512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colman AD, Liebold KE, Boren JJ. A method for studying altruism in monkeys. Psychol Rec. 1969;19:401–405. [Google Scholar]
- 20.Silk JB, et al. Chimpanzees are indifferent to the welfare of unrelated group members. Nature. 2005;437:1357–1359. doi: 10.1038/nature04243. [DOI] [PubMed] [Google Scholar]
- 21.Jensen K, Hare B, Call J, Tomasello M. What’s in it for me? Self-regard precludes altruism and spite in chimpanzees. Proc Biol Sci. 2006;273:1013–1021. doi: 10.1098/rspb.2005.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vonk J, et al. Chimpanzees do not take advantage of very low cost opportunities to deliver food to unrelated group members. Anim Behav. 2008;75:1757–1770. doi: 10.1016/j.anbehav.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto S, Tanaka M. The influence of kin relationship and the reciprocal context on chimpanzees’ other-regarding preferences. Anim Behav. 2010;79:595–602. [Google Scholar]
- 24.de Waal FBM, Leimgruber K, Greenberg AR. Giving is self-rewarding for monkeys. Proc Natl Acad Sci USA. 2008;105:13685–13689. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakshminarayanan VR, Santos LR. Capuchin monkeys are sensitive to others’ welfare. Curr Biol. 2008;18:R999–R1000. doi: 10.1016/j.cub.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 26.Cronin KA, Schroeder KKE, Snowdon CT. Prosocial behaviour emerges independent of reciprocity in Cottontop tamarins. Proc Biol Sci. 2010;277:3845–3851. doi: 10.1098/rspb.2010.0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warneken F, Tomasello M. Varieties of altruism in children and chimpanzees. Trends Cogn Sci. 2009;13:397–402. doi: 10.1016/j.tics.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Brosnan SF, Schiff HC, de Waal FBM. Tolerance for inequity may increase with social closeness in chimpanzees. Proc Biol Sci. 2005;272:253–258. doi: 10.1098/rspb.2004.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonnie KE, Horner V, Whiten A, de Waal FBM. Spread of arbitrary conventions among chimpanzees: A controlled experiment. Proc Biol Sci. 2007;274:367–372. doi: 10.1098/rspb.2006.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horner V, Proctor D, Whiten A, Bonnie KE, de Waal FBM. Prestige affects cultural learning in chimpanzees. PLoS ONE. 2010;5:e10625. doi: 10.1371/journal.pone.0010625. doi:10610.11371/journal.pone.0010625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boysen ST, Berntson GG. Responses to quantity: Perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) J Exp Psychol Anim Behav Process. 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- 32.de Waal FBM. With a little help from a friend. PLoS Biol. 2007;5:e190. doi: 10.1371/journal.pbio.0050190. 10.1371/journal.pbio.0050190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horner V, de Waal FBM. Controlled studies of chimpanzee cultural transmission. Prog Brain Res. 2009;178:3–15. doi: 10.1016/S0079-6123(09)17801-9. [DOI] [PubMed] [Google Scholar]
- 34.Vigilant L, Hofreiter M, Siedel H, Boesch C. Paternity and relatedness in wild chimpanzee communities. Proc Natl Acad Sci USA. 2001;98:12890–12895. doi: 10.1073/pnas.231320498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Waal FBM. The chimpanzee’s service economy: Food for grooming. Evol Hum Behav. 1997;18:375–386. [Google Scholar]
- 37.Koyama NF, Caws C, Aureli F. Interchange of grooming and agonistic support in chimpanzees. Int J Primatol. 2006;27:1293–1309. [Google Scholar]
- 38.Gomes CM, Mundry R, Boesch C. Long-term reciprocation of grooming in wild West African chimpanzees. Proc Biol Sci. 2009;276:699–706. doi: 10.1098/rspb.2008.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes CM, Boesch C. Wild chimpanzees exchange meat for sex on a long-term basis. PLoS ONE. 2009;4:e5116. doi: 10.1371/journal.pone.0005116. 10.1371/journal.pone.0005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Waal FBM. Chimpanzee Politics. New York: Harper & Row; 1982. [Google Scholar]
- 41.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge, MA: Harvard Univ Press; 1968. [Google Scholar]
- 42.Mitani JC, Merriwether DA, Zhang C. Male affiliation, cooperation and kinship in wild chimpanzees. Anim Behav. 2000;59:885–893. doi: 10.1006/anbe.1999.1389. [DOI] [PubMed] [Google Scholar]
- 43.Mitani JC, Watts DP. Why do chimpanzees hunt and share meat? Anim Behav. 2001;61:915–924. [Google Scholar]
- 44.Romero T, Castellanos MA, de Waal FBM. Consolation as possible expression of sympathetic concern among chimpanzees. Proc Natl Acad Sci USA. 2010;107:12110–12115. doi: 10.1073/pnas.1006991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilby IC. Meat sharing among the Gombe chimpanzees: Harassment and reciprocal exchange. Anim Behav. 2006;71:953–963. [Google Scholar]
- 46.de Waal FBM. Food sharing and reciprocal obligations in chimpanzees. J Hum Evol. 1989;18:433–459. [Google Scholar]
- 47.Sokal RR, Rohlf FJ. Biometry: The Principles and Practices of Statistics in Biological Research. 3rd Ed. New York: WH Freeman and Company; 1994. [Google Scholar]