Abstract
Pavlovian olfactory learning in Drosophila produces two genetically distinct forms of intermediate-term memories: anesthesia-sensitive memory, which requires the amnesiac gene, and anesthesia-resistant memory (ARM), which requires the radish gene. Here, we report that ARM is specifically enhanced or inhibited in flies with elevated or reduced serotonin (5HT) levels, respectively. The requirement for 5HT was additive with the memory defect of the amnesiac mutation but was occluded by the radish mutation. This result suggests that 5HT and Radish protein act on the same pathway for ARM formation. Three supporting lines of evidence indicate that ARM formation requires 5HT released from only two dorsal paired medial (DPM) neurons onto the mushroom bodies (MBs), the olfactory learning and memory center in Drosophila: (i) DPM neurons were 5HT-antibody immunopositive; (ii) temporal inhibition of 5HT synthesis or release from DPM neurons, but not from other serotonergic neurons, impaired ARM formation; (iii) knocking down the expression of d5HT1A serotonin receptors in α/β MB neurons, which are innervated by DPM neurons, inhibited ARM formation. Thus, in addition to the Amnesiac peptide required for anesthesia-sensitive memory formation, the two DPM neurons also release 5HT acting on MB neurons for ARM formation.
Keywords: brain, olfaction, aversive conditioning, neurotransmitter
Pavlovian olfactory learning in Drosophila involves coincidence detection of a conditioned stimulus (CS), an odor, and an unconditioned stimulus (US), an electric shock (1). After one session of aversive olfactory conditioning, flies can form short- and intermediate-term memories but not long-term memory (LTM), which requires repetitive spaced training and is dependent on protein synthesis (2, 3). The intermediate-term memory has been dissected further into an anesthesia-sensitive form (ASM) that requires a gene called “amnesiac” and an anesthesia-resistant form (ARM) that requires the radish gene (4–6). Three hours after one session of training, ARM and ASM contribute equally to performance and can be distinguished by application of a short cold shock-induced anesthesia that impairs ASM but not ARM. Importantly, although ARM is consolidated, in the sense that it is resistant to cold shock, it is not thought to be a protein synthesis-dependent memory because it is resistant to cycloheximide (CXM) (2). One day after repetitive spaced training, both ARM and LTM are thought to contribute to memory performance. In contrast, repetitive massed training without rest intervals induces only radish-dependent ARM without detectable cAMP response element binding protein-dependent LTM measured 1 d after training (2, 3) (but see ref. 2 for an alternative model). Thus, more than one genetically distinct memory-storage system contributes to performance both at intermediate time points (e.g., 3 h after one training session) and at later time points (e.g., 24 h after repetitive training). Given the complexity of memory consolidation observed at the genetic level, much effort has been directed toward attempts to integrate conceptually the genetically defined memory phases with biochemical signaling and neural circuits.
In adult Drosophila, each of the paired mushroom bodies (MBs) consists of ∼2,500 intrinsic Kenyon cells whose segregated dendritic fields in the calyx receive olfactory information from the antennal lobes (8–10). Based on birth timing and axon configurations, Kenyon cells fall into three major classes: the early-born γ neurons forming a single medial lobe; α′/β′ neurons branching into a vertical α′ and a medial β′ lobe; and the late-born α/β neurons branching into a vertical α and a medial β lobe (11, 12). A coherent picture has begun to emerge in which CS and US information converges on MBs, and coincidence detection within the MBs leads to the plasticity that underlies learning (13–15). Formation of aversive olfactory memory requires normal expression of many genes in the MBs (13), including the rutabaga gene encoding Ca2+-responsive adenylyl cyclase (16). Importantly, two dorsal paired medial (DPM) neurons that innervate all MB lobes are believed to release the Amnesiac peptide required for modulation of the cAMP-signaling pathway in the MB neurons (17). In contrast, it remains unknown which presynaptic neurons and which neurotransmitter modulate ARM formation, although blocking MB α/β and γ output after learning abolishes retrieval of the intermediate-term memory which includes both ASM and ARM (18–21).
We investigated the role of serotonin (5HT), a monoamine neurotransmitter synthesized from the amino acid l-tryptophan by a metabolic pathway, with two enzymes: tryptophan hydroxylase and amino acid decarboxylase (DDC) (22). In mammals, 5HT and its receptors play a key role in memory formation through modulation of enzymes including the cAMP-dependent protein kinase, the Ca2+ calmodulin-dependent protein kinase II, and MAPKs (23). 5HT also modulates glutamate, GABA, and acetylcholine which is involved in various aspects of cognitive functions including learning and memory (24). In Aplysia, when 5HT application is coupled with synaptic activity, PKC is activated, and a form of intermediate-term facilitation is induced that lasts for hours after stimulation (25). In Drosophila, four types of 5HT receptors have been reported: d5HT1A, d5HT1B, d5HT2, and d5HT7 (26). d5HT1B, expressed in the eight clock neurons, is required for normal entrainment of circadian rhythm (27), and d5HT1A, expressed in the MB, is required for normal sleep (28). Because of the broad serotonergic innervations and differential expression of the distinct 5HT receptors, it is unclear how 5HT modulates specific behavioral functions. With genetic tools for spatiotemporal manipulation, we found that two DPM neurons synthesize and release 5HT acting via d5HT1A receptors on the α/β neurons for ARM formation without affecting learning, ASM, or LTM.
Results
5HT Is Required for ARM Formation.
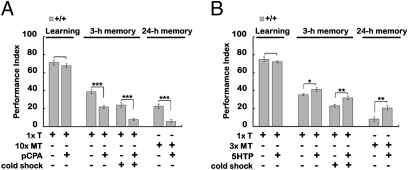
To address whether 5HT is required for aversive olfactory associative memory in Drosophila, we started by manipulating 5HT levels pharmacologically. Feeding wild-type flies with dl-p-chlorophenylalanine (pCPA), an inhibitor of 5HT synthesis (29), produced a significant suppression of intermediate-term memory, measured 3 h after training, without affecting immediate short-term memory, measured 2 min after training (Fig. 1A). This memory suppression predominantly impacted ARM, because cold-induced anesthesia that abolishes ASM but not ARM could suppress memory further in the pCPA-fed flies. The specificity of the 5HT effect on ARM was strengthened further by the result that pCPA impaired 24-h memory after massed training that produced only ARM. In contrast, feeding wild-type flies with l-5-hydroxytryptophan (5HTP), a 5HT precursor (30), enhanced intermediate-term memory without affecting learning after only one session of olfactory conditioning (Fig. 1B). Again, cold-induced anesthesia did not alter the enhancement of intermediate-term memory, suggesting that ARM rather than ASM was enhanced by 5HTP. The 5HT-dependent ARM enhancement was confirmed again using a shortened massed training protocol that induced lower ARM formation 1 d after learning. The effectiveness of pharmacological manipulation was verified by 5HT-antibody immunohistochemistry showing an elevated immunoreactive signal in the central brain after 5HTP feeding and a reduced immunoreactive signal after pCPA feeding (Fig. S1 A–C). Also, 5HT immunolabeling showed that the elevated 5HT immunoreactive signal did not occur in tyrosine hydroxylase (TH) neurons (Fig. S2), excluding the possibility of ectopic serotonin synthesis in dopamine neurons caused by 5HTP feeding as reported in larval CNS (31). Together, through manipulation of 5HT levels, our results suggest that 5HT is required specifically for the formation of ARM and not for the acquisition of ASM.
Fig. 1.
Effects of pharmacological manipulation of 5HT level on aversive olfactory memory. (A) Learning and memories in flies fed with pCPA, a tryptophan hydroxylase inhibitor. (B) Learning and memories in flies fed with 5HTP, a 5HT precursor. Flies were subjected to 1 session of training (1× T), 3 sessions of massed training (3× MT), 3 sessions of spaced training (3× ST), or 10 sessions of massed training (10× MT). Memory was measured immediately (Learning), 3 h, or 24 h after training. Cold-induced anesthesia was performed 2 h after training. The mean ± SEM was plotted for each treatment; n = 8–10 values for each group. *P < 0.05; **P < 0.01; ***P < 0.001.
Reduced 5HT Synthesis Impairs ARM Similar to the Effect of radish Mutant.
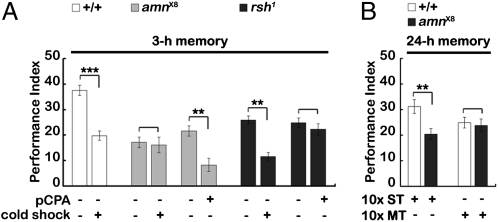
Cold-induced anesthesia during memory consolidation blocks ASM formation and is dependent on amnesiac gene expression in the two DPM neurons. In contrast, Amnesiac protein is not required for ARM formation, which instead is dependent on the radish gene (6, 17, 32, 33), whose product is expressed in MB (33). To address whether 5HT and Radish protein are part of the same pathway for ARM formation, we performed the following experiments (Fig. 2). We first confirmed that 3-h memory impairment after cold-induced anesthesia was reduced further in the radish mutant (rsh1) but not in the amnesiac mutant (amnX8) (Fig. 2A). In contrast, reducing 5HT levels by pCPA feeding reduced 3-h memory in amnX8 but had no effect in rsh1, suggesting that 5HT and Radish protein are in the same pathway for ARM formation (Fig. 2A). Furthermore, 24-h memory in amnX8 was impaired after 10 sessions of spaced training but not after massed training, suggesting that ARM remains intact in amnX8 (Fig. 2B).
Fig. 2.
Effects of pCPA feeding in amnX8 and rsh1 mutants. (A) Effects on 3-h memory after a single session of training. Cold-induced anesthesia was performed 2 h after training. (B) Effects on 24-h memory after 10 sessions of spaced training (10× ST) or massed training (10× MT). The mean ± SEM was plotted for each treatment; n = 8 values for each group. **P < 0.01; ***P < 0.001.
5HT Acts on α/β Neurons for ARM Formation.
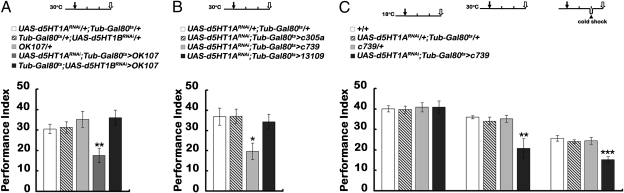
Serotonergic neurons project to most brain regions in Drosophila (34). Both Radish protein (33) and the d5HT1A serotonin receptors are expressed in the MBs (28), prompting us to examine whether 5HT acts on MB neurons for ARM formation. We addressed this question by RNAi-mediated down-regulation of specific 5HT receptors in all or subsets of MB neurons (Fig. S3). Using OK107-Gal4 to drive expression in the whole MB, we found that normal expression of the d5HT1A receptor but not the d5HT1B receptor in the MB neurons is necessary for the formation of 3-h memory (Fig. 3A). Next, by knocking down the expression of the d5HT1A receptor locally, we found that it was required only in the α/β neurons (c739), but not in the α′/β′ neurons (c305a) or γ neurons (13109), for normal 3-h memory (Fig. 3B). To minimize any developmental contribution, we confirmed the necessity of d5HT1A in α/β neurons by temporally regulated reduced expression in adults with Gal80ts (Fig. 3C) after showing that control flies kept at low temperature had normal formation of 3-h memory. Finally, cold-induced anesthesia further suppressed 3-h memory caused by down-regulation of the d5HT1A receptor in α/β neurons (Fig. 3C), an effect similar to that seen in reducing 5HT levels (Fig. 1A) or in the radish mutant (Fig. 2). Thus, these data suggest that 5HT and its d5HT1A receptors acts via the Radish pathway for ARM formation in the α/β neurons.
Fig. 3.
Requirement of 5HT receptor in MB neurons for intermediate-term memory formation. (A) Temporal down-regulation of 5HT receptors with UAS-d5HT1ARNAi or UAS-d5HT1BRNAi in OK107 neurons impairs 3-h memory. (B) Effects of temporal down-regulation of d5HT1A receptors in different subsets of MB neurons. (C) The role of d5HT1A receptors in α/β neurons. For temporal expression of RNAi, Tub-Gal80ts inhibition of Gal4 expression was removed by maintaining flies at 30 °C for 5 d before training. Control flies were maintained at 18 °C at all times. OK107-Gal4, all MB neurons; c305a-Gal4, α′/β′ neurons; c739-Gal4, α/β lobe neurons; 13109-Gal4, γ lobe neurons. Dark arrows indicate the start training. Open arrows indicate testing time. The arrowhead indicates cold-shock time. In all cases, memory was measured 3 h after a single session of training. The mean ± SEM was plotted for each genotype; n = 8 values for each group. *P < 0.05; **P < 0.01; ***P < 0.001.
Two DPM Neurons Are Serotonergic.
Next, we asked which neurons release 5HT to modulate ARM formation in the α/β neurons. To manipulate specific subsets of serotonergic neurons, we used five different Gal4 drivers with preferential expression in serotonergic neurons: Trh247-Gal4, Trh493-Gal4, Trh819-Gal4, Trh996-Gal4 (Materials and Methods), and Ddc-Gal4 (35) (Fig. S4). Whole-mount immunostaining showed 52.3 ± 2.0 (n = 15) 5HT-antibody immunoreactive neurons in the central brain and suboesophageal ganglion. Each of these drivers labeled 25–30 serotonergic neurons, as indicated by double labeling with UAS-nls::GFP and 5HT antibody (Table S1). We found that Trh493-Gal4 expressed GFP only in fibers that also were 5HT-antibody immunoreactive in the MB lobes (Fig. S4 A5–E5). These serotonergic fibers ramify extensively within all MB lobes but were absent from the posterior half of the peduncle, a morphology similar to the fibers of previously reported DPM neurons (17, 36), suggesting that they were not derived from Kenyon cells (Fig. S4B 5 and 6). Other Trh-Gal4 drivers (i.e., Trh247-Gal4, Trh819-Gal4, Trh996-Gal4, and Ddc-Gal4) did not express in DPM neurons (Fig. S4).
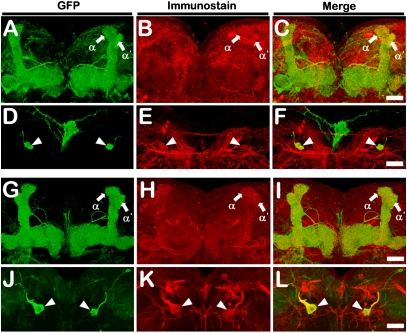
To determine if DPM neurons are the only source of 5HT in the MB lobes, we performed 5HT-antibody immunostaining using four DPM drivers: c316-Gal4 (Fig. S5 A–D), L0111-LexA (Fig. S5 E–H), 2721-Gal4 (Fig. S5 I–L), and VT64246-Gal4 (Fig. S5 M–P). VT64246-Gal4 is specific for DPM neurons, with no detectable expression elsewhere in the brain. In all cases, 5HT-antibody immunoreactive signals were found in the cell bodies and fibers of the DPM neurons (Fig. 4 A–F and Fig. S5 A–P). Tracing the DPM neuron from the cell body to all its terminals in the MBs with high-resolution 3D imaging, we confirmed that all 5HT-antibody immunoreactive signals in the MB lobes are localized on the DPM fibers (Movie S1 and Fig. S5 Q–T). Finally, we showed that the DPM neurons also are immunoreactive with antibody against DDC, another essential enzyme for making 5HT (Fig. 4 G–L).
Fig. 4.
The two DPM neurons are serotonergic. (A–F) 5HT-antibody immunostaining. (G–L) DDC-antibody immunostaining. The two DPM neurons labeled by GFP (A, D, G, J) are immunopositive to both 5HT antibody (B, E) and DDC antibody (H, K) in flies carrying the UAS-mCD8::GFP;c316-Gal4 transgene. Each close-up image is a projection of several neighboring optical slices through mushroom bodies (A–C, G–I) or the soma of the two DPM neurons (D–F, J–L). Arrows indicate the tip of an α or α′ lobe. Arrowheads indicate the soma of the two DPM neurons. (Scale bars: 20 μm.)
5HT from DPM Neurons Is Required for ARM Formation.
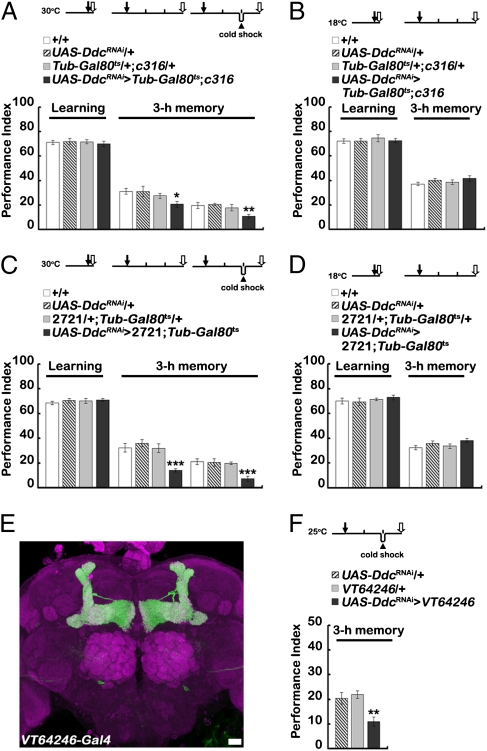
We then investigated the role of 5HT in ARM formation by direct manipulation of 5HT production with DdcRNAi expression in the two DPM neurons without affecting other serotonergic neurons or vice versa. To avoid developmental defects, Gal4 expression was suppressed using temperature-sensitive Gal80ts, keeping flies at 18 °C until adult eclosion, and then raising the temperature to 30 °C for 5 d before learning trials. These adult flies carrying UAS-DdcRNAi/+;Tub-Gal80ts/+;c316-Gal4/+ transgenes exhibit normal olfactory learning but show a significant defect in 3-h memory (Fig. 5A). In contrast, adult flies kept continuously at 18 °C exhibited normal learning and 3-h memory (Fig. 5B). The impairment in 3-h memory independent of cold shock at 2 h after training was confirmed again using 2721-Gal4 as a more specific DPM driver for the same RNA knockdown approach (Fig. 5 C and D). An additive effect of DdcRNAi and cold shock was unlikely to have been derived from nonspecific performance effects of the treatment, because cold shock had no effect on amnX8 mutant flies (Fig. 2A), as previously reported (6). Furthermore, 3-h memory was abolished completely by blocking neurotransmission with shits1 followed immediately by cold shock 1 h after training, the time when memory was more sensitive to anesthesia (Fig. S6). Thus, the DdcRNAi-induced impairment of 3-h memory, similar to the impairment in the rsh1 mutant flies (Fig. 2A) and the effects of manipulating 5HT levels and d5HT1A receptors (Figs. 1–3), suggests that the 5HT from DPM neurons is necessary for ARM formation.
Fig. 5.
The role of 5HT synthesis in DPM neurons in learning and memory. (A) Effects of temporal expression of DdcRNAi in the c316-Gal4 neurons. (B) Control c316-Gal4 flies kept at 18 °C have normal learning and 3-h memory. (C) Effects of postdevelopment temporal induction of DdcRNAi in the 2721-Gal4 neurons. (D) Control 2721-Gal4 flies kept at 18 °C have normal learning and 3-h memory. (E) VT64246-Gal4 expression pattern reported by UAS-mCD8::GFP (green). Brain structure was counterstained with Discs large-antibody immunostain (magenta). (Scale bar: 20 μm.) (F) Impairment of 3-h memory by driving DdcRNAi expression in VT64246-Gal4 and cold shock. Black arrows indicate the start of training. Open arrows indicate testing time. The arrowhead indicates cold-shock time. The mean ± SEM was plotted for each genotype; n = 8–15 values for each group. *P < 0.05; **P < 0.01; ***P < 0.001.
DDC also is required for dopamine synthesis, but several lines of evidence indicate that DdcRNAi specifically down-regulates serotonin production in DPM neurons. First, the effectiveness of the DdcRNAi after heat-shock induction was confirmed by Western blot showing a reduced amount of DDC (Fig. S7A). Second, serotonin production was reduced significantly in flies carrying elav-Gal4>UAS-DdcRNAi transgenes (Fig. S7B). Third, ThRNAi-induced down-regulation of TH mRNA encoding another enzyme required for dopamine synthesis in the DPM neurons did not affect learning and 3-h memory (Fig. S7C). The effectiveness of ThRNAi was confirmed by its expression in Th-Gal4 neurons, leading to impaired olfactory learning (Fig. S7D). Fourth, DPM neurons were immunonegative for antibody staining of TH (Fig. S7 E–G). Fifth, spatial specificity of the requirement of DDC in the DPM neuron for normal ARM was confirmed by driving DdcRNAi with VT64246-Gal4, a DPM-specific Gal4 driver (Fig. 5 E and F). Finally, by temporal induction after development of both DdcRNAi and TrhRNAi in the c316-Gal4 neurons, we confirmed again that 5HT from DPM neurons is required for ARM formation (Fig. S7H).
Other Serotonergic Neurons Are Not Involved in ARM Formation.
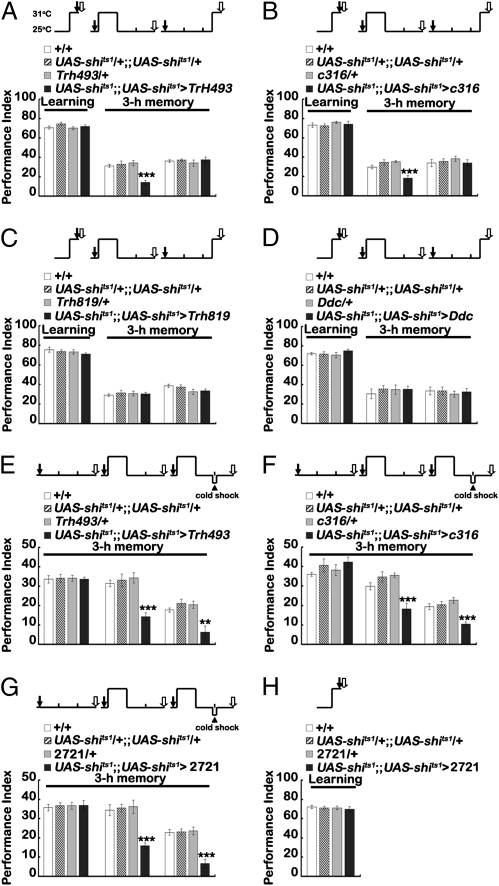
Multiple Gal4 drivers expressed in different subsets of serotonergic neurons provide an opportunity to address whether other non-DPM serotonergic neurons also are required for aversive olfactory memory formation. Using L0111-LexA as a reference for labeling the two DPM neurons, we first confirmed that c316-Gal4, L0111-LexA, and Trh493-Gal4 contained the same DPM neurons that were not labeled by Trh819-Gal4 and Ddc-Gal4 (Fig. S8). By blocking neurotransmission with UAS-shits1at the restrictive temperature (37), we found that outputs from Trh493-Gal4 and c316-Gal4 neurons were required during memory consolidation but were not required during learning or retrieval of 3-h memory (Fig. 6 A and B) (38–40). In contrast, blocking neurotransmission from Trh819-Gal4 and Ddc-Gal4 neurons did not affect learning, consolidation, or retrieval of 3-h memory (Fig. 6 C and D). These results suggest that only the two DPM neurons, and not any other serotonergic neurons expressed in Trh819-Gal4 and Ddc-Gal4, are required for 3-h memory.
Fig. 6.
Effects of DPM neuron outputs on ARM. (A) Trh493-Gal4, (B) c316-Gal4, (C) Trh819-Gal4, and (D) Ddc-Gal4 were used as drivers to express UAS-shits1 for temporal manipulation of serotonergic outputs. Blocking neurotransmission from Trh493-Gal4 neurons or c316-Gal4 neurons impairs 3-h memory during formation but not during learning or retrieval. The same manipulation on Trh819-Gal4 neurons or Ddc-Gal4 neurons has no effect. (E) Trh493-Gal4, (F) c316-Gal4, and (G) 2721-Gal4 were used as drivers to express UAS-shits1 in DPM neurons. (H) Blocking neurotransmission from 2721-Gal4 neurons does not affect learning. Dark arrows indicate start training. Open arrows indicate testing time. The arrowhead indicates cold-shock time. The mean ± SEM was plotted for each genotype; n = 8 values for each group. **P < 0.01; ***P < 0.001.
Next, we asked if blocking neurotransmission from DPM neurons specifically impairs ARM rather than ASM. We used three independent Gal4 drivers with preferential DPM expression. In each case, we measured 3-h memory performance after blocking neurotransmission from DPM neurons with shits1 immediately after training for 1 h. In each case, we observed the expected decrement in 3-h memory performance. Also, in each case, performance was reduced further by blocking ASM via application of cold-induced anesthesia 2 h after training (Fig. 6 E–G). The outputs from 2721-Gal4 were not required during the learning assay (Fig. 6H), consistent with the original reports of DPM cell temporal requirements (17). These results are consistent with the effects of manipulation of 5HT biosynthesis, d5HT1A receptor expression, and the radish gene (Figs. 1–3). Together, these convergent data indicate that the two DPM neurons are essential for both ASM and ARM, and are the only serotonergic neurons required for ARM formation.
Discussion
5HT in Memory Formation.
The key finding of our study is that 5HT from the two DPM neurons innervating all MB lobes is required specifically for ARM formation in aversive olfactory conditioning in Drosophila. This conclusion is supported by five independent lines of evidence. First, pharmacological inhibition of 5HT synthesis reduces memory formation that is additive to impaired ASM (i.e., with the amnesiac mutant or in flies subjected to cold-induced anesthesia) but is occluded in the radish mutant in which ARM already is abolished (Figs. 1A and 2). Second, pharmacological increase of 5HT enhances memory formation that is unaffected by cold-induced anesthesia (Fig. 1B). Third, the two DPM neurons are immunoreactive to 5HT (Fig. 4) but not to TH (Fig. S7 E–G). Fourth, temporal reduction of 5HT synthesis in the DPM neurons specifically abolishes ARM (Fig. 5). Fifth, blocking neurotransmission from the DPM neurons but not other serotonergic neurons specifically abolishes ARM without affecting acquisition or retrieval (Fig. 6 and Fig. S8). Although it also has been suggested that DPM neurons are cholinergic, because Cha3.3kb-Gal80 suppresses c316-Gal4 expression (38), further validation by more reliable methods such as immunostaining before and after down-regulation by ChaRNAi is necessary. Our results show that 5HT from the DPM neurons is the primary neurotransmitter involved in ARM formation because DdcRNAi inhibition of 5HT synthesis or blocking neurotransmission with the UAS-shits1 transgene results in a similar degree of memory impairment that is damaged further by cold-induced anesthesia (Figs. 5 and 6 and Fig. S6). On the other hand, 5HT is necessary for Drosophila place memory, as evidenced by genetic manipulation of the 5HT level and neurotransmission from the Ddc-Gal4–positive neurons (41). Whether the two serotonergic DPM neurons are involved in place memory requires further examination, because Ddc-Gal4 does not express in the DPM neurons (Figs. S4 and S8).
ARM Circuit.
Three lines of evidence suggest that ARM forms within the MB α/β neurons. First, ARM formation requires normal expression of d5HT1A only in the α/β neurons (Fig. 3). Second, Radish protein required for ARM formation is expressed preferentially in the α/β and γ neurons, but not in the α′/β′ neurons (33). Third, ARM retrieval requires neurotransmission from α/β neurons (7). Although the molecular basis for ARM formation is largely unknown, ARM formation generally is believed not to require new protein synthesis and may involve posttranslational modifications of existing proteins (2, 3). Thus, far, two proteins have demonstrated roles in ARM formation in Drosophila aversive olfactory conditioning: Radish (6, 33) and a persistently active, truncated, atypical PKC, atypical protein kinase M (PKM), (42). Mutant Radish flies show normal learning but fail to form ARM. Atypical PKM appears to act downstream of Radish, because induction of atypical PKM can rescue the radish mutant phenotype (42). Persistent activation of PKCs during memory formation has been shown in other animals, including honey bee and Aplysia (43, 44). Together, these data indicate that, through activation of d5HT1A receptor, 5HT released from the DPM neurons sets Radish and atypical PKM in action to form ARM in the α/β neurons.
Spatiotemporal Control of Amnesiac and 5HT.
How does the DPM neuron regulate its release of the Amnesiac peptide and 5HT to modulate ASM and ARM formation? Previous studies have shown that the DPM neuron is responsive to both US and CS in aversive olfactory conditioning and exhibits a delayed memory trace dependent on the normal function of Amnesiac (36). Blocking DPM neuron output with the UAS-shits1 transgene produces an effect similar to mutation of the amnesiac gene, one that abolishes half or more of the intermediate-term memory (38). In contrast, our findings combining UAS-shits1 transgene expression and cold-induced anesthesia (Fig. 6) suggest that the temporal defect of the dominant negative shits1 blocks 5HT release. However, rescue experiments clearly indicate a role for the amnesiac gene within the DPM cells. Amnesiac is a putative neuropeptide whose release probably would be impacted by shits1 on a slower time scale. Indeed there is some evidence that longer-duration blocking of shits1 in DPM neurons can ablate performance fully (39, 40), an effect that, by definition, includes both ASM and ARM.
It also is particularly interesting that formation of olfactory memory requires sequential involvement of different subsets of MB neurons (20). The early memory trace has been visualized only in the α′/β′ neurons in which neurotransmission outputs are required for memory acquisition and consolidation but not for retrieval (20, 45). Consistent with this notion, developmental disruption of DPM neurons, so that their fibers project mostly but not entirely to the α′/β′ lobes, produces significantly higher memory than the amnesiac mutant (39), suggesting that the Amnesiac peptide acts primarily on α′/β′ neurons. However, DPM neurons ramify not only in the α′/β′ lobes but throughout all MB lobes, and their memory traces show no evidence of MB lobe specificity (36). Together with our findings, these results suggest that DPM neurons modulate two memory mechanisms by release of Amnesiac and 5HT separately in both time and place: Amnesiac modulates an α′/β′ activity needed to support the plasticity in γ and α/β neurons that underlies ASM, whereas 5HT acts on its receptor on α/β neurons for ARM formation. Nevertheless, the presence of 5HT in all terminals of the DPM, as revealed by high-resolution 3D imaging (Fig. S5 Q–T), suggests that it plays additional functions in other MB lobes as well.
It has been suggested that in mammals 5HT and its receptors play key roles in psychosis, cognition, and mood via modulation of complex intracellular signaling pathways. During memory formation, activation of the 5HT1A receptor inhibits calcium channels, increases potassium conductance, inhibits adenylyl cyclase activity, reduces cAMP accumulation, and activates phospholipase C (24, 46). Interestingly, radish mutants with impaired ARM formation show attention-deficit and hyperactivity-like behavior that can be rescued by Ritalin, a drug used to treat human attention-deficit hyperactivity disorder that involves dysfunctions of the dopamine and 5HT systems (47, 48). Given the purported role of 5HT in depression and many other brain disorders that affect memory, Drosophila ARM facilitated by only two serotonergic DPM neurons provides a simple reductionist model, with sophisticated genetic tools that will allow dissection of the interactions between different memory systems.
Materials and Methods
Fly Strains.
Fly stocks were raised on standard cornmeal-yeast-agar medium at 25 °C and 60–70% relative humidity under a 12/12-h light/dark cycle. Ddc-Gal4, UAS-mCD8::GFP, Tub-Gal80ts, 13109-Gal4 (BG00015), and 2721-Gal4 (GawB 5015) were obtained from Bloomington Stock Center. Th-Gal4 was from Drosophila Genomics Resource Center. UAS-d5HT1ARNAi, UAS-d5HT1BRNAi, UAS-DdcRNAi, UAS-TrhRNAi, and UAS-ThRNAi lines were from Vienna Drosophila RNAi Center. UAS-Dicer and VT64246-Gal4 were from Barry Dickson. LexAop-GFP was from Tzumin Lee. Trh247-Gal4 (III), Trh493-Gal4 (III), Trh819-Gal4 (III), and Trh996-Gal4 (X) were generated by Trh and Gal4 fusions, with the numbers indicating the remaining number of 5′ flanking bases. Trh-GAL4 deletions containing the indicated amount of 5′ flanking DNA were constructed by primer mutagenesis starting with the Trh-GAL4 construct. To achieve acute and temporal down-regulation of the expression of Ddc, Trh, Th, d5HT1A, or d5HT1B without causing significant developmental defect, flies carrying specific RNAi were raised at 18 °C until eclosion and then were transferred to 30 °C for 5 d before behavior assays. To block neurotransmission acutely, flies carrying the UAS-shits1;;UAS-shits1 transgenes crossed with specific Gal4 drivers were raised at 18 °C until eclosion and then were transferred to 25 °C for 3 d to allow sufficient Shibire protein expression before experiments were performed.
Statistics.
Statistical analyses were performed using KaleidaGraph 4.1 (Synergy Software). Behavioral data were evaluated via one-way ANOVA followed by planned comparisons among the relevant groups with a Tukey Honestly Significant Difference test. All data are presented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Details of cold shock and drug feeding, behavior assay, immunohistochemistry, quantitative measurement of serotonin, and Western blot are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Barry Dickson for sharing the DPM-specific driver VT64246-Gal4; the Bloomington Stock Center, Drosophila Genomics Resource Center, and the Vienna Drosophila RNAi Center for the fly stocks; and the Developmental Studies Hybridoma Bank for the 4F3 anti-discs large antibody developed by Corey Goodman. We also thank members of the A.-S.C. laboratory for discussions and sharing transgenic tools. This work was supported by grants from the Ministry of Education and National Science Council (to A.-S.C.), National Science Council Grant 98-2311-B-260-001-MY3 (to T.-F.F.), National Institutes of Health Grant GM 84128 (to J.H.), and grants from the National Institutes of Health, Beckman Foundation, and Human Frontier Science Program (to J.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019483108/-/DCSupplemental.
References
- 1.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 2.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 3.Yin JC, et al. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Tully T, Kalderon D. Effects of a conditional Drosophila PKA mutant on olfactory learning and memory. Learn Mem. 1996;2:320–333. doi: 10.1101/lm.2.6.320. [DOI] [PubMed] [Google Scholar]
- 5.Dubnau J, Tully T. Gene discovery in Drosophila: New insights for learning and memory. Annu Rev Neurosci. 1998;21:407–444. doi: 10.1146/annurev.neuro.21.1.407. [DOI] [PubMed] [Google Scholar]
- 6.Folkers E, Drain P, Quinn WG. Radish, a Drosophila mutant deficient in consolidated memory. Proc Natl Acad Sci USA. 1993;90:8123–8127. doi: 10.1073/pnas.90.17.8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isabel G, Pascual A, Preat T. Exclusive consolidated memory phases in Drosophila. Science. 2004;304:1024–1027. doi: 10.1126/science.1094932. [DOI] [PubMed] [Google Scholar]
- 8.Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Lin HH, Lai JS, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- 12.Yu HH, Lee T. Neuronal temporal identity in post-embryonic Drosophila brain. Trends Neurosci. 2007;30:520–526. doi: 10.1016/j.tins.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Keene AC, Waddell S. Drosophila olfactory memory: Single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- 14.Fiala A. Olfaction and olfactory learning in Drosophila: Recent progress. Curr Opin Neurobiol. 2007;17:720–726. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Berry J, Krause WC, Davis RL. Olfactory memory traces in Drosophila. Prog Brain Res. 2008;169:293–304. doi: 10.1016/S0079-6123(07)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 17.Waddell S, Armstrong JD, Kitamoto T, Kaiser K, Quinn WG. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. doi: 10.1016/s0092-8674(00)00183-5. [DOI] [PubMed] [Google Scholar]
- 18.Dubnau J, Tully T. Functional anatomy: From molecule to memory. Curr Biol. 2001;11:R240–R243. doi: 10.1016/s0960-9822(01)00115-4. [DOI] [PubMed] [Google Scholar]
- 19.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 20.Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- 22.Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Cammarota M, Bevilaqua LR, Medina JH, Izquierdo I. ERK1/2 and CaMKII-mediated events in memory formation: Is 5HT regulation involved? Behav Brain Res. 2008;195:120–128. doi: 10.1016/j.bbr.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 24.Ogren SO, et al. The role of 5-HT(1A) receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Sossin WS. Isoform specificity of protein kinase Cs in synaptic plasticity. Learn Mem. 2007;14:236–246. doi: 10.1101/lm.469707. [DOI] [PubMed] [Google Scholar]
- 26.Tierney AJ. Structure and function of invertebrate 5-HT receptors: A review. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:791–804. doi: 10.1016/s1095-6433(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Q, Lin F, Zheng X, Sehgal A. Serotonin modulates circadian entrainment in Drosophila. Neuron. 2005;47:115–127. doi: 10.1016/j.neuron.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Yuan Q, Joiner WJ, Sehgal A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr Biol. 2006;16:1051–1062. doi: 10.1016/j.cub.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Paul V, Balasubramaniam E, Kazi M. The neurobehavioural toxicity of endosulfan in rats: A serotonergic involvement in learning impairment. Eur J Pharmacol. 1994;270:1–7. doi: 10.1016/0926-6917(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 30.Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet. 2007;39:678–682. doi: 10.1038/ng2029. [DOI] [PubMed] [Google Scholar]
- 31.Lundell MJ, Hirsh J. eagle is required for the specification of serotonin neurons and other neuroblast 7-3 progeny in the Drosophila CNS. Development. 1998;125:463–472. doi: 10.1242/dev.125.3.463. [DOI] [PubMed] [Google Scholar]
- 32.Quinn WG, Dudai Y. Memory phases in Drosophila. Nature. 1976;262:576–577. doi: 10.1038/262576a0. [DOI] [PubMed] [Google Scholar]
- 33.Folkers E, Waddell S, Quinn WG. The Drosophila radish gene encodes a protein required for anesthesia-resistant memory. Proc Natl Acad Sci USA. 2006;103:17496–17500. doi: 10.1073/pnas.0608377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao X, et al. Localization of serotonin/tryptophan-hydroxylase-immunoreactive cells in the brain and suboesophageal ganglion of Drosophila melanogaster. Cell Tissue Res. 2010;340:51–59. doi: 10.1007/s00441-010-0932-5. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Chaney S, Roberts IJ, Forte M, Hirsh J. Ectopic G-protein expression in dopamine and serotonin neurons blocks cocaine sensitization in Drosophila melanogaster. Curr Biol. 2000;10:211–214. doi: 10.1016/s0960-9822(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 36.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123:945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 38.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Keene AC, Krashes MJ, Leung B, Bernard JA, Waddell S. Drosophila dorsal paired medial neurons provide a general mechanism for memory consolidation. Curr Biol. 2006;16:1524–1530. doi: 10.1016/j.cub.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 40.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28:3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sitaraman D, et al. Serotonin is necessary for place memory in Drosophila. Proc Natl Acad Sci USA. 2008;105:5579–5584. doi: 10.1073/pnas.0710168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drier EA, et al. Memory enhancement and formation by atypical PKM activity in Drosophila melanogaster. Nat Neurosci. 2002;5:316–324. doi: 10.1038/nn820. [DOI] [PubMed] [Google Scholar]
- 43.Grünbaum L, Müller U. Induction of a specific olfactory memory leads to a long-lasting activation of protein kinase C in the antennal lobe of the honeybee. J Neurosci. 1998;18:4384–4392. doi: 10.1523/JNEUROSCI.18-11-04384.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C. J Neurosci. 2004;24:3600–3609. doi: 10.1523/JNEUROSCI.1134-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saudou F, Boschert U, Amlaiky N, Plassat JL, Hen R. A family of Drosophila serotonin receptors with distinct intracellular signalling properties and expression patterns. EMBO J. 1992;11:7–17. doi: 10.1002/j.1460-2075.1992.tb05021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Swinderen B, Brembs B. Attention-like deficit and hyperactivity in a Drosophila memory mutant. J Neurosci. 2010;30:1003–1014. doi: 10.1523/JNEUROSCI.4516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD) Prog Brain Res. 2008;172:543–565. doi: 10.1016/S0079-6123(08)00926-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.