Abstract
The role of the hippocampus in imagining the future has been of considerable interest. Preferential right hippocampal engagement is observed for imagined future events relative to remembered past events, and patients with hippocampal damage are impaired when imagining detailed future events. However, some patients with hippocampal damage are not impaired at imagining, suggesting that there are conditions in which the hippocampus may not be necessary for episodic simulation. Given the known hippocampal role in memory encoding, the hippocampal activity associated with imagining may reflect the encoding of simulations rather than event construction per se. The present functional (f)MRI study investigated this possibility. Participants imagined future events in response to person, place, and object cues. A postscan cued-recall test probing memory for detail sets classified future events as either successfully encoded or not. A contrast of successfully versus unsuccessfully encoded events revealed anterior and posterior right hippocampal clusters. When imagined events were successfully encoded, both anterior and posterior hippocampus showed common functional connectivity to a network including parahippocampal gyrus, medial parietal and cingulate cortex, and medial prefrontal cortex. However, when encoding was unsuccessful, only the anterior hippocampus, and not the posterior, exhibited this pattern of connectivity. These findings demonstrate that right hippocampal activity observed during future simulation may reflect the encoding of the simulations into memory. This function is not essential for constructing coherent scenarios and may explain why some patients with hippocampal damage are still able to imagine the future.
Keywords: episodic memory, autobiographical memory, future thinking, prospection
It is now firmly established that both remembering past experiences and imagining future events rely on a common network of brain regions, including medial prefrontal, medial and lateral parietal, and medial and lateral temporal regions (1–4) (for reviews, refs. 5, 6). However, despite this overlap, some regions within this network—particularly the right anterior hippocampus—are preferentially engaged by imagining future events relative to remembering past events (2, 7, 8). Furthermore, some patients with hippocampal damage, in addition to showing impaired episodic memory, also have difficulty imagining detailed and coherent future events (9–12). These findings suggest that the hippocampus is important for imagining the future, although its involvement is not a reflection of future orientation per se, but rather of the content and phenomenology of episodic simulations (4). Recently, however, an adult developmental amnesic patient (13), a group of developmental amnesic school-aged children (14), and a group of patients with bilateral hippocampal damage but spared remote episodic memory (15), were all shown to be unimpaired at imagining detailed future events, implying that a fully intact hippocampus may not be required for episodic simulation. The role of the hippocampus in imagining the future, which has emerged recently as a critical issue in the cognitive neuroscience of memory, future thinking, and imagination (5, 6, 16), is therefore currently controversial (15, 17).
One way to reconcile these conflicting findings is to consider that different regions of the hippocampus may play different roles in the process of simulation. Even though a general function of the hippocampus with respect to memory is to integrate distinct representations of objects and people with contextual information into coherent scenarios (18), the anterior and posterior aspects of the hippocampus may differ in terms of their contributions to this process (19–21). Current models propose that the posterior hippocampus is important for reinstatement of an episode in its original form, whereas the anterior hippocampus is involved in more “flexible” encoding of associative information (22–25). The involvement of the posterior hippocampus in episodic reinstatement may also be reflected in the processing of familiar spatial contexts and storage of cognitive maps for the purpose of navigation (26, 27). With respect to episodic simulation, an imagined event is situated within some spatial framework. Without such a platform upon which to build the scenario, the imagined event would lack a vital sense of coherence (9), suggesting that the posterior hippocampus should be critical for episodic simulation in general. This idea is supported by the finding that activity in the posterior hippocampus is modulated by participant ratings of detail—including spatial and contextual details—for both remembered past and imagined future events (28).
However, the anterior aspect of the hippocampus is differentially activated by future events relative to past events (2, 7, 8). The aforementioned hippocampal models would predict that such activity reflects the binding together of episodic details into novel and flexible arrangements and/or the encoding of these representations, and consistent with this prediction, anterior hippocampal activity has been shown to correlate with the amount of detail comprising a future event (28). Moreover, the disparateness of the details being integrated can modulate engagement of this region. For instance, Weiler et al. (7) found that right anterior hippocampal activity was associated with simulating unlikely future events for which the degree of flexible processing may be particularly amplified.
Whereas differential engagement of the anterior hippocampus may reflect the process of recombining details to construct a scenario, it may also result from encoding. An inherent characteristic of newly imagined future events is that they have not occurred (or been imagined) and as such, they are yet to be encoded. If a simulation is to serve a functional role in future behavior, it must be retained in memory so that it can be referred to if and when the imagined event is occurring. This issue was recognized by Ingvar (29), who suggested that the process of encoding and retaining a simulation of future behavior constituted a “memory for the future.” This is a critical aspect of the adaptive significance of episodic simulations, and virtually nothing is known about the processes that support their successful encoding and retention.
If a major function of the hippocampus during simulation is to encode the imagined scenarios, hippocampal damage would not necessarily prevent the events from being constructed initially, which may explain some of the conflicting results found in amnesic patients. Indeed, when children with hippocampal damage are asked to imagine future events, they can do so as well as controls can. However, when asked to recall these imagined events the next day, they are less accurate than controls at describing the original details (14). Therefore, the exact location of damage within the hippocampus may be critical as to whether the construction of simulations is affected. On the basis of the literature implicating the anterior hippocampus in associative encoding, and on the fact that some patients with hippocampal damage can still imagine the future but not recall their simulations, we hypothesized that the anterior hippocampus would play a role in encoding imagined events, thereby providing empirical evidence directly relevant to Ingvar’s (29) early claim.
To this end, we used a unique approach incorporating both experimental recombination (30) and subsequent memory (31) paradigms (Fig. S1). In a functional (f)MRI session, participants were presented with random recombinations of person, location, and object details extracted from their own memories, and for each, imagined a novel future event involving the three details, as per the experimental recombination paradigm (30). The amount of detail generated for each simulation was rated on a 4-point scale. In the control task, participants were presented with three common nouns and asked to construct a sentence that ordered the objects in size. Participants completed an unexpected postscan cued recall test, in which memory for each imagined event was probed by testing recall of the person, location, and object details. We examined hippocampal activity related to successful encoding by contrasting events for which the details were later recalled in the postscan test with those for which the details were later forgotten. We expected hippocampal activity to be higher for events that were successfully encoded and later remembered relative to events for which the key details were later forgotten. A partial least squares (PLS) analysis of functional connectivity was also conducted on hippocampal seeds yielded by the encoding analysis. Our findings provide evidence that both the anterior and posterior aspects of the hippocampus are important for encoding imagined events.
Results
Behavioral Results.
The number of trials in the control (M = 45, SE = 0), later-remembered (M = 46.52, SE = 2.16), and later-forgotten (M = 43.48, SE = 2.16) future conditions were not significantly different, F2,48 = 0.49, P = 0.613, suggesting that contrasts between these conditions should be unbiased. Later-remembered future events were rated as significantly more detailed (M = 2.16, SE = 0.07) than later-forgotten ones (M = 1.67, SE = 0.09) on a 4-point scale (0 = low detail, 3 = high detail). The type of detail tested affected rates of recall, F2,48 = 8.17, P = 0.001. Pairwise comparisons revealed that the number of successfully recalled events was significantly lower when participants were cued with the person-location details (M = 13.92, SE = 0.96) than when they were cued with the location-object (M = 17.2, SE = 0.90, t24 = −3.58, P = 0.002) or person-object (M = 16.08, SE = 0.73, t24 = 2.80, P = 0.01) details. Recall did not differ significantly for the location-object and person-object cues, t24 = −1.44, P = 0.163. The average temporal distance of future events was 1 y into the future (SE = 0.28).
fMRI Results.
Regions engaged by imagining future events.
Each fixed-effects model included regressors for three conditions of interest: (i) later-remembered and (ii) later-forgotten future trials and (iii) control trials. For the two future conditions, we also included linear parametric modulation regressors of detail ratings. A contrast image for each condition was entered into a random-effects flexible factorial ANOVA. To examine whether future simulation activated the common core network (1–4, 30, 32–34), we performed a random-effects contrast of all future simulation trials (irrespective of encoding success) with all control trials. For all whole-brain analyses, we applied a voxel-level threshold of P = 0.005 combined with a spatial extent threshold of 145 voxels, which together yielded a threshold of P < 0.05, corrected for multiple comparisons (as determined by a Monte Carlo simulation; Methods). At this corrected level of significance, this contrast revealed a large bilateral network composed of the medial prefrontal and parietal cortex, medial temporal lobes (including bilateral hippocampus), bilateral angular gyrus and lateral temporal cortex, and right inferior frontal gyrus (Table S1 and Fig. S2).
Encoding-related hippocampal activity.
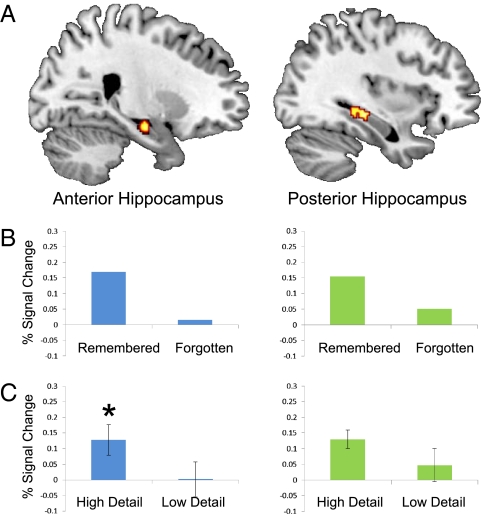
Hippocampal activity related to successful encoding was examined using a random-effects contrast of later-remembered versus later-forgotten future events, applied within the flexible factorial model described above. With the inclusion of detail as a parametric modulation regressor in the fixed-effects model, the contrast images for later-remembered and later-forgotten events entered into the random-effects analysis reflect the effect of these conditions while controlling for any effects of detail, given the hippocampal activity during future simulation has also been linked to increasing detail (28). At the corrected threshold of P < 0.05, this encoding contrast resulted in activity in some regions of the core network, including bilateral precuneus, parahippocampal gyrus and cerebellum, left inferior frontal gyrus, and right posterior hippocampus (Table 1). Because the hippocampus was an a priori region of interest, we computed a corrected threshold on the basis of the volume of the bilateral hippocampus (1,878 2 × 2 × 2 mm voxels): a voxel-level threshold of P = 0.005 combined with a spatial extent threshold of 26 voxels yielded a threshold of P < 0.05, corrected for multiple comparison (Methods and ref. 35). This analysis revealed activity in the right posterior hippocampus, as expected given the identification of this cluster in the whole-brain analysis; 54 voxels of the cluster identified in the whole-brain analysis were located within the hippocampus itself (xyz = 34 −26 −8, voxel-level P < 0.001, Z = 3.36, kHC = 54 voxels, where kHC is the number of voxels in the cluster that fall within the bilateral hippocampal mask). Additionally, this regional analysis showed another more anterior cluster of encoding-related activation in the right hippocampus (xyz = 20 −12 −16, voxel-level P < 0.001, Z = 3.34, kHC = 49 voxels; Fig. 1A). The opposite contrast of later-forgotten versus later-remembered future events yielded no significant clusters, either in the whole-brain analysis or the regional analysis.
Table 1.
Regions activated by successful future event encoding
| MNI coordinates |
||||||
| k | Brain region | x | y | z | Z score | P value |
| 706 | R precuneus (BA 31) | 22 | −58 | 20 | 3.91 | <0.001 |
| R retrosplenial cortex (BA 30) | 10 | −50 | 18 | 3.24 | 0.001 | |
| 694 | L parahippocampal gyrus (BA 30) | −18 | −48 | −2 | 3.71 | <0.001 |
| L parahippocampal gyrus (BA 36) | −26 | −44 | −8 | 3.22 | <0.001 | |
| R parahippocampal gyrus (BA 36) | 36 | −34 | −14 | 3.27 | 0.001 | |
| R fusiform gyrus (BA 37) | 28 | −44 | −16 | 2.84 | 0.002 | |
| 375* | L inferior frontal gyrus (BA 47) | −20 | 32 | −12 | 3.97 | <0.001 |
| L anterior cingulate cortex (BA 32) | −12 | 34 | −10 | 3.71 | <0.001 | |
| 214 | L precuneus (BA 31) | −16 | −58 | 16 | 3.48 | <0.001 |
| 189 | R hippocampus | 34 | −26 | −8 | 3.36 | <0.001 |
| 173 | L cerebellum | −24 | −48 | −30 | 3.98 | <0.001 |
| 149* | R cerebellum | 6 | −48 | −32 | 3.09 | 0.001 |
All clusters are significant at P < 0.05, corrected for multiple comparisons. Cluster size (k) indicates the number of voxels comprising the cluster; only clusters with a minimum extent of 145 voxels are reported. Voxel-level P value is provided. MNI, Montreal Neurological Institute; BA, Brodmann area; L, left; R, right.
*Cluster extends bilaterally.
Fig. 1.
(A) A subsequent memory analysis revealed clusters in anterior right hippocampus (Left, xyz = 20 12 16) and posterior right hippocampus (Right, 34 26 8). Activations are corrected for multiple comparison (P < 0.05) and are shown at a voxelwise threshold of P < 0.005 uncorrected (masked to only show voxels within the bilateral hippocampus). (B) For illustrative purposes, percentage of signal change is shown for later-remembered and later-forgotten trials (note that error bars are not included as this effect is not independent of voxel selection). (C) Percentage of signal change is broken down, according to the level of detail of the simulations (high versus low). Error bars show SEM. *P < 0.05.
Mean percentage of signal change was extracted from the two hippocampal clusters identified in the encoding analysis; these data for later-remembered and later-forgotten trials are displayed in Fig. 1B for descriptive purposes. Note that these data quantify the effect of the encoding conditions while controlling for any linear effects of detail (due to the inclusion of the parametric regressors in the model). We were interested in whether signal from these encoding clusters would also exhibit an effect of detail. Because these clusters were selected on the basis of an encoding analysis, and moreover that analysis was controlled for the effect of detail (achieved by including detail ratings as a parametric modulation regressor), the contrast of interest (the effect of detail) is independent of voxel selection. We extracted signal from these clusters during trials rated high in detail (i.e., ratings of 2 and 3) and trials rated low in detail (i.e., ratings of 0 and 1). A detail (high, low) by cluster (anterior, posterior) ANOVA showed a significant overall effect of detail (F1,24 = 5.74, P = 0.03), whereas the detail by cluster interaction was not significant (F1,24 = 0.691, P = 0.414). Pairwise comparisons revealed that, whereas a numerical difference between high and low detail was evident in both clusters (Fig. 1C), this effect was significant in the anterior (t24 = 2.42, P = 0.02) but not the posterior (t24 = 1.68, P = 0.11) cluster.
Seed PLS Results.
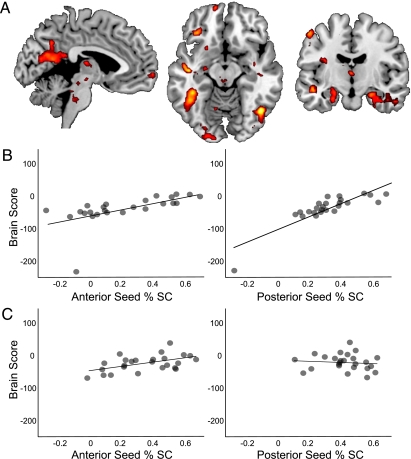
We conducted a seed PLS analysis to determine whether the two hippocampal clusters exhibited similar patterns of functional connectivity, both within the hippocampus (i.e., between these two seeds) and with the rest of the core network. We also examined whether functional connectivity differed according to encoding success. This analysis identified a significant latent variable (LV), (P = 0.006) explaining 57.35% of the covariance, which indicated that functional connectivity for these hippocampal regions were modulated by encoding success. The pattern of functional connectivity peaked at repetition time (TR) 3, and results from this TR are reported here (Table 2). When constructing a simulation that was later remembered, both seeds were strongly functionally connected with each other and with the same distributed pattern of activity in regions such as the bilateral parahippocampal gyrus, medial parietal/cingulate cortex, medial prefrontal cortex, left inferior frontal gyrus, and left inferior parietal lobule (Fig. 2A). This strong connectivity during successful encoding reflects positive correlations between activity in both seed regions and the whole-brain network identified by the LV (anterior seed, r = 0.58; posterior seed, r = 0.85; Fig. 2B). However, when constructing a simulation that was later forgotten, the posterior hippocampal seed region was no longer functionally connected with the anterior hippocampal seed or with the wider network of regions, illustrated by an absence of correlation between posterior hippocampal activity and the whole-brain pattern (r = −0.10; Fig. 2C). However, the connectivity for the anterior hippocampal seed and the wider network was evident irrespective of encoding success (r = 0.50; Fig. 2C).
Table 2.
Regions functionally connected with hippocampal seed regions
| k | Brain region | MNI coordinates |
BSR | ||
| x | y | z | |||
| 8044 | L medial parietal/cingulate cortex (BA 29)* | −12 | −50 | 10 | 15.62 |
| 753 | R inferior occipital gyrus (BA 19) | 50 | −76 | −6 | 14.75 |
| 290 | L precentral gyrus (BA 6) | −38 | −2 | 26 | 11.89 |
| 221 | L superior temporal gyrus (BA 22) | −48 | −12 | −12 | 14.19 |
| 137 | R inferior temporal gyrus (BA 20) | 46 | −14 | −24 | 9.12 |
| 126 | L medial frontal gyrus† (BA 10) | −4 | 66 | −2 | 10.13 |
| 123 | R thalamus* | 0 | −10 | 6 | 7.45 |
| 119 | L cerebellum | −32 | −34 | −32 | 8.14 |
| 104 | L middle frontal gyrus (BA 8) | −40 | 16 | 58 | 8.96 |
| 93 | R parahippocampal/inferior temporal gyrus (BA 20/36) | 36 | −14 | −26 | 6.16 |
| 82 | R fusiform gyrus (BA 20) | 48 | −34 | −22 | 6.84 |
| 78 | L middle frontal gyrus (BA 8) | −28 | 16 | 42 | 9.39 |
| 72 | L thalamus | −12 | −30 | 6 | 8.90 |
| 71 | L inferior frontal gyrus (BA 47) | −38 | 30 | −14 | 9.62 |
| 68 | R middle frontal gyrus (BA 10) | 38 | 54 | 30 | 8.43 |
| 42 | L inferior parietal lobule (BA 40) | −42 | −38 | 32 | 9.06 |
| 40 | L cerebellum* | −6 | −40 | −22 | 5.95 |
| 37 | L precentral gyrus (BA 4) | −54 | −10 | 56 | 6.16 |
| 33 | L parahippocampal gyrus (BA 36) | −22 | 0 | −38 | 7.85 |
| 24 | R cerebellum | 16 | −56 | −34 | 6.10 |
| 23 | R hippocampus | 30 | −30 | −8 | 5.60 |
Only clusters evident during the peak timepoint (TR 3) with a bootstrap ratio of greater than ±4.5 (roughly equivalent to a P value of P < 0.0001) are reported. Cluster size (k) indicates the number of voxels comprising the cluster; only clusters with a minimum extent of 20 voxels are reported. BA, Brodmann area; MNI, Montreal Neurological Institute; BSR, bootstrap ratio; L, left; R, right.
*Cluster extends bilaterally.
Fig. 2.
(A) Seed PLS analysis showed that two hippocampal seed regions (see Methods for coordinates) exhibited functional connectivity with each other and with a distributed pattern of brain regions at TR 3. Warm colors indicate regions significantly correlated with the seeds and the whole-brain pattern and represent the relative strength of this correlation (thresholded at P < 0.0001). (B) Both seeds exhibited connectivity during successful encoding, as indicated by strong positive correlations between seed activity and brain scores (a weighted average of activation across regions exhibiting significant functional connectivity). (C) During unsuccessful encoding, the anterior seed continued to show this connectivity pattern but the posterior seed did not. %SC, percentage of signal change.
Discussion
The aim of this study was to establish whether hippocampal activity evident during episodic simulation of the future reflects the events being encoded into memory. Because prior models suggest that the anterior hippocampus is particularly important for the encoding of novel associative combinations (23, 24), we hypothesized that the anterior aspect of the right hippocampus would be particularly important for the encoding of imagined events. However, our findings suggest that both the anterior and posterior regions of the right hippocampus contribute to the encoding of imagined future events. In fact, our PLS analysis suggests that connectivity of the posterior hippocampus to the anterior hippocampus and other core autobiographical regions might be most critical to successful encoding.
The finding that the right anterior hippocampus is responsive to successful encoding fits with the encoding-retrieval distinction along the long axis of the hippocampus that was proposed over a decade ago (36, 37). More recently, Spaniol et al. (38) conducted a metaanalysis of 26 studies of encoding success and 30 studies of retrieval success and confirmed that the anterior hippocampus was more reliably activated during successful encoding than during successful retrieval. Moreover, the anterior hippocampus appears to be particularly responsive to the encoding of associative (21) and novel (39) information, both of which characterize imagined future events.
The posterior hippocampus was also responsive to encoding success, and functional connectivity analyses showed that this region may be particularly critical for subsequent memory of imagined future events. The connectivity of the posterior hippocampus with the anterior hippocampus and a distributed network of regions was only evident when an imagined future event was successfully encoded. Given that the posterior hippocampus has been shown to be particularly important for the processing of spatial relations (20, 27, 40, 41), the importance of this area for episodic simulation may be in the domain of creating a spatiotemporal context in which to ground the event. Imagining future events requires recall or generation of spatial locations in which to set them and may also include simulated navigation through these contexts, both of which may be supported by the hippocampus (42).
The posterior hippocampus has also been implicated previously in the successful encoding of future-related representations using a different paradigm. Poppenk et al. (43) had participants view scenes in the scanner and either generate future intentions or present actions associated with the scenes. Postscan, participants viewed scene cues and recalled whether the cues were seen in the scanner and if so, under what task conditions (i.e., generating an intention or a present action). Results revealed a right posterior hippocampal activity covaried with overall subsequent memory performance.
The fact that highly detailed imagined events were more likely to be encoded suggests that detail can influence the process of encoding. Relevant here is that constructing a memorable imagined event will depend to some degree on how well the event details could be retrieved from memory. Because the cues for the imagined future events consisted of familiar people, places, and objects, retrieval of these familiar items was necessary before event construction. The posterior hippocampus has been associated with retrieval (24, 37) and the reinstatement of previous conditions (19, 25). Moreover, according to the constructive episodic simulation hypothesis (44), episodic details need to be retrieved from memory to build a coherent scenario, and the posterior hippocampus has been found to respond to the amount of episodic detail comprising both past and future events (28). Indeed, our behavioral results demonstrate that more detailed events were more likely to be successfully encoded. Activity in regions supporting retrieval of contextual and visuospatial information, such as the parahippocampal gyrus (45, 46) and the precuneus (47), also exhibited an encoding effect and/or connectivity with the hippocampal seed regions. It is, however, a challenge to tease apart neural activity related to the retrieval of details from episodic memory and the integration of these details into a coherent imagined scenario. Developing paradigms that can disambiguate these processes is an important focus for future research.
Activity in both the anterior and posterior hippocampal clusters was modulated by participant ratings of event detail, although this effect only reached significance in the anterior cluster. This finding suggests that the contribution of these hippocampal regions to encoding success might depend, at least in part, on the ability to construct a detailed and therefore memorable simulation. A right anterior hippocampal response to detail recombination was also found by Staresina and Davachi (48), who observed that a right anterior hippocampal cluster was more responsive when participants had to integrate spatiotemporally separated details than when details were presented in a combined form. A key component of our study is the integration of event details taken from disparate times and locations into a coherent future simulation, and its similar findings bolster the idea the hippocampus is involved in detail integration.
Our results have important implications for the debate on whether hippocampal damage disrupts the ability to imagine the future (9–15, 17). It is possible that with a damaged hippocampus, the ability to construct detailed scenarios may remain intact, whereas encoding of these representations is disrupted. This appears to be the case both for the children with hippocampal damage who were less accurate in later recalling their imagined events (14) and for the patients with hippocampal damage whose imagined events were described as repetitive, as if they could not recall the portions of the event that they had already constructed (15). Depending on the nature and location of damage—whether it is confined to the anterior/posterior regions identified here, whether it affects the entire hippocampus, and whether it is confined to the hippocampus or also affects other neighboring regions—differential impairments may be seen in tasks that require the generation of imagined episodic details, the encoding of imagined events, or both.
In summary, this study provides a more comprehensive understanding of hippocampal contributions to the construction and encoding of detailed future simulations. We have localized two regions of the right hippocampus involved in this process: one anterior and one posterior, with the connectivity between these and with other parts of the core autobiographical network being particularly necessary for successful encoding. Furthermore, hippocampal activity was modulated by event detail, suggesting that the generation of episodic details and their storage into memory may be related processes. Future thinking confers an adaptive benefit: simulating solutions to potential obstacles increases chances for success and survival (49). Thus, being able to generate detailed simulations and encode these for later use are important aspects of this ability.
Methods
Participants.
Twenty-nine young adults (12 males, aged 18–35) were recruited; all were right handed, fluent in English, and did not meet exclusionary criteria (neurological/psychiatric conditions, ferromagnetic implants, and psychotropic medication use). Four participants (1 male) were excluded due to neurological abnormalities, excessive movement, and task noncompliance; data from 25 participants were analyzed.
Procedure.
We used an adapted version of the episodic recombination (30) and subsequent memory (31) paradigms (Fig. S1) consisting of three phases: a prescan session in which the memory details were collected, a scan session in which participants imagined future events, and a postscan cued recall test for the future event details.
Prescan Session.
Participants described 110 personal episodic events from the past 10 y. For each event, three main details were isolated: a person and object that featured in the event and the specific location of the event. To ensure each detail was distinct, participants could not duplicate details across different events; extensive piloting confirmed that young participants could generate this many details. The memory details were then randomly recombined, resulting in 110 recombined detail sets containing a person, location, and object, each from a different memory. These recombined sets of details were used as cues for the imagined future events during the scan session.
Scan Session.
Participants were presented with three types of trials: future, reimagine, and control. During the 90 future trials, participants were shown the recombined sets of memory details for 8 s (the average time needed to construct a future event, ref. 2) and instructed to imagine (from a field perspective) specific future events that integrated all three detail cues. This was followed by a detail rating scale shown for 4 s (0 = low detail; 3 = vivid detail). In the 45 trials of the control task (30), participants had 8 s in which to incorporate three presented nouns into a sentence of the form “X is bigger than Y is bigger than Z,” making relative size judgments in the process. They then rated task difficulty (0 = no difficulty; 3 = extreme difficulty). During the scanning session, 45 reimagine trials were also presented. SI Methods contains more information. One-fifth of scan time was composed of jittered null (fixation-cross) trials (range, 4–16 s). Optseq2 (50) was used to determine the optimal sequence of experimental and null trials for estimation of the hemodynamic response function.
Postscan Session.
Ten minutes after scanning, participants completed an unexpected cued-recall task that followed the format of Jones’s procedure (51) for testing memory for events with multiple components. Participants were presented with two of the details from each future trial event imagined in the scanner and they recalled the missing detail. As participants were instructed to integrate all three details into an event, memory for the three integrated details gives an indication of the extent to which they were bound and encoded into a coherent scenario. The particular detail tested (person, location, or object) was counterbalanced. This subsequent memory task yielded approximately equal numbers of remembered and forgotten trials, ideal for a subsequent memory analysis. On the basis of this test, each future trial from the scan session was classified as either successfully or unsuccessfully encoded.
MRI Acquisition, Preprocessing, and General Linear Model Analysis.
Full 3T scanning parameters and preprocessing protocol are provided as SI Methods. Each event was modeled by SPM8’s canonical hemodynamic response function (i.e., a stick function) applied at stimulus onset. Trials were modeled as stick functions to capture early activity reflecting the construction phase of future simulation and to be consistent with previous studies identifying construction-related right hippocampal activity (e.g., refs. 2, 8). Each fixed-effects models comprised (i) three regressors of interest: later-remembered future events, later-forgotten future events, and control trials; (ii) parametric modulation regressors for both future event conditions (detail ratings and modeled linearly); and (iii) two regressors of no interest (reimagine condition and rating task). Importantly, with the inclusion of the detail regressors in the model, the contrast images for the later-remembered and later-forgotten conditions quantify the effect of these conditions while controlling for any linear effects of detail. Contrast images for each regressor of interest (relative to implicit baseline) were entered into a random-effects flexible factorial model with two factors, condition and subject. Two contrasts were computed: all imagine future trials > control trials and later-remembered > later-forgotten future events.
For whole-brain analyses, a combined voxelwise threshold of P = 0.005 and a spatial extent threshold of 145 voxels was used to achieve an α of 0.05, corrected for multiple comparisons. The minimum cluster size required for corrected significance was determined using a Monte Carlo simulation (10,000 iterations) implemented using Analysis of Functional Neuroimages’s 3dClustSim program to estimate the overall probability of false positives within the 3D whole-brain search volume (178,888 2 × 2 × 2 mm voxels). We also computed the required cluster size for the correction of multiple comparisons within our a priori region of interest–the bilateral hippocampus (35). Using an anatomical mask of the bilateral hippocampus (generated using MARINA; ref. 52) with a search volume of 1,878 2 × 2 × 2 mm voxels, the Monte Carlo simulation (10,000 iterations) indicated that a voxelwise threshold of P = 0.005 combined with a spatial extent threshold of 26 voxels was required to correct for multiple comparisons at P < 0.05. Information regarding localization and visualization of activation (including extraction of percentage of signal change) are provided in SI Methods.
Functional Connectivity: Seed PLS Analysis.
Two significant clusters within the right hippocampus emerged in the encoding analyses: one anterior (xyz = 20 −12 −16) and one posterior (34 −26 −8). We were interested in whether these regions exhibited similar patterns of functional connectivity, both with each other and with the rest of the core network and whether functional connectivity differed according to encoding success. We used spatiotemporal PLS (53) (for more detail, SI Methods) to assess functional connectivity over an 18-s temporal window. We used the mean percentage of signal change extracted from these regions as “seeds”; correlations were computed between signal in each seed and all other voxels for each condition across subjects. The resulting correlation maps were stacked and analyzed with singular value decomposition, producing a set of orthogonal LVs, each containing a linear contrast between the seeds and the conditions (coding for the effect depicted by voxels) and a singular image of voxel weights, which are proportional to the covariance of activity with the linear contrast. The statistical significance of each LV was assessed using permutation tests (500 iterations) with a threshold of P < 0.05. The reliability of the voxel saliences was computed using bootstrap estimation of the SE (300 iterations). Clusters of five or more voxels in which bootstrap ratios were greater than ±4.5 (roughly equal to P < 0.0001), were considered reliable.
Supplementary Material
Acknowledgments
We thank A. Gilmore, H. Koschwanez, L. Pan, and R. Roberts for assistance and A. Preston and anonymous reviewers for helpful comments. This work was supported by the Royal Society of New Zealand Marsden Grant UOA0810 (to D.R.A.), National Institute of Mental Health Grant MH060941 (to D.L.S.), and a New Zealand International Doctoral Scholarship (awarded to V.C.M.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the SumsDB database (accession no. Martin_PNAS11).
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105816108/-/DCSupplemental.
References
- 1.Okuda J, et al. Thinking of the future and past: The roles of the frontal pole and the medial temporal lobes. Neuroimage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 2.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104:642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyberg L, Kim ASN, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proc Natl Acad Sci USA. 2010;107:22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 6.Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philos Trans R Soc Lond B Biol Sci. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiler JA, Suchan B, Daum I. Foreseeing the future: Occurrence probability of imagined future events modulates hippocampal activation. Hippocampus. 2010;20:685–690. doi: 10.1002/hipo.20695. [DOI] [PubMed] [Google Scholar]
- 8.Addis DR, Cheng T, Roberts R, Schacter DL. Hippocampal contributions to the episodic simulation of specific and general future events. Hippocampus. 2011 doi: 10.1002/hipo.20870. 10.1002/hipo.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Race E, Keane MM, Verfaellie M. Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci. 2011;31:10262–10269. doi: 10.1523/JNEUROSCI.1145-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andelman F, Hoofien D, Goldberg I, Aizenstein O, Neufeld MY. Bilateral hippocampal lesion and a selective impairment of the ability for mental time travel. Neurocase. 2010;16:426–435. doi: 10.1080/13554791003623318. [DOI] [PubMed] [Google Scholar]
- 12.Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Maguire EA, Vargha-Khadem F, Hassabis D. Imagining fictitious and future experiences: Evidence from developmental amnesia. Neuropsychologia. 2010;48:3187–3192. doi: 10.1016/j.neuropsychologia.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JM, Vargha-Khadem F, Gadian DG, Maguire EA. The effect of hippocampal damage in children on recalling the past and imagining new experiences. Neuropsychologia. 2011;49:1843–1850. doi: 10.1016/j.neuropsychologia.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Squire LR, et al. Role of the hippocampus in remembering the past and imagining the future. Proc Natl Acad Sci USA. 2010;107:19044–19048. doi: 10.1073/pnas.1014391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 2010;61:27–48, C1–C8. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 17.Maguire EA, Hassabis D. Role of the hippocampus in imagination and future thinking. Proc Natl Acad Sci USA. 2011;108:E39. doi: 10.1073/pnas.1018876108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giovanello KS, Schnyer D, Verfaellie M. Distinct hippocampal regions make unique contributions to relational memory. Hippocampus. 2009;19:111–117. doi: 10.1002/hipo.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woollett K, Maguire EA. Navigational expertise may compromise anterograde associative memory. Neuropsychologia. 2009;47:1088–1095. doi: 10.1016/j.neuropsychologia.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17:1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- 24.Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- 26.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 27.Maguire EA, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proc Natl Acad Sci USA. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Addis DR, Schacter DL. Constructive episodic simulation: Temporal distance and detail of past and future events modulate hippocampal engagement. Hippocampus. 2008;18:227–237. doi: 10.1002/hipo.20405. [DOI] [PubMed] [Google Scholar]
- 29.Ingvar DH. “Memory of the future”: An essay on the temporal organization of conscious awareness. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- 30.Addis DR, Pan L, Vu M-A, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Wagner AD, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 32.Botzung A, Denkova E, Manning L. Experiencing past and future personal events: Functional neuroimaging evidence on the neural bases of mental time travel. Brain Cogn. 2008;66:202–212. doi: 10.1016/j.bandc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. J Neurosci. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summerfield JJ, Hassabis D, Maguire EA. Differential engagement of brain regions within a ‘core’ network during scene construction. Neuropsychologia. 2010;48:1501–1509. doi: 10.1016/j.neuropsychologia.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yassa MA, Stark CEL. Multiple signals of recognition memory in the medial temporal lobe. Hippocampus. 2008;18:945–954. doi: 10.1002/hipo.20452. [DOI] [PubMed] [Google Scholar]
- 36.Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 37.Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 38.Spaniol J, et al. Event-related fMRI studies of episodic encoding and retrieval: Meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 39.Köhler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: A comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- 40.Ryan L, Lin C-Y, Ketcham K, Nadel L. The role of medial temporal lobe in retrieving spatial and nonspatial relations from episodic and semantic memory. Hippocampus. 2010;20:11–18. doi: 10.1002/hipo.20607. [DOI] [PubMed] [Google Scholar]
- 41.Hassabis D, et al. Decoding neuronal ensembles in the human hippocampus. Curr Biol. 2009;19:1–9. doi: 10.1016/j.cub.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poppenk J, Moscovitch M, McIntosh AR, Ozcelik E, Craik FIM. Encoding the future: Successful processing of intentions engages predictive brain networks. Neuroimage. 2010;49:905–913. doi: 10.1016/j.neuroimage.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 44.Schacter DL, Addis DR. The cognitive neuroscience of constructive memory: Remembering the past and imagining the future. Philos Trans R Soc Lond B Biol Sci. 2007;362:773–786. doi: 10.1098/rstb.2007.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 46.Szpunar KK, Chan JCK, McDermott KB. Contextual processing in episodic future thought. Cereb Cortex. 2009;19:1539–1548. doi: 10.1093/cercor/bhn191. [DOI] [PubMed] [Google Scholar]
- 47.Cavanna AE, Trimble MR. The precuneus: A review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 48.Staresina BP, Davachi L. Mind the gap: Binding experiences across space and time in the human hippocampus. Neuron. 2009;63:267–276. doi: 10.1016/j.neuron.2009.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30:299–313, discussion 313–351. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- 50.Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones GV. A fragmentation hypothesis of memory: cued recall of pictures and of sequential position. J Exp Psychol Gen. 1976;105:277–293. [Google Scholar]
- 52.Walter B, et al. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses. 2003 In: 9th International Conference on Functional Mapping of the Human Brain. New York, NY. [Google Scholar]
- 53.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.